Abstract
The study identified relations among traumatic stressors, HIV-related trauma symptoms, comorbid medical conditions, and health related quality of life (HRQL) in individuals with HIV. Participants (N = 118) completed a structured clinical interview on HIV as a traumatic stressor and other severe traumatic stressors and completed the Impact of Event Scale to assess HIV-related trauma symptoms and the Medical Outcomes Study 36-item Short Form (SF-36) to assess HRQL. Medical chart reviews determined comorbid conditions. Path analysis findings indicated participants with prior severe traumatic stressors experienced their HIV diagnosis as traumatic and in turn were more likely to have current HIV-related trauma symptoms which were negatively related to HRQL. HIV as a traumatic stressor was related to coronary artery diseases and HRQL. Traumatic stressors and HIV-related trauma symptoms impact health in individuals with HIV and highlight the need for psychological interventions prior to diagnosis and throughout treatment.
Keywords: Health related quality of life, HIV, Traumatic stress, Medical comorbidity, Path analysis
Introduction
With the introduction of combination antiretroviral therapy (ART), HIV was transformed from a fatal disease to a manageable chronic disease [1]. Consequently, health-related quality of life (HRQL) [2, 3] and the management of comorbid chronic medical conditions [4–6] have been identified as essential components of overall healthcare for individuals living with HIV. HRQL refers to a patient’s perceived well-being across a number of areas including physical, social, and mental functioning [7] and is a significant predictor of survival among individuals with HIV [8]. A number of factors have been identified that influence HRQL in individuals living with HIV including disease status, HIV symptoms, functional status, and psychological functioning [9]. Individuals with HIV have poorer HRQL than individuals living with other chronic conditions [10]. Furthermore, in non-HIV research, there is a strong link between trauma exposure and poor HRQL [11].
Research indicates that individuals with HIV are more likely than the general U.S. population to have significant trauma histories [12–15] and comorbid medical conditions [16], both of which are negatively related to HRQL [8, 11, 17–19]. As many as 95% of those living with HIV report at least one severe traumatic stressor [13, 20] and up to 54% meet criteria for posttraumatic stress disorder (PTSD) [13, 21]. Further, between 30 and 40% of individuals living with HIV and PTSD identify receiving or living with HIV as the index traumatic stressor for their PTSD [22–24]. Experiencing one’s HIV diagnosis as a traumatic stressor and ongoing HIV-related trauma symptoms likely negatively impact health and HRQL in ways that are unique to this population [21–25]. The cumulative negative effect of trauma in people with HIV, including increased biological and psychological vulnerability to elevated stress response [26–28], increased likelihood of perceiving stress as traumatic [29], and depleted psychological and physical resources for coping [30], can be severe. Furthermore, serious long-term negative health consequences accompany trauma exposure, including chronic medical conditions [31–33] and reduced HRQL [8, 18]. Several studies have found that those with PTSD report a greater number of chronic pain conditions [32], are at an increased risk of developing pulmonary diseases [32, 34], and developing and dying of coronary artery diseases (CAD) [31, 35]. Moreover, research indicates PTSD is a stronger predictor of mortality and morbidity than objective measures of health [18].
Schnurr and colleagues [33] propose a causal model for the relationship between trauma and negative health outcomes. Specifically, that trauma is indirectly related to poor health outcomes including the development of chronic medical conditions and poor HRQL through behavioral and physiological changes that prevent effective coping and positive health behaviors. This model recognizes that both trauma exposure and the development of trauma symptoms are predictors of poor health outcomes [33].
