Abstract
Background
Androgen deprivation therapy (ADT) in localized prostate cancer improves overall survival and is recommended by National Comprehensive Cancer Network guidelines in certain situations. However, ADT is without benefit in other situations and can actually cause harm. This study examines recent trends in the ADT use and quantifies the cost of guideline-discordant ADT.
Patients and methods
Patients, aged 66–80 years, in the Surveillance Epidemiology and End Results-Medicare database with non-metastatic prostate cancer diagnosed between 2004 and 2007 were included for analysis. Prostate-specific antigen, Gleason score, and stage were used to define D'Amico risk categories. Logistic regression was used to examine factors associated with guideline-discordant ADT. Annual direct cost was estimated using 2011 Medicare reimbursement for ADT.
Results
Of 28 654 men included, 12.4% received guideline-discordant ADT. In low-risk patients, 14.9% received discordant ADT, mostly due to simultaneous ADT with radiation. Discordant use was seen in 7.3% of intermediate and 14.9% of high-risk patients, mostly from ADT as primary therapy. The odds of receiving guideline-discordant ADT decreased over time (2007 versus 2004; OR 0.69; 95% CI 0.62–0.76). The estimated annual direct cost from discordant ADT is $42 000 000.
Conclusion
Approximately one in eight patients received ADT discordant with published guidelines. Elimination of discordant use would result in substantial savings.
Keywords: drug costs, gonadotropin-releasing hormone, health services, prostatic neoplasms, SEER program
introduction
Prostate cancer affects ∼240 000 men each year in the United States, and 81% will present with a localized and potentially curable disease [1]. This cancer disproportionately affects the Medicare population with 62% of all cases being diagnosed in those of age 65 years or older [2]. It is common practice to categorize patients based on clinical stage, Gleason score, and the prostate-specific antigen (PSA) level into low-, intermediate, and high-risk diseases [3]. This risk grouping allows for treatment recommendations based on cancer aggressiveness, including for some men no treatment [4]. There is increasing recognition of overtreatment in prostate cancer including the use of androgen deprivation therapy (ADT) in settings where it is not clinically indicated [5–7].
ADT has a well-studied and established role in localized prostate cancer treatment. For men with intermediate or high-risk disease treated with external beam radiation therapy (RT), multiple randomized trials have demonstrated that adding ADT significantly improves overall survival compared with RT alone, and ADT is currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines in this setting [8–13]. These guidelines do not recommend the use of ADT with RT in low-risk cancer, the addition of ADT to prostatectomy or ADT after brachytherapy. Further, ADT as primary therapy (without surgery or radiation) has been found to have no significant benefit when compared with no treatment, and is not recommended for patients in any risk category [14]. An important concern in guideline-discordant uses of ADT is the potential harm to the patient without clear benefit, as incident diabetes, cardiovascular disease, fractures, mental health conditions and other health problems have all been associated with ADT [15–24].
Previous research demonstrated that in the Medicare population, use of ‘inappropriate’ ADT dramatically decreased after a Medicare policy change in 2004–2005 reduced the financial incentive to physicians for providing ADT [6]. The addition of PSA and Gleason score information to SEER-Medicare starting in 2004 allowed for quantification of guideline discordant ADT use by risk group, which previously was impossible with SEER-Medicare studies including the prior publication. The goal of the current study is to examine recent patterns of ADT use, quantify guideline concordant versus discordant use by risk group, and to estimate the financial burden of discordant use on Medicare.
patients and methods
data source
This study was exempted by the University of North Carolina Institutional Review Board. We used the Surveillance, Epidemiology, and End Results cancer registry linked to Medicare claims data (SEER-Medicare) to identify patients diagnosed with prostate cancer. SEER-Medicare is widely used to examine patterns of care in the over 65-year-old population [25].
patient selection
We included men diagnosed with clinically localized prostate cancer between 2004 and 2007 with no other malignancies and excluded men in whom the diagnosis was first made on the death certificate or autopsy. The year cutoffs were used as PSA, and Gleason score were not available in SEER-Medicare before 2004, precluding risk stratification, and 2007 was the most recent year with available data. To allow for the assessment of baseline comorbidity and initial treatment, we excluded men not continuously enrolled in Medicare part A and B and those enrolled in a HMO, for 1 year before and after diagnosis. This resulted in 54 703 patients considered potentially eligible for inclusion.
