Abstract
Background
Information on cytokine profiles in fungal sepsis (FS), an important cause of mortality in extremely low birthweight infants (ELBW), is lacking. We hypothesized that cytokine profiles in the 1st 21 days of life in ELBW with FS differ from those with bacterial sepsis (BS) or no sepsis (NS).
Methods
In a secondary analyses of the NICHD Cytokine study, three groups were defined - FS (≥1 episode of FS), BS (≥1 episode of BS without FS), and NS. Association between 11 cytokines assayed in dried blood spots obtained on days 0-1, 3±1, 7±2, 14±3, and 21±3 and sepsis group was explored.
Results
Of 1066 infants, 89 had FS and 368 had BS. Compared to BS, FS was more likely to be associated with lower birthweight, vaginal delivery, patent ductus arteriosus, postnatal steroids, multiple central lines, longer respiratory support and hospital stay, and higher mortality (p<0.05). Analyses controlling for covariates showed significant group differences over time for IFN-γ, IL-10, IL-18, TGF-β and TNF-α (p<0.05).
Conclusion
Significant differences in profiles for IFN-γ, IL-10, IL-18, TGF-β and TNF-α in FS, BS or NS in this hypothesis-generating secondary study require validation in rigorously designed prospective studies and may have implications for diagnosis and treatment.
Introduction
Although advances in neonatal intensive care have led to improved survival, sepsis continues to be a major cause of mortality in extremely low birthweight infants (ELBW) 1. Fungal sepsis (FS) is particularly problematic because of difficulty in diagnosis and poor prognosis among infected infants. Infants with FS have a higher mortality rate compared with bacterial sepsis (BS), higher incidence of severe retinopathy of prematurity, periventricular leukomalacia (PVL), bronchopulmonary dysplasia, and significant neurodevelopmental impairment (57%) resulting in increased length of stay and hospital costs of ∼$30,000,000/year in the US2-4.
ELBW are at risk for invasive FS because of an immature protective layer of skin, the need for invasive medical devices and defects in cellular and humoral responses1. A definitive diagnosis, based on blood, cerebrospinal fluid (CSF), or urine culture, is usually reached only after a delay of 2–3 days5. Rapid progression of an untreated infection may greatly increase morbidity and mortality. Although cytokine levels and acute-phase reactants have been used as markers of neonatal sepsis in general, the response of these biomarkers to FS has not been studied.
The first step in the innate immune response to fungal infections is recognition of fungal polymers (β-1,3/β-1,6 glucans, glucuronoxylomannan, phospholipomannan and galactomannan) by innate immune effector cells6. In animal models and human adults, adaptive antifungal immunity includes T helper (Th)1-biased responses which are protective; and Th2-biased responses which are maladaptive or deleterious7. While CD4+ T cells have been classically divided as either Th1 or Th2, a new T helper effector subset, Th17, has been reported to play a seminal role in antifungal defense8-13. Knowledge of the cytokine response in ELBW with and without FS is important to corroborate the information from animal models and human adults, to understand immune regulation and host defense in these infants, and to develop novel methods for diagnosis and treatment of FS.
The primary objective of this study was to assess cytokine levels and their temporal trends in dried blood spots (DBS) collected over the 1st three weeks after birth in ELBW with FS, BS, or no sepsis (NS). Eleven cytokines identified as being important in the development of FS were investigated: (i) Th-1 Cytokines and their inducers: TNF-α, IL-1β, IL-12, IL-18, IFN-γ; (ii) Th-2 Cytokines and their inducers: IL-4, IL-5, IL-6, IL-10; and (iii) Th-17 Cytokines and their inducers: IL-17, TGF-β. We hypothesized that FS in ELBW will be associated with decreased Th-1 cytokines, IL-17 or TGF-β and/or elevated Th-2 cytokines in the first three weeks of life compared to infants with BS or NS.
Results
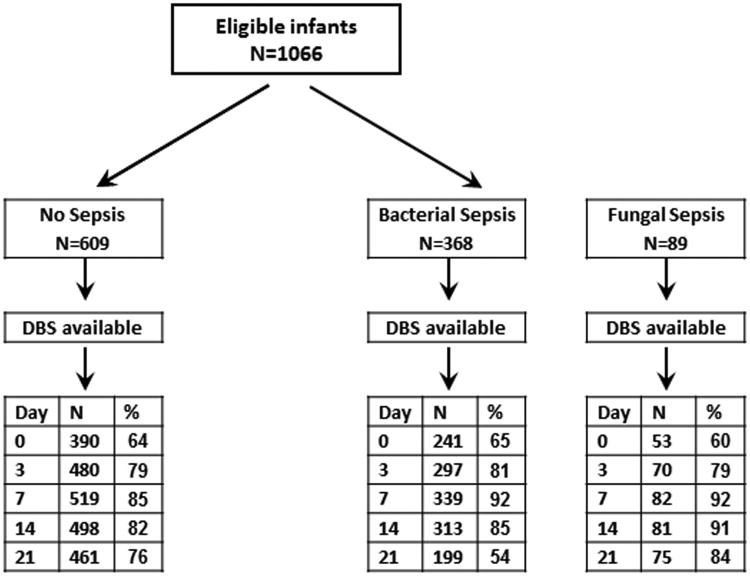
Of 1066 eligible infants, 89 developed FS, 368 developed BS without FS and 609 did not have any episode of culture positive sepsis (Figure 1). DBS were available for 684 infants on D0, 847 infants on D3, 940 infants on D7, 892 infants on D14 and 735 infants on D21. Sepsis was diagnosed on basis of positive blood culture in 91-92%, CSF culture in 5-6% and both blood and CSF culture in 2-3% of infants in both BS and FS groups. In the FS group, 61 infants also had positive bacterial cultures (68.5%) either before (n=32) or after (n=29) after the 1st positive fungal blood and/or CSF culture.