Due to the negative health consequences of trauma exposure, the current study used path analysis to explore the relations among prior severe traumatic stressors, HIV diagnosis as a traumatic stressor, HIV-related trauma symptoms, comorbid chronic medical conditions, and HRQL. The model utilizes a trauma and health conceptual framework [33] to empirically examine the links between the experience of trauma and health in individuals living with HIV. Specifically, it was hypothesized that: (1) participants with a prior history of severe traumatic stressors (traumatic stressors meeting Criterion A of the PTSD diagnosis) would be more likely to experience their HIV diagnosis as a traumatic stressor, (2) experiencing one’s HIV diagnosis as a traumatic stressor would be directly associated with current HIV-related trauma symptoms, (3) greater levels of current HIV-related trauma symptoms would be negatively related to HRQL, (4) experiencing one’s HIV diagnosis as a traumatic stressor would be directly associated with a number of chronic health conditions, (5) experiencing one’s HIV diagnosis as a traumatic stressor would be negatively related to HRQL, and (6) current HIV-related trauma symptoms would mediate the relationship between HIV diagnosis as a traumatic stressor and HRQL.
Methods
Participants and Procedures
This study took place at an urban medical center in an HIV/AIDS clinic over a 6-month recruitment period in 2006. Research assistants approached 146 patients as they were in the waiting area and described the study. Twenty-eight patients (19%) declined to participate for various reasons including lack of interest and time. One-hundred-eighteen patients were interested and were eligible to participate (HIV-positive adults receiving care at the clinic); thus, they were escorted to a private room and informed consent was obtained. A structured interview to obtain demographic information including gender, age, ethnicity, sexual orientation, and education and to obtain information regarding HIV diagnosis as a potentially traumatic stressor and history of severe traumatic stressors was conducted. Participants then completed the self-report questionnaires. A $15 gift card was provided to each participant upon completion. With consent, a medical chart review was also conducted. All procedures were approved by affiliated Institutional Review Boards, and a Certificate of Confidentiality was obtained from the National Institute of Mental Health.
Measures
Demographics
A structured interview was developed to obtain information on gender, age, ethnicity, sexual orientation, and education.
HIV Diagnosis as a Traumatic Stressor
A structured clinical interview, using Criterion A for PTSD based on the DSM-IV [36], assessed HIV diagnosis as a traumatic stressor. Participants were requested to recall the date they were diagnosed with HIV. If they were unable to recall the date, the research assistant probed further with questions regarding age at diagnosis, season of the year when diagnosed, etc. Participants responded to two “Yes/No” questions: (1) “Did you perceive this experience as a threat to your life or a threat to your physical well-being?” and (2) “Did your response involve intensive fear, helplessness, or horror?” The use of Criterion A has been deemed a “feasible definition of trauma” that can “serve a wide range of clinical and research needs” [37]. Endorsement of both criteria indicated that receiving an HIV diagnosis was experienced as a traumatic stressor and thus coded a dichotomous variable.
History of Severe Traumatic Stressors
Participants were requested to recall severe traumatic stressors that occurred over their lifetime that also satisfied DSM-IV PTSD Criterion A [36]. The traumatic stressor, date and/or age at which each stressor occurred were documented. Only traumatic stressors occurring prior to HIV diagnosis were included in the current study, which were summed to create a count variable.
HIV-Related Trauma Symptoms
The Impact of Event Scale (IES; [38]) was used to measure current trauma symptoms and anchored to “receiving an HIV-positive diagnosis” as the referent traumatic stressor. This measure assesses intrusive and avoidant traumatic stress symptoms using 15-items, each rated on a 4 point scale from 0 (not at all), 1 (rarely), 3 (sometimes), to 5 (often) based on how often each symptom was experienced within the past week. An example of an intrusive item is ‘I had dreams about it’ and an example of an avoidant item is ‘I stayed away from reminders of it.’ A total score is derived from a summation of all items, scores range from 0 to 75 with higher scores indicating greater traumatic stress. The IES has good test–retest reliability (0.87) and predictive validity [38]. In the current study the alpha for the total scale score was 0.91.
