Abstract
Background
Treatments of non-small-cell lung cancer (NSCLC)—particularly of the squamous subtype—are limited. In this article, we describe the immunomodulatory environment in NSCLC and the potential for therapeutic targeting of the immune system through cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death-1 (PD-1) immune-checkpoint pathway blockade.
Materials and methods
We searched PubMed and presented abstracts for publications describing the clinical benefit of checkpoint blockade in NSCLC.
Results
Antibody-mediated checkpoint molecule blockade is being investigated in NSCLC, and of these approaches, the anti-CTLA-4 antibody ipilimumab has undergone the most extensive clinical study. By targeting the immune system rather than specific antigens, checkpoint blockade agents differ from vaccine therapy. In a phase II study in advanced NSCLC, phased ipilimumab with chemotherapy demonstrated the greatest efficacy in squamous NSCLC. A phase I study of nivolumab, an anti-PD-1 antibody, has suggested that this agent is also active against squamous and non-squamous NSCLC. Ongoing phase III studies are evaluating the therapeutic potential of these agents.
Conclusions
Although treatment options for NSCLC are limited, a better understanding of the immune profile of this disease has facilitated the development of immunotherapeutics that target checkpoint blockade molecules, and clinical evaluation to date supports combining checkpoint blockade with chemotherapy for squamous NSCLC.
Keywords: CTLA-4 blockade, immunomodulators, ipilimumab, non-small-cell lung cancer, squamous histology
introduction
‘Lung cancer’, which is usually diagnosed at an advanced stage, has a dismal prognosis and is the leading cause of cancer deaths worldwide (over 1.4 million deaths in 2008) [1, 2]. In the United States, it is estimated that over 221 000 new cases of lung cancer were diagnosed in 2011, and that ∼157 000 people died of the disease [3]. In Europe, lung cancer was the third most common cancer in 2006 [4], with a crude incidence of 52.5/100 000 per year, and the mortality 148 000 per year [5]. Thus, the disease burden of lung cancer is considerable.
The two major types of lung cancer are non-small-cell lung cancer (NSCLC), which accounts for 80%–85% of all cases; and small-cell lung cancer, which accounts for the remaining 15%–20% [1, 3, 5, 6] NSCLC is further classified into subtypes based on histology, with ∼30% of cases being squamous-cell carcinoma. The remaining 70% may collectively be classified as non-squamous NSCLC; most of these tumors are adenocarcinomas, but this group also includes large-cell carcinomas and less well-differentiated variants [1, 7, 8].
Recent advances in targeted therapy for NSCLC have augmented treatment choices for non-squamous NSCLC [9], but chemotherapy remains the therapeutic mainstay for squamous NSCLC. The use of pemetrexed in doublet chemotherapy regimens is only recommended for non-squamous NSCLC, based on a phase III randomized trial of gemcitabine–cisplatin versus pemetrexed–cisplatin, which demonstrated superior survival for pemetrexed–cisplatin in advanced NSCLC of non-squamous (but not squamous) histology [10]. Other targeted therapies with substantial clinical benefit in non-squamous NSCLC may have limited use in the squamous subtype due to a greater risk of serious toxicity (bevacizumab) [11, 12] or the possibility of reduced efficacy (erlotinib and gefitinib) [13, 14]. Thus, chemotherapy with carboplatin–paclitaxel (Taxol, Bristol-Myers Squibb) remains the standard first-line treatment for squamous NSCLC in the United States [9], whereas in Europe, platinum-based combination therapy with a third-generation agent (i.e. gemcitabine) is preferred and widely used [6].
Ongoing research is revealing clinical and molecular differences between squamous and non-squamous NSCLC which may help to explain these divergent phenotypes and may impact treatment decisions. These findings have also provided the impetus for researchers to explore novel therapeutic approaches to treating squamous NSCLC, and one of these approaches is immunotherapy. Circulating immune cells may have the ability to recognize, infiltrate and eliminate some incipient cancer cells, but some evade immune surveillance and immune system-mediated cell death [15]. Recent studies have improved our understanding of the molecular basis for this phenomenon, and have aided in the identification of anticancer approaches that act by modulating the immune system directly. In this review, following a brief discussion of the immunomodulatory environment in NSCLC, we will focus on the potential to target the immune system by blockade of two immune-checkpoint pathways in squamous-cell NSCLC: cytotoxic T-lymphocyte antigen 4 (CTLA-4) [16, 17] and programmed death-1 (PD-1) [18].
the role of the immune system in carcinogenesis
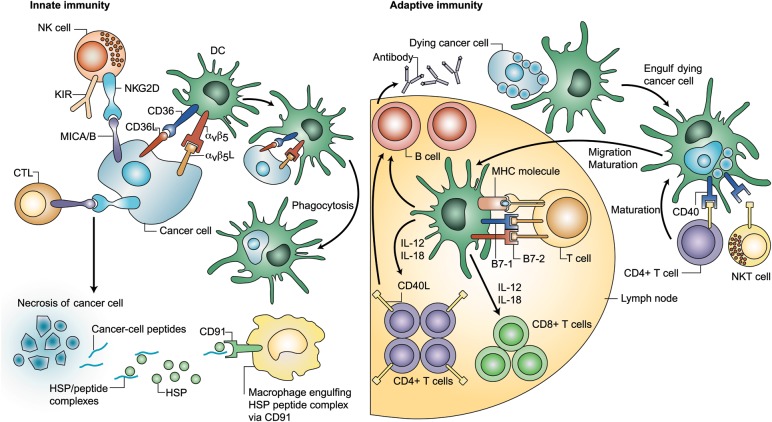
Immune surveillance involves cell types in both branches of the immune system: innate [e.g. natural killer (NK) cells] and adaptive [e.g. CD8+ cytotoxic T-lymphocytes (CTLs), CD4+ T-helper cells] (Figure 1) [19, 20]. The observed tumor-infiltrating lymphocytes (TILs) altered cytokine profiles in NSCLC suggest that a host-mounted, antitumor immune response against the tumor occurs, but growth and progression continue in spite of this response [21].