We further restricted the cohort by excluding patients over age 79 as the optimal treatment strategy in this population is unclear (N = 8879). Patients with locally advanced disease who are unlikely curable (T3b-T4, lymph node positive) were also excluded (N = 1088) as were patients with missing information for risk stratification (N = 10 563) or other important variables (N = 1021). We restricted the analysis to African American and Caucasian patients due to the small numbers of patients in other racial groups (N = 4498). This resulted in a final cohort of 28 654 patients.
outcome and control variables
We used SEER patient-level data for the year of diagnosis and to categorize patients into D'Amico risk groups: low risk (clinical T stage T1-T2a, Gleason score ≤6, and PSA ≤10 ng/ml), intermediate risk (T2b, or Gleason score 7, or PSA >10 and ≤20 ng/ml), and high risk (T2c or T3a, or PSA >20 ng/ml, or Gleason score ≥8) [3]. SEER also provided data on race, age, marital status, a census tract measure of socioeconomic status (education), SEER region, and population density of the patient's residence (urban versus rural).
Treatment received was assessed using Current Procedural Technology/Healthcare Common Procedure Coding System (CPT/HCPCS) codes in Medicare data to identify prostatectomy, RT, brachytherapy, and ADT received within 12 months of diagnosis (supplementary Table S1, available at Annals of Oncology online). Medicare also provided data on an individual level socioeconomic measure (Medicaid dual-eligibility), and claims from the 12 months before prostate cancer diagnosis to calculate a modified Charlson comorbidity index score validated specifically in claims data, the National Cancer Institute Combined Index score [26].
According to the NCCN guidelines, discordant ADT included ADT as primary therapy in all risk groups and its use with RT in low-risk disease. The use of ADT before brachytherapy was considered concordant because it is often used to downsize a large prostate to facilitate brachytherapy; however, ADT after brachytherapy has no clear benefit (discordant). In addition, ADT given before prostatectomy was guideline discordant, as multiple randomized trials have demonstrated no benefit [27–29]. In order to be conservative, we categorized ADT after prostatectomy as ‘guideline-concordant’ because these patients may have had a biochemical recurrence.
statistical and cost analysis
We present descriptive statistics for the initial treatment received by patients stratified by risk group. Bivariate and multivariate logistic regression models were used to examine the potential association between covariates and guideline-discordant ADT as the dependent variable. Covariates included risk group, race (Caucasian or African American), age at diagnosis (66–69, 70–74, 75–79 years), year of diagnosis, marital status, comorbidity score (stratified by the median value of 0), census-tract education, Medicaid eligibility, SEER region, and population density (urban versus rural). All tests were two sided and reported with confidence intervals, with P < 0.05 considered statistically significant. Analyses were carried out with SAS 9.2 (Cary, NC).
Direct cost estimates were calculated based on the cost of ADT from the Medicare code J9217 (which accounts for most of Medicare's ADT claims) in the fourth quarter of 2011. This reported cost of $221.07 was multiplied by the estimated yearly incidence of localized prostate cancer in the over 65-year-old population as provided by SEER cancer statistics [1], by the proportion of this population receiving discordant ADT based on findings from this study, and then by an average duration of ADT. We considered ADT durations of 6 months, 1 year, and 2 years in the cost estimates, with 1 year used as the base estimate. Additionally, we examined the cost from discordant ADT use specifically in low-risk men, as these patients may not need any treatment at all.
results
The baseline characteristics of the cohort are listed in Table 1. Approximately one-third of patients were in each risk group: low, intermediate, and high. Overall, 11.2% of the cohort was African American, and 67.8% had a low comorbidity score.
Table 1.