Health Information
An in-depth systematic review of participants’ medical charts was conducted using a data extraction worksheet including (1) date first diagnosed with HIV, (2) current anti-retroviral treatment status, (3) most recent CD4 count, and (4) current physician and/or nurse practitioner diagnosed medical conditions receiving treatment within the past 12-month period including: (a) Coronary Artery Disease (i.e. myocardial infarction, angina pectoris), (b) Chronic Pain Conditions (i.e. severe arthritis, rheumatism, or other joint or bone disease), (c) Renal/Kidney Disease, and (d) Respiratory/Pulmonary Disease (i.e. asthma, emphysema, chronic obstructive pulmonary disease). All chart reviews were conducted by research assistants and checked a second time for accuracy by the PI. These categories of medical conditions were chosen for several reasons, namely: (i) they are diseases that are generally not part of the HIV disease process; for example we intentionally did not include peripheral neuropathy and tuberculosis, (ii) they are conditions that are chronic and require ongoing medical care, (iii) they are conditions that often present in an aging population, and (iv) are increasingly of concern in the care of individuals living with HIV due to ART side effects [4–6, 16, 39].
Health Related Quality of Life
The Medical Outcomes study 36-item Short Form (SF-36; [7]) was used to assess HRQL. This is a widely used self-report measure of general physical and mental health functioning across several domains including: physical functioning, role-physical (limitations in the kinds/amount of work/activities due to physical functioning), bodily pain, general health, vitality, social functioning, role-emotional (limitations to the kinds/amounts of work/activities due to emotional functioning), and mental health. A number of validation studies have been conducted in the general population and with various medically ill populations [7]. A physical component summary score (Physical HRQL), was calculated with the physical functioning, role-physical, bodily pain, and general health subscales and a mental component summary score (Mental HRQL), was calculated with the vitality, social functioning, role-emotional, and mental health subscales [40]. Higher scores are indicative of greater HRQL. Internal consistency for the current study data were Physical HRQL = 0.86, Mental HRQL = 0.73.
Data Analysis
Descriptive statistics were performed using SPSS version 18 [41] and model testing was performed using AMOS version 17 [42]. Information obtained during the interview, demographics, HIV as a traumatic stressor, and prior severe traumatic stressors, did not have any missing data points. A missing data analysis for items on the IES and the SF-36 was conducted. Missing data were replaced using regression imputation for the IES. Further, scales for the SF-36 were calculated using recommended SF-36 procedures [7] in which missing values were replaced by scale means when there were valid responses for at least half of the scale items. Using these procedures, all missing values were replaced. Descriptive statistics, including frequencies and percents were used for categorical data and means and standard deviations were used for continuous data. Correlation analyses using appropriate coefficients including Pearson, Spearman, and Phi were used to examine bivariate relations between variables. Path analysis was used to test the hypotheses. Maximum likelihood was used to estimate path coefficients and results are presented in standardized form. Further, several fit indices were used to test the hypothesized path models, including: (1) comparative fit index (CFI), (2) root mean square error of approximation (RMSEA), (3) Tucker-Lewis Coefficient (TLI), and (4) Incremental Fit Index (IFI). A nonsignificant χ2 value indicates the hypothesized model does not differ from the covariance structure of the data. Values for CFI, TLI, and IFI above 0.95 and RMSEA values of 0.06 or less are indicative of a good model fit [43]. Due to small sample size we used the bootstrap-corrected Maximum Likelihood estimation method [44]. Finally, we examined the significance of indirect effects of HIV as a traumatic stressor on HRQL through bootstrapping and obtained bias-corrected confidence intervals.
Results
Missing Values Analysis
One hundred five participants completed the IES with no missing data points. The mean number of items missing per participant was 0.14 (SD = 0.43). The rate of missing values was below one percent for all items. Results indicated there was less than one percent missing data and that data were missing at random. Missing data for the SF-36 indicated 75 (64%) completed it with no missing data points. Overall, the mean number of missing items per participants was 0.69 (SD = 1.50). The rate of missing values was below five percent for all items.
Collinearity analyses indicated multicollinearity was not a concern [45]. Analyses were conducted to assess assumptions of normality and revealed IES data and CD4 count were positively skewed; therefore, data were Winsorized to the 95th percentile [46]. The SF-36 did not violate normality assumptions.
Sample Demographics
Participants were predominately Black American (89%), men (73%), and represented all sexual orientations (55% heterosexual, 31% gay/lesbian, and 11% bisexual). The average age was 45 years (SD = 9), range 23–69. Demographic characteristics are presented in Table 1.