Figure 1.
Crosstalk between immune cells and cancer cells [20]. (Adapted and reprinted with permission from Copyright Clearance Center [20]) DC, dendritic cell; HSP, heat shock protein; IL, interleukin; MHC, major histocompatibility complex; NK, natural killer.
Tumors may induce immune tolerance or resist destruction by activated immune effector cells through multiple proposed mechanisms [21]. A number of human tumors (including lung tumors) repress modulation of the adaptive immune system [e.g. reduced expression of class I major histocompatibility complex (MHC) molecules] [21] and the innate immune system (e.g. downregulation of activating NK ligands such as NKG2D [22] and overexpression of membrane complement regulators, particularly CD46, CD55 and CD59) [23]. Moreover, some tumor cell lines avoid Fas-induced apoptosis through downregulating Fas receptor cell surface expression, which renders them ‘invisible’ to NK cells or CTLs; by altering their expression of proteins that inhibit Fas-induced apoptosis; [24, 25] or by secreting a Fas ligand, which binds to Fas on NK cells and CTLs and initiates apoptosis in the immune cells [24].
Characterization of TILs in lung tumors has revealed that these immune cells, initially thought to be exclusively CTLs, also include regulatory T cells (Tregs). Some tumor cells secrete the chemokine CCL, [22] which attracts Tregs to the tumor site [26]. As a consequence, antitumor activity of effector T cells may be blunted [26]. Clinically, the prognosis of several solid tumors (including ovarian, endometrial, nasopharyngeal, and NSCLC) is correlated to the ratio of CTLs to Tregs within the TIL population [27–30].
Tumor cells also promote an anti-inflammatory state that supports tumor growth. For example, tumor cells may secrete interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), which have immunosuppressive effects on effector T-lymphocytes and macrophages [31, 32]. Tumor-secreted cytokines may also stimulate the recruitment of macrophages, which have phenotype-contingent anti- and pro-tumor activity (M1 versus M2) [33]. Early in tumor development, M1 macrophages exposed to interferon-gamma (IFN-γ) have tumoricidal activity, but macrophages activated in response to tumor cell products, including IL-10, have the M2 phenotype and may promote tumor growth [33]. Some products of macrophages (IL-1, TNF and IL-6) may also promote metastasis [33].
immunomodulatory mechanisms in NSCLC
Tissue samples from patients with NSCLC are duplicitous, displaying signs not only of an ongoing immune response to the tumor, but also indications of an immunosuppressive microenvironment that may serve to limit this response [34]. The non-tumoral stroma, composed of fibroblasts and extracellular matrix components, endothelial cells, and angiogenic factors, also contains inflammatory cells [35]. Two-thirds of these inflammatory cells are lymphocytes, primarily T cells (80%) [35]. The nature of the immune cells within the tumor tissue may have profound clinical implications. Higher numbers of TILs have been strongly associated with a survival benefit regardless of disease subtype [36–39]. Tregs may be a particularly important prognostic indicator, as NSCLC tumors have more Tregs than normal lung tissue, and the Treg/CTL ratio is a significant predictor of recurrence [30]. In squamous NSCLC, a higher proportion of Tregs significantly increased the risk of tumor recurrence (p = 0.0148) [30]. It is thought that the antitumor response and tumor progression associated with high numbers of Tregs in NSCLC occurs through production of IL-10 and TGF-β, which may inhibit reactive T cells [40–44].
subtype specificity of NSCLC and the immune system: squamous NSCLC
Emerging evidence points to an immunologic profile for NSCLC that is at least partially subtype specific. Squamous-cell carcinoma displays a highly consistent immune profile, in terms of expression of such molecules as p63/CK5/6/34bE-12 and non-expression of thyroid transcription factor-1. In contrast, adenocarcinoma shows more heterogeneity for these and other immune elements [45]. Several separate analyses of NSCLC tumor samples from clinical trials have identified that squamous tumors more frequently express known tumor antigens [i.e. melanoma-associated antigen (MAGE)-A3, MAGE-A4 and NY-ESO-1, compared with non-squamous tumors] [46, 47]. Other tissue sample studies have reported a less extensive infiltration of Tregs and a more extensive infiltration of CD8+ effector cells in squamous tumors compared with non-squamous tumors [48, 49].