Patient characteristics of men with localized prostate cancer in SEER-Medicare 2004–2007, N = 28 654
| Characteristics | N (%) |
|---|---|
| Risk group | |
| Low | 9404 (32.8) |
| Intermediate | 9452 (32.9) |
| High | 9798 (34.2) |
| Race | |
| Caucasian | 25 445 (88.8) |
| African-American | 3209 (11.2) |
| Age at diagnosis (years) | |
| 66–69 | 9708 (33.9) |
| 70–74 | 11 060 (38.6) |
| 75–79 | 7886 (27.5) |
| Year of diagnosis | |
| 2004 | 7056 (24.6) |
| 2005 | 6875 (24.0) |
| 2006 | 7382 (25.8) |
| 2007 | 7341 (25.6) |
| Marital status | |
| Married | 20 701 (72.2) |
| Not married/unknown | 7953 (27.8) |
| NCI comorbidity index score | |
| 0 | 19 413 (67.8) |
| >0 | 9241 (32.2) |
| Proportion of non-HS graduates in census tract | |
| 0–25% | 7345 (25.6) |
| 26–50% | 7281 (25.4) |
| 51–75% | 7069 (24.7) |
| 76–100% | 6959 (24.3) |
| State Medicaid dual-eligibility | |
| No | 26 189 (91.4) |
| Yes | 2465 (8.6) |
| Geographic region | |
| Northeast | 6948 (24.2) |
| South | 5936 (20.7) |
| Central | 5077 (17.7) |
| West | 10 693 (37.3) |
| Residence | |
| Rural | 4417 (15.4) |
| Urban | 24 237 (84.6) |
Geographic regions are Northeast (Connecticut, New Jersey), South (Atlanta, Rural Georgia, Kentucky, Louisiana), Central (Detroit, Iowa, Utah, New Mexico), and West (California, Seattle, Hawaii).
NCI, National Cancer Institute; HS, high school.
Table 2 shows the frequency of guideline-discordant and concordant ADT stratified by risk group. Overall, 12.4% of patients received guideline-discordant ADT, including 14.9% of low-risk patients, 7.3% of intermediate risk patients, and 14.9% of high-risk patients. Most of the guideline-discordant use in high-risk (1228/1456 patients) and intermediate risk men (492/689) was due to ADT as primary therapy. On the other hand, the majority of guideline-discordant ADT in the low-risk group was due to its addition to external beam RT or after brachytherapy (1040/1405).
Table 2.
Frequency of guideline-discordant versus concordant ADT according to NCCN guidelines by risk group for men with localized prostate cancer
| Discordant N (%) | Concordant N (%) | |
|---|---|---|
| Low risk (N = 7404) | 1405 (14.9) | 7999 (85.1) |
| No ADT | 7412 (78.8) | |
| ADT only | 309 (3.3) | |
| ADT + RT | 722 (7.7) | |
| ADT + BTa | 318 (3.4) | 562 (6.0) |
| ADT + surgeryb | 56 (0.6) | 25 (0.3) |
| Intermediate risk (N = 8189) | 689 (7.3) | 8763 (92.7) |
| No ADT | 6331 (67.0) | |
| ADT only | 492 (5.2) | |
| ADT + RT | 1644 (17.4) | |
| ADT + BTa | 66 (0.7) | 650 (6.9) |
| ADT + surgeryb | 131 (1.4) | 138 (1.5) |
| High risk (N = 8687) | 1456 (14.9) | 8342 (85.1) |
| No ADT | 4470 (45.6) | |
| ADT only | 1228 (12.5) | |
| ADT + RT | 2810 (28.7) | |
| ADT + BTa | 40 (0.4) | 730 (7.4) |
| ADT + surgeryb | 188 (1.9) | 332 (3.4) |
aADT is concordant when used before brachytherapy (e.g. for prostate downsizing), but discordant after brachytherapy. Combination brachytherapy with external beam radiation is considered as external beam radiation and consistent with clinical practice and the NCCN guidelines.
bADT is concordant when used after surgery (e.g. for possible recurrence) but discordant before surgery.