Table 1.
Demographic characteristics of participants (N = 118)
| Category | Number (Percent) |
|---|---|
| Gender | |
| Male | 86 (73) |
| Female | 32 (27) |
| Age | |
| 20–29 | 8 (7) |
| 30–39 | 26 (22) |
| 40–49 | 55 (47) |
| 50+ | 29 (24) |
| Ethnicity | |
| African American | 105 (89) |
| Caucasian | 4 (3) |
| Hispanic | 2 (2) |
| Other | 7 (6) |
| Education | |
| Less than high school | 28 (24) |
| High school graduate | 37 (31) |
| Some college | 39 (33) |
| College graduate | 14 (12) |
Descriptive Information
On average, participants had been living with HIV for 10.4 (SD = 5.97) years (range <1–23 years). Ninety-two participants (78%) were prescribed ART at the time of the study and the average participant CD4 count was 500 (SD = 394).
Overall, 39 (33%) of the participants reported a history of prior severe traumatic stressor [M = 0.48 (SD = 0.85), range 0–4] prior to learning of one’s HIV diagnosis. More detailed information about the specific traumatic stressors is provided in Table 2. Further, 37 (31%) of the participants reported experiencing their HIV diagnosis as a traumatic stressor. The average HIV-related trauma symptoms score was 24.45 (SD = 17.41). Scores 26 and greater are indicative of moderate to severe traumatic stress [38] and 51 (43%) participants scored in this range.
Table 2.
Descriptive information for study participants (N = 118)
| Variable | Frequency (%) |
Mean (SD) | Range |
|---|---|---|---|
| Prior severe trauma | 39 (33) | 0.48 (0.85) | 0–4 |
| Serious accident or fire | 20 (17) | ||
| Community violence with guns | 11 (10) | ||
| Childhood abuse | 7 (6) | ||
| Life-threatening medical emergency | 4 (3) | ||
| Military combat | 2 (2) | ||
| Adult sexual assault | 1 (1) | ||
| Intimate partner violence | 1 (1) | ||
| HIV as a traumatic stressor | 37 (31) | ||
| Current HIV-related trauma symptoms | 24.45 (17.41) | 0–58 | |
| Pulmonary disease | 32 (27) | ||
| Chronic pain condition | 25 (21) | ||
| Coronary artery disease | 13 (11) | ||
| Renal disease | 11 (9) | ||
| Physical HRQL | 57.45 (23.50) | 5–100 | |
| Mental HRQL | 63.28 (21.66) | 24–100 |
Sixty-two participants (53%) had at least one of the physician diagnosed comorbid chronic disease of interest in the current study. Further examination revealed 46 (39%) had one comorbid condition, 13 (11%) had two comorbid conditions, and 3 (3%) had three comorbid conditions. Information about the specific comorbid chronic conditions is provided in Table 2. The average physical HRQL was 57.45 (SD = 23.50) and the average mental HRQL was 63.28 (SD = 21.66).
Study Variable Relationships
Bivariate analysis examined the relations among study variables and demographic variables. Participants with pulmonary diseases were more likely to be female (Pearson χ2: 6.15, p = 0.013) and have less education (Spearman’s rho: −0.19, p = 0.042). Those with CAD were older (Spearman’s rho: 0.22, p = 0.019). Participants with greater levels of mental HRQL had more education (Pearson’s r: 0.26, p = 0.004). Years since diagnosis and ART status were not significantly related to study variables.
Bivariate analysis examined the relationships between predictor variables and outcome variables. Physical HRQL was significantly related to prior trauma history (Spearman’s rho: −0.217, p = 0.019), HIV diagnosis as traumatic stressor (Spearman’s rho: −0.227, p = 0.014), current trauma symptoms (Pearson’s r: −0.311, p < 0.001), renal disease (Spearman’s rho: −0.193, p = 0.037), and chronic pain conditions (Spearman’s rho: −0.314, p < 0.001). Mental HRQL was significantly related to prior trauma history (Spearman’s rho: −0.221, p = 0.016), current trauma symptoms (Pearson’s r: −0.393, p < 0.001), pulmonary disease (Spearman’s rho: −0.300, p < 0.001), and chronic pain conditions (Spearman’s rho: −0.322, p < 0.001).