Figure 1.
Flow diagram of preterm neonates with available DBS by sepsis group. DBS=Dried blood spots.
The most commonly identified bacteria included coagulase negative staphylococci (n=189), Staphylococcus aureus (n=34), Streptococci (n=35; GBS=4), Escherichia coli (n=20), and Klebsiella (n=17). Identified fungal elements included Candida albicans (n=44), Candida parapsilosis (n=33), Candida (species not specified, n=9), Candida glabrata (n=2) and Saccharomyces (n=1).
Maternal and Neonatal Characteristics
Median (interquartile range (IQR)) age at first sepsis episode was 18 (11–30.5) days in BS and 20 (14-32) in FS groups. In 55% of infants, sepsis was diagnosed between day 7 and 21 of age. Maternal characteristics that differed significantly across sepsis group included preterm labor, hypertension, use of intrapartum antibiotics, and mode of delivery (Table 1). Compared to infants with NS, infants with BS or FS were more likely to have lower birthweight and GA, require delivery room intubation, treatment with postnatal steroids, have 3 or more central lines, undergo assisted ventilation and treatment with supplemental oxygen, have higher mortality and longer hospital stays in survivors. They were also more likely to develop necrotizing enterocolitis and PDA and receive treatment with histamine-2 receptor (H2) blockers.
Table 1. Maternal and Neonatal Characteristics.
| Characteristic | Sepsis | P* | P** | ||
|---|---|---|---|---|---|
|
| |||||
| None N=609 | Bacterial N=368 | Fungal N=89 | |||
|
| |||||
| Preterm labor (%) | 65 | 72 | 81 | 0.0013 | 0.0939 |
| Hypertension (%) | 28 | 23 | 7 | <0.0001 | 0.0004 |
| Maternal antibiotics prior to delivery (%) | 66 | 71 | 81 | 0.0087 | 0.0603 |
| Mode of delivery – C. section (%) | 61 | 58 | 44 | 0.0067 | 0.0147 |
| Gestational age (wks) (mean±SD) | 26±2 | 26±2 | 25±2 | <.0001 | 0.0001 |
| Birth weight (gm) (mean±SD) | 777±138 | 749±141 | 699±138 | <.0001 | 0.0022 |
| 5 minute Apgar ≤ 5 (%) | 6 | 11 | 7 | 0.0329 | NS |
| Delivery room intubation (%) | 73 | 77 | 87 | 0.0143 | 0.0465 |
| Postnatal steroids (%) | 20 | 31 | 39 | <0.0001 | NS |
| Ventilator days (mean±SD) | 20±23 | 33±2 | 46±29 | <.0001 | <0.0001 |
| Days in oxygen (mean±SD) | 48±38 | 64±39 | 74±34 | <.0001 | 0.0306 |
| Early Sepsis (%) | 0 | 4 | 3 | <0.0001 | NS |
| Late Sepsis (%) | 0 | 96 | 97 | <0.0001 | NS |
| 3 or more central lines (%) | 44 | 63 | 83 | <0.0001 | 0.002 |
| Grade III or IV IVH† (%) | 15 | 18 | 24 | 0.0991 | NS |
| BPD (%) | 42 | 56 | 63 | <0.0001 | NS |
| NEC (≥ Stage 2) (%) | 8 | 15 | 10 | 0.0025 | NS |
| Deaths (%) | 16.2 | 19.6 | 29.2 | 0.0100 | 0.0480 |
| Age at Discharge for survivors (days) (mean±SD) | 90±32 | 113±49 | 129±54 | <0.0001 | 0.0241 |
| Patent ductus arteriosus (%) | 42 | 57 | 75 | <0.0001 | 0.0014 |
| H2 Blockers (%) | 33 | 47 | 49 | <0.0001 | NS |
| Age at 1st Positive Culture (days) (mean±SD) | 25±20 | 20±16 | 0.033 | ||
comparison of all three sepsis groups
Bacterial vs. fungal sepsis
Intraventricular hemorrhage
Characteristics that did not show significant differences across Sepsis groups included: premature prolonged rupture of membranes, use of antenatal corticosteroids or tocolytics, gender, race, and Cystic PVL
Bivariate Analysis of Mean Cytokine Levels by Sepsis Group at Each Time Point
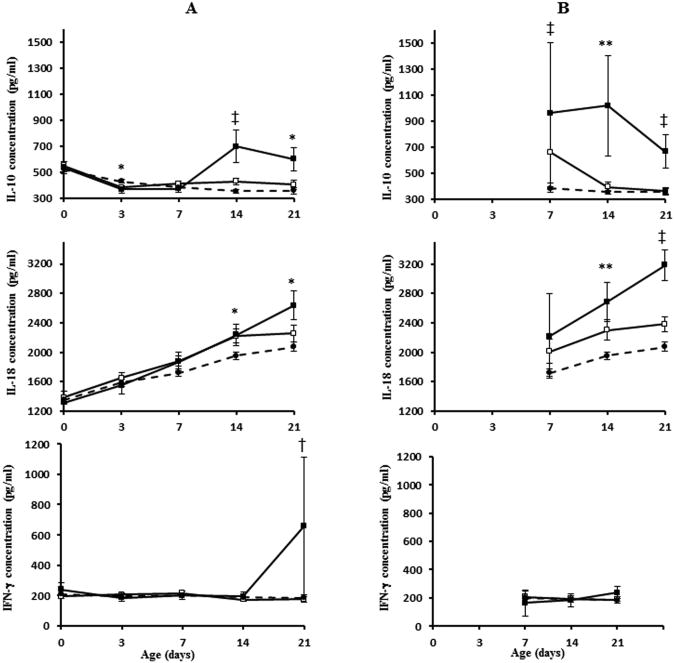
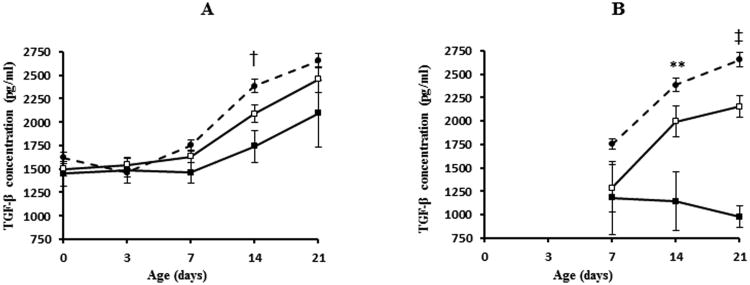