ADT, androgen deprivation therapy; RT, radiotherapy; BT, brachytherapy.
Bivariate and multivariate logistic regression results are presented in Table 3. On multivariate analysis, patients who were older [Odds ratio (OR) 1.33 for age 70–74 years, OR 2.32 for age 75–79 years], or had a higher baseline comorbidity (OR 1.32) were significantly more likely to receive guideline-discordant ADT. African American race was borderline significantly associated with receipt of guideline-discordant ADT. There was a decrease in guideline discordant use from 2004 to 2007(OR 0.69, for 2007 versus 2004).
Table 3.
Logistic regression models of guideline-discordant ADT in localized prostate cancer
| Bivariate |
Multivariate |
|||
|---|---|---|---|---|
| Covariate | OR | 95% CI | OR | 95% CI |
| Risk group | ||||
| Low | – | – | – | – |
| Intermediate | 0.45 | 0.41–0.49 | 0.42 | 0.38–0.47 |
| High | 0.99 | 0.92–1.08 | 0.85 | 0.78–0.92 |
| Race | ||||
| Caucasian | – | – | – | – |
| African-American | 1.38 | 1.25–1.53 | 1.12 | 1.00–1.26 |
| Age at diagnosis (years) | ||||
| 66–69 | – | – | – | – |
| 70–74 | 1.33 | 1.21–1.45 | 1.33 | 1.21–1.46 |
| 75–79 | 2.26 | 2.07–2.48 | 2.32 | 2.11–2.54 |
| Year of diagnosis | ||||
| 2004 | – | – | – | – |
| 2005 | 0.88 | 0.80–0.97 | 0.89 | 0.80–0.98 |
| 2006 | 0.78 | 0.71–0.86 | 0.78 | 0.71–0.86 |
| 2007 | 0.67 | 0.61–0.74 | 0.69 | 0.62–0.76 |
| Marital status | ||||
| Married | – | – | – | – |
| Not married/unknown | 1.55 | 1.44–1.67 | 1.35 | 1.25–1.46 |
| NCI comorbidity index score | ||||
| 0 | – | – | – | – |
| >0 | 1.48 | 1.38–1.59 | 1.32 | 1.23–1.42 |
| Proportion of non-HS graduates in census tract | ||||
| 0–25% | – | – | – | – |
| 26–50% | 1.16 | 1.04–1.29 | 1.05 | 0.94–1.17 |
| 51–75% | 1.34 | 1.21–1.48 | 1.16 | 1.04–1.29 |
| 76–100% | 1.72 | 1.56–1.91 | 1.31 | 1.18–1.47 |
| State Medicaid dual-eligibility | ||||
| No | – | – | – | – |
| Yes | 2.15 | 1.94–2.39 | 1.73 | 1.55–1.94 |
| Geographic region | ||||
| Northeast | – | – | – | – |
| South | 1.41 | 1.27–1.56 | 1.26 | 1.13–1.41 |
| Central | 1.02 | 0.91–1.14 | 0.94 | 0.84–1.06 |
| West | 0.94 | 0.86–1.04 | 0.94 | 0.85–1.04 |
| Residence | ||||
| Rural | – | – | – | – |
| Urban | 0.67 | 0.61–0.73 | 0.75 | 0.68–0.84 |
Geographic regions are Northeast (Connecticut, New Jersey), South (Atlanta, Rural Georgia, Kentucky, Louisiana), Central (Detroit, Iowa, Utah, New Mexico), and West (California, Seattle, Hawaii).
Multivariable analysis adjusted for: risk group, race, age, year of diagnosis, marital status, NCI comorbidity index, measure of education by census tract, Medicaid dual-eligibility, geographic region, and population density.
OR, odds ratio; CI, confidence interval; NCI, National Cancer Institute; HS, high school.