Path Analysis
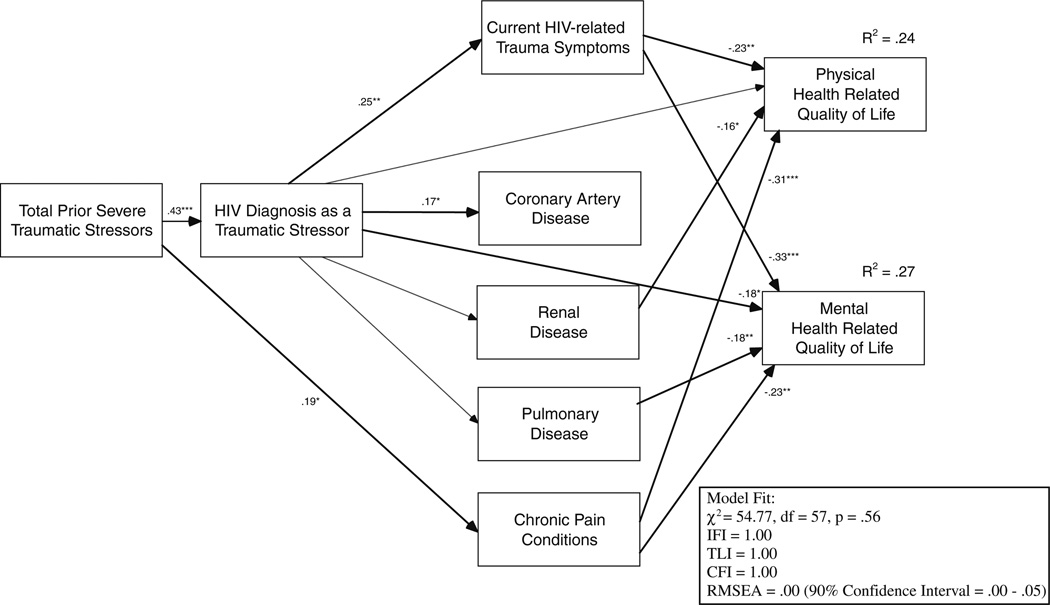
Based on bivariate analysis, a pathway between prior severe traumatic stressors and chronic pain was established rather than a pathway between HIV diagnosis as a traumatic stressor. The hypothesized model was not a good fit with the study data, χ2 (26, N = 118) = 103.79, p = 0.00 (RMSEA = 0.16, 95% CI = 0.13–0.19; CFI = 0.48, IFI = 0.51, TLI = 0.27). Modification indices suggested adding covariance between the error terms for physical and mental HRQL. Further, pathways were also included from renal and chronic pain conditions to physical HRQL and from pulmonary and chronic pain conditions to mental HRQL, these modifications were included for both statistical and conceptual purposes and significantly improved the model fit. Figure 1 presents the final path model. Though not shown, the effects of all significant covariates were controlled for in the model. Overall, the final model was a good fit with the study data, χ2 (57, N = 118) = 54.77, p = 0.56 (RMSEA = 0.00, 95% CI = 0.00–0.05; CFI = 1.00, IFI = 1.00, TLI = 1.00). The model accounted for 24% of the variance in physical and 27% of the variance in mental HRQL. For the first hypothesis, participants with more past severe traumatic stressors were more likely to have experienced their HIV diagnosis as a traumatic stressor (p < 0.001). For the second hypothesis, those who experienced their HIV diagnosis as a traumatic stressor had greater levels of current HIV-related trauma symptoms (p = 0.006). For the third hypothesis, greater current HIV related trauma symptoms was significantly related to poorer physical HRQL (p = 0.005) and poorer mental HRQL (p < 0.001). For the fourth hypothesis, those who experienced their HIV diagnosis as a traumatic stressor were more likely to have CAD (p = 0.045). HIV diagnosis as a traumatic stressor was not significantly related to the other comorbid chronic conditions. However, history of prior severe traumas was significantly related to chronic pain condition (p = 0.033). For the fifth hypothesis, experiencing one’s HIV diagnosis as a traumatic stressor was not directly significantly related to poorer physical HRQL (p = 0.135); however, it was significantly related to poorer mental HRQL (p = 0.029). For the sixth hypothesis on mediation, the indirect effects of HIV as a traumatic stressor was significant for physical HRQL, = −0.072 (p = 0.020) but was not significant for mental HRQL (p = 0.087).