Recent studies suggest that various immune markers may have prognostic values in some subtypes of NSCLC but not others [50]. Although not fully characterized, these differences may affect the clinical activity of at least some of the immunotherapeutic approaches discussed in the next section.
modulating immune responses against NSCLC
Immunotherapy boosts the host antitumor immune response by ‘enhancing the enhancers’ (enabling components of the immune system to mount or maintain an effective response) or ‘inhibiting the inhibitors’ (suppressing factors that dampen or prevent the immune response) [51]. The former approach includes administration of effector cytokines, such as IL-2 or IFN-α, dendritic cell vaccination, injection of CTLs or activated NK cells, or vaccination with tumor-associated antigen peptides or vectors, with the goal of generating or strengthening the antitumor immune response [51].
Examples of approaches to inhibit the inhibitors include blocking Treg cell function and blocking cytokine signaling pathways, with the goal of overturning peripheral tolerance to the tumor. Another promising investigational approach involves augmenting the existing antitumor immune responses through blockade of inhibitory ‘checkpoint pathways’ (i.e. natural mechanisms that serve to limit the immune response), and this approach will comprise the remainder of this article [51].
immune checkpoint blockade
A major component of immune defense against tumors occurs in the lymph nodes, where T cells encounter tumor antigens. If this encounter leads to activation, the activated T cells then circulate to the tumor site where they can recognize, and hopefully eliminate, tumor cells [19, 21]. At least two receptor–ligand interactions are required for full T-cell activation: (i) T-cell receptor recognition of its cognate ligand presented in the context of an MHC and (ii) a second co-stimulatory signal transmitted from the antigen-presenting cells (APCs) expressing the MHC-peptide to the T cell [52]. This second signal is transmitted from the B7-1 and/or B7-2 molecules on the APC to the CD28 molecule on the T-cell [52].
There are several checkpoints in place to moderate this nascent immune response, with the goal of suppressing inappropriate responses to ‘self’ antigens and damage to normal tissue. Because some tumors also engage these checkpoint pathways to escape antitumor immune responses, checkpoint molecule blockade is being pursued as a therapeutic anticancer strategy. Two examples of immune-checkpoint pathways with the potential for therapeutic anticancer targeting include the PD-1 and CTLA-4 pathways [53].
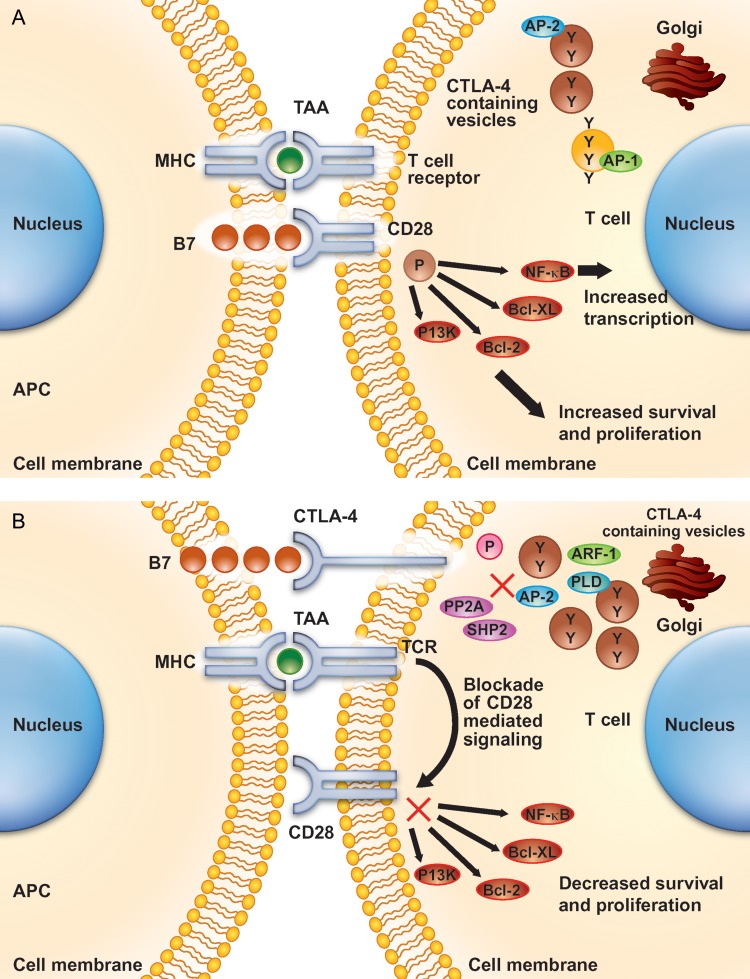
PD-1 and CTLA-4 are similar in structure and are both expressed on activated T cells (CTLA-4 is also constitutively expressed on Treg cells) [53]. Following the initial T-cell activation signal, both molecules interact with ligands on APCs to abrogate the resulting immune response. Despite these similarities, PD-1 and CTLA-4 have distinct ligand specificities and biologic functions; those related to CTLA-4 have been studied more extensively (Figure 2) [54, 55].
Figure 2.
The action of CTLA-4 in the T cell. In the resting state (A) CTLA-4 is stored in intracellular vesicles, but upon activation (B) is expressed on the cell surface. Binding of CTLA-4 to the B7 ligand on the antigen-presenting cell (APC) prevents B7 from binding to CD28, and blocks intracellular signaling that leads to T-cell proliferation [57]. (Adapted and reprinted with permission from the American Association for Cancer Research: Salama AK and Hodi FS, Cytotoxic T-lymphocyte-associated antigen-4, Clinical Cancer Research, 2011, Vol. 17, pp. 4622–4628.) APC, antigen-presenting cell; CTLA-4, cytotoxic T-lymphocyte antigen-4; HLA B7, human leukocyte antigen B7; TCR, T-cell receptor.