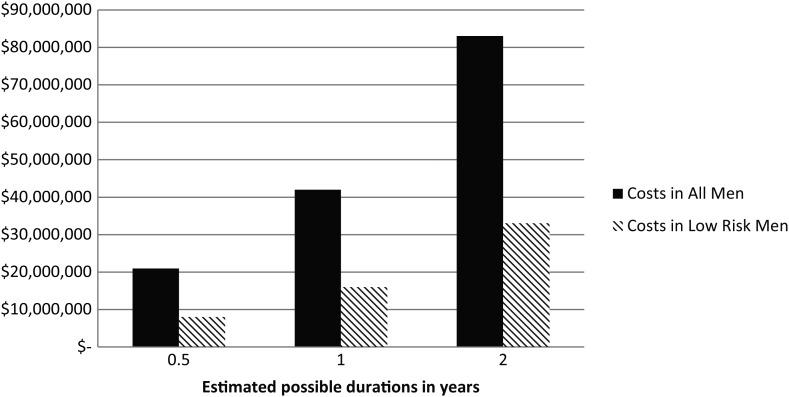
The direct cost to Medicare from discordant ADT is estimated at $42 000 000 per year if we conservatively assume an average duration of therapy of 1 year. The cost changes proportionally if we assume shorter or longer durations of ADT (Figure 1). The direct cost from the low-risk group alone is $16 000 000 per year assuming an average duration of 1 year of therapy.
Figure 1.
Direct annual costs of discordant ADT use to Medicare assuming the different average durations of therapy. ADT, androgen deprivation therapy. Cost calculated from fourth quarter of 2011 Medicare's most common ADT claim (J9217, $221.07), multiplied by the estimated number of Medicare patients with localized prostate cancer (126 911), the prevalence of guideline discordant therapy (12.4% for all patients, 14.9% low risk), and different potential durations of ADT use (6 months, 1, and 2 years).
discussion
In this population-based study of a recent cohort of men with localized and potentially curable (non-metastatic, not locally advanced) prostate cancer, we found that approximately one in eight (12.4%) received guideline-discordant ADT within the first year after diagnosis. We were able to further examine the source of guideline-discordant use based on risk-based subgroups and found that in intermediate and high-risk patients the source of guideline-discordance was mostly due to ADT use as primary therapy. On the other hand, in the low-risk population, the majority of guideline-discordance was due to ADT given with radiation. This study is unique as the first to quantify the contemporary patterns of discordant ADT use based on published guidelines, which was not possible previously due to lack of PSA and Gleason information in SEER. This discordant use of ADT results in a large expenditure to Medicare.
While ADT has demonstrated efficacy in prostate cancer treatment, there are also well-described long-term side-effects. Keating et al. [17] have shown in a population-based study of veterans that ADT is associated with a 28% increase in the risk of incident diabetes and an ∼20% increased risk in vascular morbidity, including coronary artery disease, stroke, and myocardial infarction [17]. A SEER-Medicare study found an increase in the risk of bone fractures in men receiving ADT [18]. Additionally, there have been other published findings on a range of adverse effects, including increased anemia, decreased vitality and sexual function, worsening of cognition and mood, as well as increased obesity and changes in body fat redistribution [21–24].
These important side-effects raise particular concern for the ADT use in situations where it has no proven benefit. Multiple randomized trials examining the addition of ADT to prostatectomy have failed to show improvements in disease control or survival [30–33]. Equally, primary ADT has no demonstrated benefit in localized prostate cancer compared with no treatment [14], and in two randomized trials primary ADT resulted in worse survival compared with RT [34, 35]. Further, while ADT when added to RT has been shown to improve overall survival in randomized trials for men with intermediate and high-risk cancers, no clear benefit has been shown when ADT is added to RT for low-risk disease [8–10, 13, 36, 37]. The ADT use in these clinical situations is not supported by existing literature and is discordant with published NCCN guidelines.
It is likely that in men with low-risk disease, the use of ADT either as primary therapy or when added to RT represents overtreatment. The issue of overtreatment in prostate cancer recently has gained more attention, and applies especially to low-risk (slow growing) disease [5]. Given the overwhelmingly good outcomes of men with low-risk disease receiving RT, BT, prostatectomy, and even on active surveillance, the addition of ADT in these patients is more likely to harm than benefit the patient.