For each infant with BS or FS, DBS were classified as being “pre-infection” if obtained prior to the microbiologic confirmation of sepsis and “post-infection” if obtained after microbiologic confirmation of sepsis (Table 2). Bivariate analysis of mean cytokine levels in the pre-infection phase revealed significant group differences for IL-10 (D3, 14, 21); IL-18 (D14, 21); IFN-γ (D21) and TGF-β (D14) (Figures 2 and 3). In the post-infection phase, mean cytokine levels were significantly different between the three groups for IL-10 (D7, 14, 21), IL-18 (D14, 21), TGF-β (D14, 21) and TNF-α (D21). IL-10, IL-18, IFN-γ and TNF-α levels were higher and TGF-β levels were lower in infants with FS compared to BS or NS (data for TNF-α not presented in Figure 2).
Table 2. Number of Infants with DBS Obtained Before and After 1st Episode of Bacterial and Fungal Sepsis.
| Infection Phase | Bacterial Sepsis | Fungal Sepsis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| D0 | D3 | D7 | D14 | D21 | D0 | D3 | D7 | D14 | D21 | |
| Pre-Infection | 240 | 283 | 320 | 209 | 136 | 52 | 69 | 78 | 65 | 40 |
| Post-Infection | 1 | 14 | 19 | 104 | 163 | 1 | 1 | 4 | 16 | 35 |
| Total | 241 | 297 | 339 | 313 | 199 | 53 | 70 | 82 | 81 | 75 |
Figure 2.
Mean concentrations (pg/ml) of cytokines significantly higher in fungal sepsis group before and after 1st episode of infection. Panels A and B represent data for pre- and postinfection phases respectively. Data for D0 and D3 not presented for postinfection period as there were very few cases. Refer to Table 2 for number of cases in each group at various time points. Vertical lines represent ± 1SE. *p<0.05, ** p<0.01, † p<0.001, ‡ p<0.0001. White squares, bacterial group; black squares, fungal group; black circles, no sepsis group.
Figure 3.
Mean concentrations (pg/ml) of cytokines significantly lower in fungal sepsis group before and after 1st episode of infection. Panels A and B represent data for pre- and postinfection phases respectively. Data for D0 and D3 not presented for postinfection period because there were very few cases. Refer to Table 2 for number of cases in each group at various time points. Vertical lines represent ± 1SE. ** p<0.01, † p<0.001, ‡ p<0.0001. White squares, bacterial group; black squares, fungal group; black circles, no sepsis group.
Mixed Model Analysis: Interaction of Sepsis Group and Cytokines over time
On mixed model analysis with adjustment for confounding variables, significant group differences and postnatal time trends were observed for IFN-γ, IL-10, IL-18, TGF-β, and TNF-α with significant interaction between group and age at cytokine evaluation (Table 3). There was a significant difference in IFN-γ, IL-10, IL-18 and TGF-β between pre- and post-infection period with a significant interaction for sepsis group. Table 4 summarizes the cytokines significantly different between sepsis groups in the pre- and post-infection periods on adjusted analyses.
Table 3. Mixed Model Analysis for Cytokines Showing Significant Sepsis Group and Time Interaction After Controlling for Significant Covariates.
| Cytokine | Effect – F Value (Probability>F) | Significant Covariates (p) | ||||
|---|---|---|---|---|---|---|
| Group | Postnatal Age | Group* Agea | Pre- vs. Post-Infection status | Pre-/Post-infection status* Groupb | ||
| IFN-γ | 0.011 | <0.0001 | <0.0001 | 0.0005 | 0.0001 | Center‡ |
| Preterm labor* | ||||||
| IL-10 | 0.0002 | <0.0001 | <0.0001 | 0.0075 | 0.0067 | Center‡ |
| Oxygen** | ||||||
| NEC* | ||||||
| IL-18 | 0.0097 | <0.0001 | 0.0283 | 0.0009 | 0.0265 | Center‡ |
| GA** | ||||||
| Hypertension** | ||||||
| TGF-β | 0.0024 | <0.001 | 0.0007 | <0.0001 | 0.0013 | Center‡ |
| GA** | ||||||
| Preterm labor** | ||||||
| PDA† | ||||||
| NEC** | ||||||
| TNF-α | 0.0162 | 0.0025 | 0.0064 | NS | NS | Center‡ |
| PDA* | ||||||
interaction of group and age
interaction of pre- or post-infection status and Group
p<0.05
p<0.01
p<0.001
p<0.0001
Table 4. Summary of Cytokines that Show statistically Significant Differences by Age and Sepsis Group on Adjusted Analyses.