PD-1 interacts with the ligands PD-L1 (B7-H1) and PD-L2 (B7-DC), which result in diminished T-cell proliferation, altered cytokine production and initiation of T-cell exhaustion and/or apoptosis [56]. CTLA-4 binds to B7-1 and B7-2, which inhibits interaction of these ligands with the co-stimulatory CD28 molecule on the surface of APCs (Figure 2) [57]. Evidence to date suggests that CTLA-4 is able to outcompete CD28 for binding to B7-1 and B7-2, and it also sequesters B7-1 and B7-2 to prevent their interaction with CD28. Moreover, independently of CD28, CTLA-4 can dephosphorylate intracellular signaling molecules required for T cell activation, and it can reduce the time and extent of the T-cell/APC interaction, which transduces a ‘reverse’ signal to APCs via the B7-1 ligand; in Treg cells, the latter mechanism may serve to inhibit the response to an antigen [53, 57].
In the context of tumor immunology, CTLA-4 signaling is more evident in limiting the initiation of a T-cell response in the lymph nodes, while engagement of PD-1 is more prominent later in the process and serves to limit T-cell activity in the tumor microenvironment [58]. In murine knockout models, this difference contributes to distinct phenotypes: CTLA-4-deficient mice die within a few weeks from multi-organ immune-related reactions, while PD-1 deficient mice develop organ-specific immune reactions over a course of several months [59, 60].
In the clinic, the divergent roles of CTLA-4 and PD-1 may influence the clinical profiles of anticancer agents that target these pathways. Indeed, preliminary clinical observations have revealed some differences in the clinical profiles of antibodies that bind to either CTLA-4 or PD-1 (discussed in detail for patients with NSCLC in the sections below), but to date we lack the necessary head-to-head clinical comparisons to fully characterize these differences or understand the underlying mechanisms. The divergent roles also suggest a potential for combining or sequencing CTLA-4 and PD-1 blockade; this possibility is under clinical investigation in patients with late-stage melanoma (NCT01024231 and NCT01176461).
clinical relevance of PD-1 inhibition in NSCLC
A phase I study recently assessed the antitumor activity and safety of BMS-936558 (now known as nivolumab), a fully human IgG4-blocking monoclonal antibody directed against PD-1, in 296 patients with advanced solid tumors—including melanoma (104), NSCLC (122), renal-cell cancer (RCC) (34), castration-resistant prostate cancer (17) and colorectal cancer (CRC) (19) [18]. All patients with NSCLC had already received platinum-based chemotherapy (94%) or TKIs (34%); 55% had received more than three lines of therapy. Patients were treated with BMS-936558 at a dose of 0.1–10.0 mg/kg of body weight every 2 weeks. Response by modified RECIST criteria was assessed after each 8-week treatment cycle, and patients received up to 12 cycles until disease progression or complete response.
A maximum-tolerated dose was not defined in this study. Eighteen grade 3/4 treatment-related adverse events were observed in 41 (14%) patients. Treatment-related adverse events of special interest included pneumonitis, vitiligo, colitis, hepatitis, hypophysitis and thyroiditis. In patients with NSCLC, 14 objective responses were observed at doses of 1.0, 3.0 or 10.0 mg/kg, with response rates of 6%, 32% and 18%, respectively. Objective responses were also observed in NSCLC of squamous [6 of 18 patients (33%)] or non-squamous histologies [7 of 56 patients [12%)]. All 14 patients with objective responses began treatment 6 months or more before data analysis, and 8 (of 14) responses lasted 6 months or more, suggesting durability of response. Although these preliminary data are encouraging, the potential to select patients for treatment based on PD-L1 expression in tumors needs to be further prospectively validated. Phase III studies of BMS-936558 in NSCLC, melanoma and RCC are underway [18].
clinical significance of CTLA-4 in NSCLC
In retrospective meta-analyses, certain polymorphisms in the gene encoding CTLA-4 may be associated with increased susceptibility to solid tumors, including lung cancer. For example, the +49 adenine–guanine polymorphism has been found to increase the risk of cancer [17] and is classified as a prognostic predictor for advanced NSCLC [61]. Similarly, the −318 cytosine–thymine polymorphism has been shown to be a risk factor for cancer [17]. While these markers have not yet been thoroughly validated in large clinical studies, the observations do implicate the CTLA-4 pathway's involvement in NSCLC.