However, in high-risk and some intermediate risk men, guideline-discordant ADT may represent undertreatment of a potentially curable malignancy, possibly due to inadequate referral patterns or perceived patient frailty [38]. There is an emerging literature describing the undertreatment of elderly patients with aggressive cancers. For example, elderly patients with colorectal cancer are less likely to receive adjuvant chemotherapy for potentially curable disease, while elderly patients with head and neck cancer receive less total radiation than younger patients [39, 40]. Our data suggest that this finding may also apply in elderly patients with high-risk prostate cancer. However, the important difference in prostate compared with other cancers is that overtreatment within prostate cancer is possible as well as undertreatment. Further complicating this is that undertreatment uses an agent that can actually be harmful to the patient. Examination of failure to use ADT in guideline concordant situations (e.g. intermediate and high-risk men receiving RT) was not done in this analysis but could be an important issue to examine in future studies.
We examined multiple demographic factors to determine which men were more likely to receive guideline-discordant therapy. In addition to older age, men with more comorbidities were more likely to receive guideline-discordant ADT. This may be due to a desire from physicians and patients to ‘do something’ for a diagnosed prostate cancer, when in fact their competing causes of mortality are higher than in younger and healthier patients [41, 42]. In this study, there was an encouraging finding of a declining rate of guideline-discordant ADT from 2004 to 2007, though there remained significant use of guideline-discordant ADT as recently as 2007.
In the current era of large increases in healthcare spending and with ongoing national discussions about the long-term fiscal viability of Medicare, we felt it was informative to make a direct estimate of the cost outlays of this therapy to the Medicare program. Conservatively assuming an average duration of 1 year of ADT, the direct cost from guideline-discordant ADT use is $42 000 000 per year. Importantly, our analysis was based on the cost of ADT alone and did not include potential costs such as facility charges for the appointment or the cost of long-term complications due to ADT use. In low-risk patients for whom active surveillance is a potential management option, the cost from discordant ADT is $16 000 000. On the other hand, for intermediate and high-risk patients, forgoing ADT alone (discordant) and instead pursuing surgical or radiation treatment may actually increase utilization and direct costs to Medicare. However, surgical and radiation treatments for aggressive prostate cancers have a curative potential and have been found to be cost effective, while ADT alone provides no proven benefit [43].
There are several limitations to this analysis. Medicare data may be subject to misclassification and the potential exists for incorrect treatment assignment. However, SEER-Medicare is commonly used and has established methodology for assessing patterns of care [44–46], and the large numbers of patients allow stable estimates of treatment patterns. Also, as the treatment decision-making process is complex, we do not have information regarding why guideline discordant therapy was given. In some patients, there could be extenuating circumstances leading to guideline discordant but not ‘inappropriate’ therapy. However, NCCN guideline concordance is an established quality of care indicator [47], and our finding that one in eight Medicare patients received guideline-discordant ADT is concerning.
In summary, this study demonstrated in a recent cohort of Medicare patients that one in eight men over the age of 65 with localized prostate cancer received ADT discordant with published practice guidelines. Primary ADT, and simultaneous ADT with radiation in low-risk cancer, are significant sources of guideline discordant use. The ADT use in these settings has no proven benefit but has well-established potential harms, and results in significant costs to Medicare.
funding
This work was supported in part by the Agency for Healthcare Research and Quality DEcIDE program (HHSA29020050040I); NRSA Post-doctoral Fellowship from the Agency of Healthcare Research and Quality, sponsored by the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill, (T32HS019442 to AK); and National Institutes of Health Clinical and Translational Science Award (UL1RR025747).
disclosure
The authors have declared no conflicts of interest. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Supplementary Material
acknowledgement
The authors thank Jane Darter for her assistance with data management.