| Before 1st episode of sepsis | After 1st episode of sepsis | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| D3 | D7 | D14 | D21 | D7 | D14 | D21 | |
|
| |||||||
| BS vs. NS | IL-10 | IL-10 | IL-10 | ||||
| IL-18 | IL-18 | IL-18 | |||||
| TGF-β | TGF-β | TGF-β | |||||
|
| |||||||
| FS vs. NS | TGF-γ | IL-10 | IL-10 | IL-10 | |||
| IL-10 | IL-10 | IL-18 | IL-18 | ||||
| TGF-β | IL-18 | IL-18 | TGF-β | TGF-β | TGF-β | ||
| TGF-β | TGF-α | TGF-α | TGF-α | ||||
|
| |||||||
| FS vs. BS | TGF-γ | IL-10 | IL-10 | ||||
| IL-10 | IL-10 | IL-18 | IL-18 | ||||
| TGF-α | TGF-β | TGF-β | |||||
| TGF-α | TGF-α | ||||||
In analysis before 1st episode of infection, no group differences in cytokines on D0 observed.
In analysis after 1st episode of infection, group differences not evaluated at D0 and D3 because of the small numbers of subjects at these time points.
All cytokines except TGF-β were elevated in BS or FS vs. NS and in FS vs. BS. TGF-β was decreased in BS or FS vs. NS and in FS vs. BS.
Receiver Operating Characteristic (ROC) Analysis
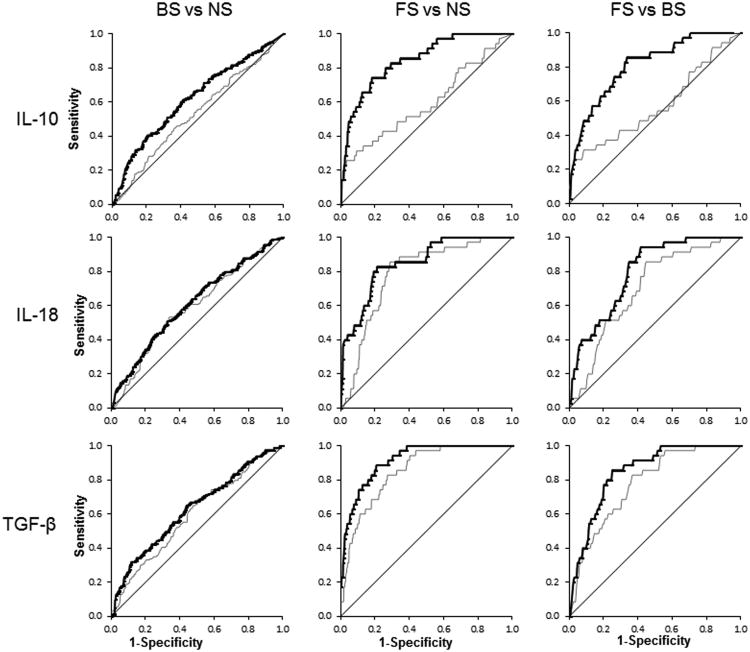
ROC analysis revealed that 3 cytokines – IL-10, IL-18 and TGF-β – had significant ability to discriminate between sepsis groups in the pre- and post-infection phase on D14 and D21 (Table 5). We did not attempt to determine cut-off values for these cytokines at different time points as in this study DBS were obtained at pre-defined postnatal ages and not in relation to timing of onset of clinical suspicion of infection or positive blood culture. We computed area under the curve (AUC) which showed that all three cytokines had moderately to high accuracy (AUC≥0.7) for differentiating between FS vs. NS and FS vs. BS in the post-infection phase on D14 and D21 DBS samples (Figure 4). Results in the pre-infection phase for these cytokines were less robust.
Table 5. Area under the Curve for Cytokines Significantly Different Between Groups in the Pre- and Post-Infection Phase.
| PRE-INFECTION PHASE | |||||||
|---|---|---|---|---|---|---|---|
| Cytokine | Analyses | D14 | D21 | ||||
| BS vs. No Sepsis | FS vs. No Sepsis | BS vs. FS | BS vs. No Sepsis | FS vs. No Sepsis | BS vs. FS | ||
| IL-10 | Unadjusted | 0.56 (0.51-0.60)** | 0.59 (0.51-0.67)** | 0.54 (0.45-0.62)** | 0.61 (0.51-0.72)* | ||
| Adjusted | 0.72 (0.69-0.77) † | 0.81 (0.76-0.86)‡ | 0.80 (0.74-0.86)* | 0.70 (0.61-0.79)* | |||
| IL-18 | Unadjusted | 0.56 (0.52-0.61)* | 0.68 (0.59-0.76)* | ||||
| Adjusted | 0.61 (0.56-0.65)* | ||||||
| TGF-β | Unadjusted | 0.56 (0.51-0.60)* | 0.64 (0.57-0.71)** | 0.67 (0.58-0.77)* | |||
| Adjusted | |||||||
| POST-INFECTION PHASE | |||||||
| Cytokine | Analyses | D14 | D21 | ||||
| BS vs. No Sepsis | FS vs. No Sepsis | BS vs. FS | BS vs. No Sepsis | FS vs. No Sepsis | BS vs. FS | ||
| IL-10 | Unadjusted | 0.73 (0.59-0.87)** | 0.71 (0.56-0.86)* | 0.58 (0.45-0.69)** | |||
| Adjusted | 0.87 (0.76-0.98)** | 0.79 (0.65-0.92)* | 0.85 (0.78-0.91)* | 0.81 (0.73-0.89)** | |||
| IL-18 | Unadjusted | 0.58 (0.51-0.64)* | 0.72 (0.62-0.82)* | 0.59 (0.54-0.64)* | 0.78 (0.72-0.85)‡ | 0.70 (0.61-0.79)** | |
| Adjusted | 0.66 (0.61-0.72)* | 0.61 (0.56-0.66)* | 0.85 (0.79-0.91)** | 0.80 (0.73-0.87)* | |||
| TGF-β | Unadjusted | 0.60 (0.53-0.66)* | 0.79 (0.66-0.92)** | 0.60† | 0.85‡ | 0.78‡ | |
| Adjusted | 0.91 (0.82-1.0)* | 0.63* | 0.92‡ | 0.85† | |||
Numbers represent AUC (95% Confidence Limits)
p<0.05
p<0.01
p<0.001
p<0.0001
Figure 4.