CTLA-4 blockade as an investigational anticancer strategy differs from antigen-specific immunotherapy vaccines in that it is designed to target the entire immune system [62, 63]. The exact mechanism by which antibody targeting of CTLA-4 leads to tumor cell death is not known. However, cellular analysis of antibody-mediated CTLA-4 blockade suggests that direct enhancement of T effector cells and concomitant inhibition of Tregs both contribute to antitumor activity [64]. Moreover, preclinical studies in murine cancer models have demonstrated the ability of anti-CTLA-4 antibodies to enhance immune responses induced by chemotherapy, radiotherapy, and depletion of CD25+ Tregs [65]. In the mouse M109 lung cancer model, the combination of an anti-CTLA-4 antibody with chemotherapeutic agents (i.e. ixabepilone, etoposide and gemcitabine) resulted in synergistic antitumor activity that was superior to each treatment alone [66, 67]. Animals treated with CTLA-4 monoclonal antibody and ixabepilone also rejected a subsequent tumor rechallenge, indicative of protective memory immune response [66]. These preclinical findings, together with the emerging immunologic profile of NSCLC as a disease, have bolstered the rationale for the clinical development of CTLA-4 antibodies in the treatment of NSCLC.
therapeutic potential of ipilimumab in NSCLC
The CTLA-4-blocking, fully human monoclonal antibody, ipilimumab [68], significantly improved survival as monotherapy or in combination with chemotherapy in patients with metastatic melanoma, with a manageable safety profile [69, 70], ultimately leading to its approval in this setting in over 40 countries. Because the mechanism of action of ipilimumab is not dependent on tumor cells expressing a particular antigenic target, it was reasoned that it may also have activity against multiple tumor types for which there is evidence of an ongoing antitumor immune response [71]. Therefore, clinical trials to evaluate ipilimumab in NSCLC were initiated, drawing from emerging knowledge of the immune profile of NSCLC and the clinical experience with ipilimumab in melanoma.
In investigations of ipilimumab in melanoma, investigators noted that in a minority of cases, response patterns that differed from those associated with chemotherapy, although responses resembling those of standard therapies were observed most of the time [70, 72, 73]. The phase III trials in advanced melanoma thus employed immune-related response criteria (irRC), derived from World Health Organization (WHO) criteria, in order to account for the typical and atypical responses to ipilimumab therapy. According to the modified WHO (mWHO) criteria, any tumor growth and/or appearance of new lesions indicate progressive disease that may mean that the treatment has failed; neither the measurement of new lesions nor their inclusion as part of the patient's total tumor burden is required. The irRC, in contrast, add measurable new lesions as they appear to baseline index lesions to determine the patient's total tumor burden, which is then compared with baseline tumor burden. This approach is designed to account for continued tumor growth during the time required for an immune response to develop, or for a tumor volume increase due to transient T-cell infiltration (with or without edema) into established, radiographically undetectable tumor deposits [72, 74].
Based on the above learning from advanced melanoma, the phase II and III trials of ipilimumab for the treatment of NSCLC were designed to evaluate the response to ipilimumab using both irRC and mWHO criteria. Trial protocols designated a confirmation of PD after 4 weeks, when the investigator deemed that an additional 4-week wait was feasible for the patient. Of note, however, the irRC have not been prospectively validated in clinical studies of ipilimumab in lung cancer. Moreover, none of the ipilimumab trials in melanoma or lung cancer that have measured outcomes by both mWHO and irRC were designed to prospectively validate the irRC, nor have they been able to consistently identify a benefit to utilizing irRC over mWHO. As such, the utility of irRC is still a subject of investigation. It is clear, however, that clinical responses to immunotherapy like ipilimumab may sometimes differ from responses to standard chemotherapy. As agents like ipilimumab becomes more established as an anticancer approach, it will be necessary to weigh the possible benefits of immunotherapy against the time required to confirm disease progression as part of clinical decision making.
In the NSCLC studies, ipilimumab has been paired with chemotherapy, specifically a doublet of paclitaxel and carboplatin. A chemo-immunotherapeutic approach was adopted for the NSCLC trials, in part because paclitaxel–carboplatin is a widely used, reasonably well-tolerated first-line regimen in NSCLC. In addition, evidence in cell lines and animal models supported the feasibility of combining chemotherapy with CTLA-4 blockade through demonstration of the following: (i) therapy with taxanes and platinum-based compounds may foster release tumor-specific antigens from dying tumor cells and improve the immunogenicity of the microenvironment [75]; (ii) chemotherapy may sensitize tumor cells to lymphocyte killing [76] and (iii) certain chemotherapeutic agents and CTLA-4 blockade display in vitro synergistic activity [77] as discussed above.
clinical data on CTLA-4 manipulation by ipilimumab in squamous NSCLC
In a phase II study [78, 79], 204 chemotherapy-naive patients with stage IIIb/IV NSCLC of all subtypes were given a combination of paclitaxel–carboplatin and either ipilimumab or placebo [79]. Patients were randomly assigned (1:1:1) to receive one of the three induction strategies: one of the two different schedules of ipilimumab and chemotherapy (phased or concurrent), or chemotherapy with placebo. In the phased schedule, designed to promote potential chemotherapy-induced antigen release before ipilimumab addition, chemotherapy was given alone for two cycles, followed by ipilimumab in combination with paclitaxel–carboplatin for the next four cycles, every 3 weeks. In the concurrent schedule, designed to ensure that ipilimumab was present as chemotherapy-induced antigen release was occurring, ipilimumab was given in combination with paclitaxel–carboplatin for the first four cycles, followed by chemotherapy alone for the next two cycles, every 3 weeks. Patients in the placebo arm received six cycles of chemotherapy with placebo. After six cycles of induction therapy, all eligible patients received a single dose of the study drug (ipilimumab or placebo) every 12 weeks as maintenance therapy [79].