references
- 1.Bethesda, MD: ational Cancer Institute; SEER Cancer Statistics Review, 1975–2008. http://seer.cancer.gov/csr/1975_2008/ 9 December 2011, ccessed) [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2011 . http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2011. 9 December 2011, ccessed)
- 3.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29(27):3669–3676. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 6.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363(19):1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 7.Swisher-McClure S, Pollack CE, Christodouleas JP, et al. Variation in use of androgen suppression with external-beam radiotherapy for nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):8–15. doi: 10.1016/j.ijrobp.2011.06.1951. doi:10.1016/j.ijrobp.2011.06.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11(11):1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 9.Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of radiation therapy oncology group protocol 85–31. J Clin Oncol. 1997;15(3):1013–1021. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 11.Granfors T, Modig H, Damber JE, et al. Long-term followup of a randomized study of locally advanced prostate cancer treated with combined orchiectomy and external radiotherapy versus radiotherapy alone. J Urol. 2006;176(2):544–547. doi: 10.1016/j.juro.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 12.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 13.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 14.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300(2):173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25(17):2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 16.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 17.Keating NL, O'Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 19.Stoch SA, Parker RA, Chen L, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001;86(6):2787–2791. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- 20.Morote J, Martinez E, Trilla E, et al. Osteoporosis during continuous androgen deprivation: influence of the modality and length of treatment. Eur Urol. 2003;44(6):661–665. doi: 10.1016/s0302-2838(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 21.Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94(6):430–437. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- 22.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18(3):237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strum SB, McDermed JE, Scholz MC, et al. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol. 1997;79(6):933–941. doi: 10.1046/j.1464-410x.1997.00234.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 25.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-medicare data: content, research applications, and generalizability to the united states elderly population. Med Care. 2002;40(8 Suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. –. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Labrie F, Cusan L, Gomez JL, et al. Downstaging by combination therapy with flutamide and an LHRH agonist before radical prostatectomy. Cancer Surv. 1995;23:149–156. [PubMed] [Google Scholar]
- 28.Goldenberg SL, Klotz LH, Srigley J, et al. Randomized, prospective, controlled study comparing radical prostatectomy alone and neoadjuvant androgen withdrawal in the treatment of localized prostate cancer. canadian urologic oncology group. J Urol. 1996;156(3):873–877. [PubMed] [Google Scholar]
- 29.Soloway MS, Sharifi R, Wajsman Z, et al. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. the lupron depot neoadjuvant prostate cancer study group. J Urol. 1995;154(2 Pt 1):424–428. [PubMed] [Google Scholar]
- 30.Dalkin BL, Ahmann FR, Nagle R, et al. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. J Urol. 1996;155(4):1357–1360. [PubMed] [Google Scholar]
- 31.Klotz LH, Goldenberg SL, Jewett MA, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170(3):791–794. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 32.Schulman CC, Debruyne FM, Forster G, et al. 4-year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2–3N0M0 prostate cancer. european study group on neoadjuvant treatment of prostate cancer. Eur Urol. 2000;38(6):706–713. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 33.Wirth MP, Weissbach L, Marx FJ, et al. Prospective randomized trial comparing flutamide as adjuvant treatment versus observation after radical prostatectomy for locally advanced, lymph node-negative prostate cancer. Eur Urol. 2004;45(3):267–270. doi: 10.1016/j.eururo.2003.10.013. discussion 270. [DOI] [PubMed] [Google Scholar]
- 34.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 35.Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50(5):1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 37.D'Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 38.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170(5):440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21(7):1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 40.Huang SH, O'Sullivan B, Waldron J, et al. Patterns of care in elderly head-and-neck cancer radiation oncology patients: a single-center cohort study. Int J Radiat Oncol Biol Phys. 2011;79(1):46–51. doi: 10.1016/j.ijrobp.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 41.Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institute for Health and Clinical Excellence. Prostate Cancer: Diagnosis and Treatment. London: National Institute for Health and Clinical Excellence; 2008. CG58. [Google Scholar]
- 44.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 45.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30(14):1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the department of veterans affairs. J Clin Oncol. 2010;28(19):3176–3181. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.