ROC curves for cytokines significantly different between sepsis groups in the postinfection period on D21. Gray and bold black lines represent ROC curve in analyses unadjusted and adjusted for significant covariates, respectively.
Discussion
To our knowledge, this is the first population study evaluating cytokine profiles in ELBW with NS, BS and FS. Although cytokine levels and acute-phase reactants have been used as markers of neonatal sepsis, the response of these biomarkers to FS has not been systematically studied14. In this exploratory, hypothesis generating study, we identified a panel of five inflammatory markers to be significantly associated with sepsis group in the 1st three weeks of life on adjusted analysis - IFN-γ, IL-10, IL-18, TGF-β and TNF-α. These cytokines are potential candidate biomarkers of neonatal BS and FS that may allow early diagnosis before blood cultures become positive and that may also differentiate between BS and FS. Cytokines were not significantly different between groups on D0 suggesting that fetal inflammatory response syndrome is neither a marker for nor predisposes to neonatal sepsis and that systematic difference in cytokine profiles do not exist between infants who later acquire sepsis (BS or FS) or not. Significant group differences in cytokines in the pre-infection period suggest that changes in levels of these cytokines may precede clinical signs and symptoms and microbiologic confirmation of diagnosis. After the microbiologic diagnosis of sepsis, group differences in cytokine levels became more prominent as compared to values before microbiologic confirmation suggesting that these differences reflect a response to infection rather than conferring an inherent predisposition to infection.
In keeping with our hypothesis, IL-10 (Th-2 cytokine) was increased and TGF- β decreased in infants with FS compared to those with BS or NS. However, contrary to our hypothesis, Th-1 cytokines, IFN-γ, IL-18, and TNF-α, were increased in infants with FS. IL-1β, IL-12, IL-4, IL-5, IL-6 and IL-17 did not show any association with sepsis group in our study. Our hypotheses were based on cytokine profiles described in sepsis in experimental models and adults. Only one observational study of FS and BS in a small group of term and preterm neonates has shown significant differences in C-reactive protein (CRP) and IL-6 levels15. Our results support the complexity of the immune response involving multiple cytokines with divergent functions and suggest that the immune response may differ in ELBW neonates.
TNF-α, initiator of the inflammatory response, has been reported to be increased early in neonatal sepsis. These studies included small number of patients, did not differentiate between FS and BS16-18, were not limited to ELBW19,20 and included infants with culture negative sepsis21,22. In our study TNF-α was significantly higher in ELBW with FS compared to BS and NS.
IL-6, one of the most studied cytokines in neonatal sepsis, increases early, but its half-life is short and its sensitivity decreases after 12 to 24 hours of infection. Most studies included small number of patients, did not differentiate between FS and BS16,18,23-25, were not limited to ELBW19,20,26-32 and included infants with culture negative sepsis33. In one study, IL-6 levels were significantly elevated in BS compared to FS in a cohort of term and preterm infants15. The lack of association of IL-6 with culture proven BS and FS in our large homogenous population of ELBW infants suggests that IL-6 may not play a role in immune response to sepsis in ELBW. Alternately, imprecision in timing of collection of samples in relation to clinical suspicion of infection in our study may have led to spuriously negative results because of the short half-life of IL-6.
IL-17, the signature cytokine of TH17 cells, has important role in induction of neutrophilmediated protective immune response against extracellular bacterial or fungal pathogens such as Klebsiella pneumonia and Candida albicans in experimental models11,34,35. However, in ELBW subjects of our study, IL-17 levels were not significantly different across sepsis group.
IL-18 is a pro-inflammatory cytokine that reduces polymorphonuclear cell apoptosis, potentiates IFN-γ production, and induces production of TNF-α, IL-1β, and IL-836. IL-18 is protective against infection caused by a number of intracellular and extracellular pathogens. In a recent comprehensive study of >140 serum analytes from neonates evaluated for late-onset sepsis (including BS, FS, culture-negative clinical sepsis), IL-18 was elevated in infected vs. non-infected neonates and in FS37. In our study as well, IL-18 emerged as a potential biomarker to differentiate FS from NS and BS.
IL-10 is an anti-inflammatory cytokine responsible for downregulation of the inflammatory process and maintaining homeostasis in vital organs36. Elevated levels of IL-10 indicate infectious disease in neonates, and IL-10 remains elevated until 24 hours after the beginning of sepsis5,26,38 In our study, IL-10 not only discriminated between BS and NS but also between FS and BS or NS.
No previous studies have investigated levels of TGF-β, a bipolar cytokine that can both trigger and inhibit the immune system, in neonatal sepsis39. In the present study, ELBW with sepsis had lower levels of TGF-β on D14 compared to infants with NS in the pre-infection period. In the post-infection period, TGF-β levels were significantly different on D14-21 between FS and BS or NS and between BS and NS.