The phased schedule of ipilimumab and paclitaxel–carboplatin met the primary end point of the study: significantly prolonged progression-free survival (PFS) over chemotherapy alone, as measured by irRC (irPFS) [5.7 months versus 4.6 months, respectively; HR = 0.72; 95% confidence interval (CI), 0.50–1.06; P = 0.05] or mWHO criteria (5.1 months versus 4.2 months, respectively; HR = 0.69; 95% CI, 0.48–1.00; P = 0.02). Patients in the group receiving phased ipilimumab had an immune-related best overall response rate (irBORR) of 32% (95% CI, 22% to 45%), compared with 21% (95% CI, 13% to 33%) in the group receiving concurrent ipilimumab, and 18% (95% CI, 10% to 30%) in the group receiving paclitaxel–carboplatin alone. Corresponding BORRs using the mWHO criteria were 32%, 21% and 14%, respectively, for the three study arms.
Higher rates of inflammatory events, such as any-grade pruritus, rash and diarrhea, were observed in patients who received ipilimumab [79]. Grade 3/4 adverse events occurred in 57% and 54% of patients with the concurrent ipilimumab and phased ipilimumab regimens, respectively, versus 40% with paclitaxel–carboplatin alone. There were two treatment-related deaths reported, one each in concurrent ipilimumab and paclitaxel–carboplatin alone arms. The hematologic abnormalities observed were those generally anticipated with paclitaxel–carboplatin. The most common (>5%) treatment-related grade 3/4 adverse events (concurrent versus phased versus paclitaxel–carboplatin alone) were fatigue (10% versus 7% versus 6%) and diarrhea (10% versus 7% versus 4%) among non-squamous patients, and nausea (0% versus 5% versus 7%), vomiting (0% versus 5% versus 7%) and dyspnea (0% versus 0% versus 7%) among squamous patients. Reported immune-mediated adverse events involving multiple organ systems included hypophysitis, enterocolitis and hypothyroidism [80]. In the ongoing phase III clinical trials of ipilimumab, these adverse events are managed using the guidelines that specify proactive monitoring and intervention, as specified in the individual protocols. Notably, there is some evidence, although not conclusive, that these adverse events may correlate with tumor response. The possible correlation between adverse events and tumor response may be clarified upon completion of ongoing larger, randomized trials.
The above results were prospectively analyzed by the baseline histology to determine whether there were safety or efficacy differences among patients with squamous-cell carcinoma (n = 57) and those with non-squamous carcinomas (predominantly adenocarcinoma; n = 146) [81]. Notably, compared with patients with non-squamous NSCLC, patients with squamous NSCLC exhibited greater improvements in irPFS and OS over chemotherapy alone upon receiving the phased-schedule ipilimumab/chemotherapy regimen (Figure 3) [80]. For the concurrent regimen, irPFS and OS were similar among patients with squamous and non-squamous histologies compared with paclitaxel–carboplatin alone. Adverse event profiles were comparable in tumors of squamous and non-squamous histologies.
Figure 3.
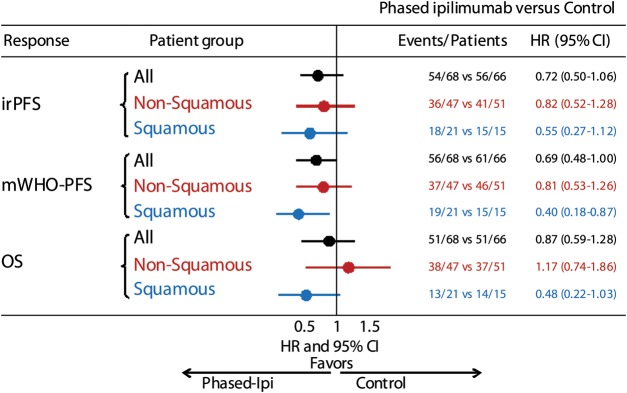
Immune-related progression-free survival (irPFS), PFS by modified WHO (mWHO) criteria and overall survival (OS) in the phase II randomized study of ipilimumab given either in a phased schedule or currently with paclitaxel–carboplatin in patients with NSCLC, analyzed by histological subtype [81]. (Figure adapted, with permission, from Thomas Lynch.) Comparison of the phased ipilimumab arm versus placebo arm. Phased ipilimumab plus paclitaxel–carboplatin appeared to have a greater effect on patients with squamous histology than those with non-squamous histology. The hazard ratio point estimates for irPFS, mWHO-PFS and OS were significantly smaller with phased ipilimumab plus paclitaxel–carboplatin in the squamous population than the non-squamous population; however, small sample size warrants caution in interpretation.
Small sample sizes in each histologic subgroup preclude definitive conclusions, but the data do suggest that ipiIimumab, given in a phased schedule with first-line chemotherapy (phased-schedule paclitaxel–carboplatin), provides greater improvement in individuals with squamous-cell NSCLC than in those with non-squamous tumors. Although these observations suggest that the timing and schedule of immunotherapeutic regimens with other therapies may have an impact on clinical benefit, it is not clear why the phased schedule appeared to provide greater benefit than the concurrent schedule. The authors on the study suggest that the exposure to chemotherapy may have enhanced the subsequent activation of T cells by ipilimumab and augmented the resulting antitumor response [79]. It is noteworthy that concomitant corticosteroids were allowed as premedication for paclitaxel infusion in this study, and the extent to which the relative timing of paclitaxel therapy (and thus corticosteroids) contributed to the differential clinical benefit in the two ipilimumab arms is not known, neither is it clear whether the phased schedule utilized in this study represents the optimal timing and scheduling for ipilimumab in lung cancer.