On ROC analysis, three cytokines demonstrated moderate to high accuracy in discriminating between FS and NS or BS after microbiologic confirmation of diagnosis despite the fact that DBS were obtained at pre-defined postnatal ages and not timed in relation to 1st positive blood culture. These robust trends suggest that these cytokines may be biomarkers of neonatal FS and BS and need to be validated in prospective studies with blood samples obtained in relation to time of clinical suspicion of infection and 1st positive blood culture.
The majority of clinical characteristics that were strongly associated with sepsis group in our study have been previously reported1. Center difference was a significant covariate in most of the analyses. This could be related to differing ethnic populations, proportions of inborn/outborn infant, clinical practices, or other unidentified factors. Wide variation in the incidence of invasive fungal infections between NICUs has been shown in multiple publications3,40. Importantly, even after controlling for these covariates, the association between FS and five cytokines remained significant suggesting their role in the pathogenesis of FS. Postnatal time trends and GA differences for cytokines have been described by our group previously41.
This is the 1st population based study to compare cytokine concentrations in the 1st three weeks of life in ELBW with FS, BS or NS. Two small studies has previously explored the cytokine signature of host response to different microbiologic agents in neonates with elevated IL-18 and CRP and lower IL-6 levels being hallmarks of fungal infections15,37. Strengths of our study include a large sample size; prospectively collected data permitting a thorough investigation of the association of sepsis group and cytokine response at pre-defined time points, standardized collection of repeated, timed blood samples for three weeks starting from birth; assay of multiple cytokines from small volumes of blood using the Luminex assay; and multivariate adjustment for control of clinical variables.
Despite the important findings of this study, there are limitations. Although infants with BS represented a pure group with no positive fungal blood/CSF cultures; 68.5% of infants with FS also had positive blood/CSF bacterial cultures before or after the 1st episode of FS. Positive blood cultures for bacteria during the period of fungal infection are characteristic of the natural history of disease of invasive fungal infections in ELBW1. Contamination of FS group with BS should have under-estimated differences between the two groups; in spite of that we were able to detect significant differences between groups for five cytokines suggesting that these reflect true differences in cytokine response in BS and FS. Secondly, blood samples were timed in relation to postnatal age and not to clinical suspicion of infection. It has been shown that cytokine levels decline rapidly after onset of infection. Therefore DBS obtained at specified postnatal ages may have occurred at different time intervals before or after onset of infection. Despite this imprecision, we obtained consistent and significant predictive values for three cytokines at 14-21 days of age, which is close to the median age of onset of confirmed sepsis, suggesting dramatic effects. Although the study design of collection of protocol driven samples at pre-defined postnatal ages in this hypothesis-generating secondary study was not ideal to assess response to infection, it did allow evaluation of cytokines levels prior to diagnosis of sepsis which may not be possible to assess in a study design where sample collection is dictated by clinical suspicion of infection. Though these results may reflect true differences between FS, BS and NS because of the robust trends and biologic plausibility, they need to be validated in carefully designed large longitudinal cohort studies with prospective blood collection in relation to sepsis evaluation. Thirdly, it is difficult to distinguish between primary and secondary mediators in the cytokines described. Additionally, circulating cytokine concentrations may not reflect their biologic activity. Although we included 11 biomarkers, other markers (procalcitonin, CRP) that have been shown to be useful for the early detection of neonatal sepsis were not studied5,37. Lastly, we did not correct for multiple comparisons since the posthoc subgroup analysis in this study is exploratory in nature and hypothesis generating. Therefore, reliance should be placed on the observed effect size rather than on statistical significance testing as a basis for decision making42.
In conclusion, in this prospective study, IL-10, IL-18, TGF-β and TNF-α were found to be the most promising indicators of FS vs. BS or NS in ELBW. These findings offer insight into immune regulation in ELBW with sepsis, the prospect of rapid differentiation of neonatal BS and FS, early institution of targeted antimicrobial therapy and development of new treatments for this disease by enhancing the production of cytokines that confer protection. These novel findings will require validation in larger rigorously designed prospective studies.
Methods
We conducted a secondary exploratory analysis using clinical and biologic data collected as part of the multicenter NICHD Cytokine study, a prospective study assessing biomarkers for adverse neurodevelopmental outcomes in preterm infants with a birth-weight of 401-1000 grams43. This study was approved by Institutional Review Boards at all 17 participating centers, and written informed consent was obtained from parent(s).
ELBW neonates who were enrolled in the NICHD Cytokine Study and had available DBS were eligible for the study. Sepsis (including early and late-onset) was defined by a blood and/or CSF culture positive for bacteria or fungi and ≥ 5 days of treatment (or intent to treat in those who died prior to 5 days). The outcome variable was Sepsis group – No (NS), bacterial (BS, (≥1 episode of bacterial sepsis without fungal sepsis), and fungal (FS, ≥1 episode of fungal sepsis). Infants with positive bacterial and fungal blood/CSF culture were included in FS group as positive blood cultures for bacteria during the period of fungal infection are characteristic of the natural history of disease of invasive fungal infections in ELBW1. Cultures positive for Corynebacterium spp., Propionibacterium spp., Alcaligenes spp., Penicillium spp., and diptheroids were considered contaminants and excluded. Cultures positive for coagulase negative Staphylococcus (CONS) were reviewed and categorized as a definite infection, possible infection or probable contaminant as described previously14. Definite and possible CONS infections were included as sepsis while probable contaminants were not. Infants who had cultures positive only for pathogens considered contaminants were included in the non-infected group.
Whole blood was collected on filter paper and frozen at five time periods (days): 0-1 (D0), 3±1 (D3), 7±2 (D7), 14±3 (D14), and 21±3 (D21). The treatment of blood samples was standardized. After the filter paper blood sample had dried, it was wrapped in stock paper cover and placed in a plastic bag which was stored in a −20°C freezer as soon as possible after the specimen was dry but no later than 24 hours. Clinical information was collected by trained research nurses on all morbidities following preterm birth and their risk factors permitting a thorough investigation of the association of inflammatory cytokines and sepsis group.
Cytokines were analyzed in stored blood spots using a multiplex Luminex assay (Luminex Corp., Austin, TX) as described previously44,45. This assay has low intra- (<10%) and inter-assay (7-23%) variation. It has been shown that cytokine measurements on DBS stored at −24 °C for >20 years are stable over time. For this secondary analysis of the primary study, 11 cytokines were identified as being important in the development of FS (TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, IL-18, IFN-γ, and TGF-β).
For each infant with BS or FS, DBS and cytokine levels were classified as pre-infection if they were obtained before the 1st episode of bacterial sepsis in the BS group, and before the 1st episode of fungal sepsis in the FS group and post-infection if they were collected after the 1st episode of infection.
Statistical Analyses
Statistical analyses were performed using SAS System 9.2 (SAS, Cary, NC). Unadjusted analysis was performed using chi-square tests for categorical outcomes and analysis of variance test (F-test) for continuous outcomes to study the association of several maternal and neonatal variables with sepsis group (NS, BS and FS). Medians and inter-quartile ranges were computed for all cytokines at each of the five time points and bivariate analyses used to assess differences across sepsis groups. For each cytokine, levels were plotted against time for each sepsis group to assess their temporal profiles over the first three postnatal weeks. Differences in temporal profiles across sepsis groups were evaluated using mixed effect models for each cytokine independently to account for the multicenter design, repeated measurement of cytokine levels, significant clinical covariates and interaction of postnatal age and sepsis group. Confounding variables included center, gestational age (GA), birthweight, race, gender, premature prolonged rupture of membranes, antenatal steroids, postnatal steroids, days in oxygen, intraventricular hemorrhage (Grades 3, 4), cystic PVL, surgically treated PDA, and necrotizing enterocolitis (Bells' stage ≥2). A p value of <0.05 was considered statistically significant. A receiver operating characteristic (ROC) curve was constructed to determine the cytokines that could predict sepsis group. Area under the curve was determined to measure accuracy of various cytokines in discriminating between sepsis groups.
Acknowledgments
The results reported in this manuscript were presented in part at the Pediatric Academic Societies' Annual Meeting on May 2, 2011 at Denver, CO.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. Data collected at participating NRN sites were transmitted to RTI International, the data coordinating center (DCC) for the NRN, which stored, managed, and analyzed the data for this study. Drs. Abhik Das (DCC PI) and Shampa Saha (DCC statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Statement of financial support: The National Institutes of Health (General Clinical Research Center Grants M01 RR30, M01 RR32, M01 RR39, M01 RR70, M01 RR80, M01 RR633, M01 RR750, M01 RR997, M01 RR6022, M01 RR7122, M01 RR8084, M01 RR16587), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (Grants U10 HD36790, U10 HD21364, U10 HD21373, U10 HD21385, U10 HD21397, U10 HD21415, U10 HD27851, U10 HD27853, U10 HD27856, U10 HD27871, U10 HD27880, U10 HD27881, U10 HD27904, U10 HD34216, U10 HD40461, U10 HD40492, U10 HD40498, U10 HD40689), and the U.S. Centers for Disease Control and Prevention (Interagency Agreement Y1-HD-5000-01) provided grant support for the Neonatal Research Network's Cytokines Study. In addition, Dr. Benjamin received support from the Thrasher Research Fund and NICHD (Grant HD44799). The funding agencies provided overall oversight for study conduct, but all data analyses and interpretation were independent of the funding agencies. Information regarding investigator/agency participation can be found as a supplementary data file in the online journal.
Category of study: Clinical Study
References
- 1.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17:638–80. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman DA, Cuff AL, Wamstad JB, et al. Fluconazole prophylaxis in extremely low birth weight infants and neurodevelopmental outcomes and quality of life at 8 to 10 years of age. J Pediatr. 2011;158:759–65. doi: 10.1016/j.jpeds.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 5.Zeitoun AA, Gad SS, Attia FM, Abu Maziad AS, Bell EF. Evaluation of neutrophilic CD64, interleukin 10 and procalcitonin as diagnostic markers of early- and late-onset neonatal sepsis. Scand J Infect Dis. 2010;42:299–305. doi: 10.3109/00365540903449832. [DOI] [PubMed] [Google Scholar]
- 6.Hohl TM, Rivera A, Pamer EG. Immunity to fungi. Curr Opin Immunol. 2006;18:465–72. doi: 10.1016/j.coi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–7. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 9.Ghilardi N, Ouyang W. Targeting the development and effector functions of TH17 cells. Semin Immunol. 2007;19:383–93. doi: 10.1016/j.smim.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 12.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappu BP, Angkasekwinai P, Dong C. Regulatory mechanisms of helper T cell differentiation: new lessons learned from interleukin 17 family cytokines. Pharmacol Ther. 2008;117:374–84. doi: 10.1016/j.pharmthera.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schelonka RL, Maheshwari A, Carlo WA, et al. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2011;53:249–55. doi: 10.1016/j.cyto.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oguz SS, Sipahi E, Dilmen U. C-reactive protein and interleukin-6 responses for differentiating fungal and bacterial aetiology in late-onset neonatal sepsis. Mycoses. 2011;54:212–6. doi: 10.1111/j.1439-0507.2009.01802.x. [DOI] [PubMed] [Google Scholar]