This observed clinical benefit led to an ongoing phase III trial in squamous NSCLC (ClinicalTrials.gov identifier NCT01285609) to determine whether the phased schedule of ipilimumab and paclitaxel–carboplatin would prolong OS over chemotherapy alone. In this trial, patients are randomly assigned to two lead-in doses of paclitaxel–carboplatin followed by induction therapy with paclitaxel–carboplatin and ipilimumab 10 mg/kg (arm A) or paclitaxel–carboplatin and placebo (arm B) every 3 weeks for up to four doses. Following the induction phase, patients who experience response or SD with tolerable toxicity receive maintenance therapy of the blinded study drug (ipilimumab or placebo), every 12 weeks until progressive disease or unacceptable toxicity occurs. The trial seeks to enroll 920 chemotherapy-naive patients with stage IV or recurrent NSCLC of squamous histology. It is hoped that this study will further characterize the benefit of the regimen in squamous NSCLC, as was observed in the phase II study [79].
conclusions
Improved understanding of the immune profile of squamous NSCLC has led to immunotherapeutic strategies, including inhibition molecules responsible for abrogating an immune response such as PD-1 and CTLA-4. Compared with non-squamous NSCLC, squamous NSCLC displays a more consistent immune profile, more frequently expresses specific tumor antigens and shows more extensive infiltration of CD8+ effector cells.
Among the checkpoint blockade molecules under investigation for NSCLC, ipilimumab has undergone the most study. A phase II study of ipilimumab in patients with locally advanced or metastatic NSCLC demonstrated activity (in combination with chemotherapy), while also maintaining a manageable toxicity profile. Sub-group analyses from this study suggested a greater improvement in patients with squamous-cell carcinoma than in those with non-squamous carcinomas. It is hoped that further studies in larger number of patients will confirm the potential for this agent in squamous NSCLC.
disclosure
The authors have declared no conflicts of interest.
acknowledgements
Professional medical writing and editorial assistance were provided by Kenyon Ogburn and Rebecca Goldstein at StemScientific. Funding for this assistance was provided by Bristol-Myers Squibb.
references
- 1.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. doi:10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/index.html. (16 November 2011, date last accessed)
- 3.American Cancer Society. Cancer facts & figures 2011. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf. (17 November 2011, date last accessed)
- 4.Brodowicz T, Ciuleanu T, Crawford J, et al. Third CECOG consensus on the systemic treatment of non-small-cell lung cancer. Ann Oncol. 2012;23:1223–1229. doi: 10.1093/annonc/mdr381. doi:10.1093/annonc/mdr381. [DOI] [PubMed] [Google Scholar]
- 5.D'Addario G, Felip E ESMO Guidelines Working Group. Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):68–70. doi: 10.1093/annonc/mdp132. [DOI] [PubMed] [Google Scholar]
- 6.D'Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. doi: 10.1093/annonc/mdq189. doi:10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 7.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. doi:10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(Suppl 1):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. doi:10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 2.2012 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl. (26 July 26 2011, date last accessed)
- 10.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. doi:10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. doi:10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Travis WB, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8(5):381–385. doi: 10.1513/pats.201107-042ST. doi:10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 13.Choi DR, Lee DH, Choi CM, et al. Erlotinib in first-line therapy for non-small cell lung cancer: a prospective phase II study. Anticancer Res. 2011;31:3457–3462. [PubMed] [Google Scholar]
- 14.Shukuya T, Takahashi T, Kaira R, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci. 2011;102:1032–1037. doi: 10.1111/j.1349-7006.2011.01887.x. doi:10.1111/j.1349-7006.2011.01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. doi:10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Karabon L, Pawlak E, Tomkiewicz A, et al. CTLA-4, CD28, and ICOS gene polymorphism associations with non-small-cell lung cancer. Hum Immunol. 2011;72:947–954. doi: 10.1016/j.humimm.2011.05.010. doi:10.1016/j.humimm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang J, Deng Y, et al. Polymorphisms in the cytotoxic T-lymphocyte antigen 4 gene and cancer risk: a meta-analysis. Cancer. 2011;117:4312–4324. doi: 10.1002/cncr.25979. doi:10.1002/cncr.25979. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. doi:10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. doi:10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. doi:10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 21.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. doi:10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Lee KM, Kim DW, et al. Elevated TGF-β1 and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 23.Gancz D, Fishelson Z. Cancer resistance to complement-dependent cytotoxicity (CDC): problem-oriented research and development. Mol Immunol. 2009;46:2794–2800. doi: 10.1016/j.molimm.2009.05.009. doi:10.1016/j.molimm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Nagata S. Fas ligand and immune evasion. Nat Med. 1996;2:1306–1307. doi: 10.1038/nm1296-1306. doi:10.1038/nm1296-1306. [DOI] [PubMed] [Google Scholar]
- 25.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. doi:10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 26.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. doi:10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 27.de Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. doi:10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Leffers N, Gooden MJM, de Jong RA, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. doi:10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang X, Xia X, Wang C, et al. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18:24–28. doi: 10.1097/PAI.0b013e3181b6a741. doi:10.1097/PAI.0b013e3181b6a741. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating FOXP3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. doi:10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 31.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. doi:10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. doi:10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. doi:10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Dy GK, Adjei AA. Emerging therapeutic targets in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:218–23. doi: 10.1513/pats.200808-099LC. doi:10.1513/pats.200808-099LC. [DOI] [PubMed] [Google Scholar]