- 16.Caldas JP, Marba ST, Blotta MH, Calil R, Morais SS, Oliveira RT. Accuracy of white blood cell count, C-reactive protein, interleukin-6 and tumor necrosis factor alpha for diagnosing late neonatal sepsis. J Pediatr (Rio J) 2008;84:536–42. doi: 10.2223/JPED.1838. [DOI] [PubMed] [Google Scholar]
- 17.Ucar B, Yildiz B, Aksit MA, et al. Serum amyloid A, procalcitonin, tumor necrosis factor-alpha, and interleukin-1beta levels in neonatal late-onset sepsis. Mediators Inflamm. 2008;2008:737141. doi: 10.1155/2008/737141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng PC, Li K, Leung TF, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52:1181–9. doi: 10.1373/clinchem.2005.062075. [DOI] [PubMed] [Google Scholar]
- 19.Hotoura E, Giapros V, Kostoula A, Spirou P, Andronikou S. Tracking changes of lymphocyte subsets and pre-inflammatory mediators in full-term neonates with suspected or documented infection. Scand J Immunol. 2011;73:250–5. doi: 10.1111/j.1365-3083.2010.02499.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurt AN, Aygun AD, Godekmerdan A, Kurt A, Dogan Y, Yilmaz E. Serum IL-1beta, IL-6, IL-8, and TNF-alpha levels in early diagnosis and management of neonatal sepsis. Mediators Inflamm. 2007;2007:31397. doi: 10.1155/2007/31397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shouman B, Badr R. Regulated on activation, normal T cell expressed and secreted and tumor necrosis factor-alpha in septic neonates. J Perinatol. 2010;30:192–6. doi: 10.1038/jp.2009.167. [DOI] [PubMed] [Google Scholar]
- 22.Ng PC, Li K, Chui KM, et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. 2007;61:93–8. doi: 10.1203/01.pdr.0000250207.95723.96. [DOI] [PubMed] [Google Scholar]
- 23.Kuster H, Weiss M, Willeitner AE, et al. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352:1271–7. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez BE, Mercado CK, Johnson L, Brodsky NL, Bhandari V. Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med. 2003;31:60–8. doi: 10.1515/JPM.2003.009. [DOI] [PubMed] [Google Scholar]
- 25.Ng PC, Li K, Wong RP, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003;88:F209–13. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancelier AC, Petronilho F, Reinke A, et al. Inflammatory and oxidative parameters in cord blood as diagnostic of early-onset neonatal sepsis: a case-control study. Pediatr Crit Care Med. 2009;10:467–71. doi: 10.1097/PCC.0b013e318198b0e3. [DOI] [PubMed] [Google Scholar]
- 27.Onal EE, Kitapci F, Dilmen U, Adam B. Interleukin-6 concentrations in neonatal sepsis. Lancet. 1999;353:239–40. doi: 10.1016/S0140-6736(05)77250-0. [DOI] [PubMed] [Google Scholar]
- 28.Hodge G, Hodge S, Haslam R, et al. Rapid simultaneous measurement of multiple cytokines using 100 microl sample volumes--association with neonatal sepsis. Clin Exp Immunol. 2004;137:402–7. doi: 10.1111/j.1365-2249.2004.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messer J, Eyer D, Donato L, Gallati H, Matis J, Simeoni U. Evaluation of interleukin-6 and soluble receptors of tumor necrosis factor for early diagnosis of neonatal infection. J Pediatr. 1996;129:574–80. doi: 10.1016/s0022-3476(96)70123-3. [DOI] [PubMed] [Google Scholar]
- 30.Romagnoli C, Frezza S, Cingolani A, et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160:345–50. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- 31.Panero A, Pacifico L, Rossi N, Mancuso G, Stegagno M, Chiesa C. Interleukin 6 in neonates with early and late onset infection. Pediatr Infect Dis J. 1997;16:370–5. doi: 10.1097/00006454-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54:1251–7. doi: 10.1016/s0895-4356(01)00400-0. [DOI] [PubMed] [Google Scholar]
- 33.Laborada G, Rego M, Jain A, et al. Diagnostic value of cytokines and C-reactive protein in the first 24 hours of neonatal sepsis. Am J Perinatol. 2003;20:491–501. doi: 10.1055/s-2003-45382. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–47. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 35.Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppenheim JJ, Ruscetti FW. Cytokines. In: Parslow TG, Stites DP, Terr AI, Imboden JB, editors. Medical Immunology. 10th Ed. New York: Lange Medical Books/McGraw-Hill Medical Publishing Division; 2001. pp. 148–66. [Google Scholar]
- 37.Kingsmore SF, Kennedy N, Halliday HL, et al. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008;7:1863–75. doi: 10.1074/mcp.M800175-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender L, Thaarup J, Varming K, Krarup H, Ellermann-Eriksen S, Ebbesen F. Early and late markers for the detection of early-onset neonatal sepsis. Dan Med Bull. 2008;55:219–23. [PubMed] [Google Scholar]
- 39.Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin DK, Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126:e865–73. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sood BG, Madan A, Saha S, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67:394–400. doi: 10.1203/PDR.0b013e3181d01a36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lagakos SW. The challenge of subgroup analyses--reporting without distorting. N Engl J Med. 2006;354:1667–9. doi: 10.1056/NEJMp068070. [DOI] [PubMed] [Google Scholar]
- 43.Carlo WA, McDonald SA, Tyson JE, et al. Cytokines and neurodevelopmental outcomes in extremely low birth weight infants. J Pediatr. 2011;159:919–25. e3. doi: 10.1016/j.jpeds.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skogstrand K, Ekelund CK, Thorsen P, et al. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]