- 35.Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol. 2011;6:824–833. doi: 10.1097/JTO.0b013e3182037b76. doi:10.1097/JTO.0b013e3182037b76. [DOI] [PubMed] [Google Scholar]
- 36.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. doi:10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. doi:10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. doi:10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 39.Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. doi:10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 40.Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T-cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunology. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. doi:10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FoxP3 expression and CD4+CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. doi:10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 44.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. doi:10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 45.Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348–1359. doi: 10.1038/modpathol.2011.92. doi:10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 46.Bolli M, Kocher T, Adamina M, et al. Tissue microarray evaluation of melanoma antigen E (MAGE) tumor-associated antigen expression: potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Ann Surg. 2002;236:785–793. doi: 10.1097/01.SLA.0000036266.09823.6C. doi:10.1097/00000658-200212000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J-H, Zo JI, Nakayama H, et al. Patient and tumor characteristics impacting on MAGE-A3 expression: screening data from the MAGRIT phase III trial. Presented at the 2011 World Congress of Lung Cancer; July 3–7; Amsterdam, Netherlands. 2011. Session MO21. [Google Scholar]
- 48.Yoshida N, Abe H, Ohkuri T, et al. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens and T cell infiltration in non-small cell lung carcinoma and their prognostic significance. Int J Oncol. 2006;28:1089–1098. [PubMed] [Google Scholar]
- 49.Kim SH, Lee S, Lee CH, et al. Expression of cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small-cell lung carcinomas and their relationship with immune cell infiltration. Lung. 2009;187:401–411. doi: 10.1007/s00408-009-9181-3. doi:10.1007/s00408-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17:524–5256. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 51.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. doi:10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 52.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. doi:10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. doi:10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. doi:10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 55.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. doi:10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 56.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. doi:10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 57.Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622–4628. doi: 10.1158/1078-0432.CCR-10-2232. doi:10.1158/1078-0432.CCR-10-2232. [DOI] [PubMed] [Google Scholar]
- 58.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. doi:10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 59.Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. doi:10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. doi:10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 61.Song B, Liu Y, Liu J, et al. CTLA-4 +49A>G polymorphism Is associated with advanced non-small cell lung cancer prognosis. Respiration. 2011;82:439–444. doi: 10.1159/000329345. doi:10.1159/000329345. [DOI] [PubMed] [Google Scholar]
- 62.Holt GE, Podack ER, Raez LE. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy. 2011;8:43–54. doi: 10.2217/thy.10.84. doi:10.2217/thy.10.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shepherd FA, Douillard JY, Blumenschein GR., Jr Immunotherapy for non-small cell lung cancer: novel approaches to improve patient outcome. J Thorac Oncol. 2011;6:1763–1773. doi: 10.1097/JTO.0b013e31822e28fc. doi:10.1097/JTO.0b013e31822e28fc. [DOI] [PubMed] [Google Scholar]
- 64.Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. doi:10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. doi:10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Jure-Kunkel MN, Masters G, Girit E, et al. Antitumor activity of anti-CTLA-4 monoclonal antibody (mAb) in combination with ixabepilone in preclinical tumor models. J Clin Oncol. 2008;26(20 suppl) Abstr 3048. [Google Scholar]
- 67.Masters G, Barreto L, Girit E, et al. Antitumor activity of cytotoxic T-lymphocyte antigen-4 blockade alone or combined with paclitaxel, etoposide, or gemcitabine in murine models. Presented at the 24th Annual Meeting of the International Society for Biological Therapy of Cancer, Washington DC, USA; October 29–31, 2009. J Immunother. 2009;32:994. [Google Scholar]
- 68.Yervoy™ (ipilimumab) [prescribing information] Princeton, NJ: Bristol-Myers Squibb; 2011. [Google Scholar]
- 69.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. doi:10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. doi:10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 71.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. doi:10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 72.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. doi:10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 73.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. doi:10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. doi:10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tesniere A, Apetoh L, Ghiringhelli F, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. doi:10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. doi:10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masters G, Barreto L, Girit E, et al. Antitumor activity of cytotoxic T-lymphocyte antigen-4 blockade alone or combined with paclitaxel, etoposide or gemcitabine in murine models. J Immunother. 2009;32:994. [Google Scholar]
- 78.Calabrò L, Danielli R, Sigalotti L, et al. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol. 2010;37:460–467. doi: 10.1053/j.seminoncol.2010.09.006. doi:10.1053/j.seminoncol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. doi:10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 80.Tomasini P, Khobta N, Greillier L, et al. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol. 2012;4:43–50. doi: 10.1177/1758834011431718. doi:10.1177/1758834011431718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lynch T, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in non-small cell lung cancer: analysis by baseline histology in a phase 2 trial. Presented at the 2011 World Congress of Lung Cancer; July 3–7; Amsterdam, Netherlands. [Google Scholar]