Fig. 1.
Path analysis examining final model including hypothesized pathways. Solid lines represent significant pathways while dashed lines represent non-significant pathways. Although not indicated in the figure, the effects of gender on pulmonary disease, age and CD4 count on coronary artery, and education on mental health related quality of life and pulmonary disease were controlled in the model. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
Our findings support the general study hypotheses and the trauma-health link [33] in individuals living with HIV. Specifically, the current study found that: (1) individuals with histories of prior severe traumatic stressors were more likely to experience their HIV diagnosis as a traumatic stressor, (2) those who experienced their HIV diagnosis as a traumatic stressor reported greater levels of current HIV-related trauma symptoms, diminished mental HRQL, and were more likely to have CAD, and (3) current HIV-related trauma symptoms had a significant negative impact on physical and mental HRQL independent of chronic medical conditions and significant covariates. Further, although not the focus of the current study, findings elucidate the seriousness of comorbid chronic medical conditions in an already immune-compromised population. Findings are discussed within biological and behavioral theoretical perspectives while emphasizing their clinical relevance.
In spite of the evidence that individuals living with HIV are likely to have significant trauma histories [12–15]; this is the first study we are aware of to examine the cumulative effect of prior traumatic stressors on the perceptions of a highly stressful event, specifically, that of receiving an HIV-positive diagnosis, and the resulting health impact. In the current study, participants with histories of prior severe traumatic stressors were more likely to experience their HIV diagnosis as a traumatic stressor. This finding supports research in non-HIV populations that suggests individuals with histories of prior traumas are vulnerable to perceiving future stressor as traumatic [29, 30]. Further, findings indicate there may be long-term health consequences of this perception.
Perceiving one’s HIV-positive diagnosis as a traumatic stressor, regardless of length of time since diagnosis, was significantly related to indicators of current psychological and physiological functioning including greater levels of current HIV-related trauma symptoms, CAD, and diminished mental HRQL. Biological models suggest that traumatic stress may result in several biological processes that can have a negative impact on health, including sympathetic over activity, endocrinologic irregularities, and altered immunologic mechanisms [27, 28]. It has also been suggested that these biological changes may allow for an accelerated aging process, the manifestation of diseases [17, 47], or lead to a disinhibition of the inflammatory process [48]. In fact, increased inflammation, a risk factor of CAD, has also been demonstrated among individuals diagnosed with PTSD [35]. Behavioral models suggest that the relationship between traumatic stress and health outcomes may also be partly explained by the effect of trauma on health behaviors. Prior studies have shown that individuals with histories of trauma and/or trauma symptoms are more likely to engage in high-risk behavior [12] and less likely to engage in health promoting behaviors [49]. Taken together, these models bring to light the multiple pathways from traumatic stress to indicators of health. Although HIV has been transformed from a fatal disease to a chronic disease, the impact of receiving the diagnosis and living with the disease continues to have a profound impact on the quality of life in individuals living with the disease. Moreover, while the ‘test and treat’ model is a good public policy, it may be problematic for people with a preexisting trauma history. Appropriate screening for the presence of trauma history and current trauma symptoms prior to HIV testing may be necessary to identify those who may require additional support following a diagnosis to avoid being traumatized, or retraumatized, by receiving an HIV diagnosis. These individuals may also be good candidates for additional mental health treatment to reduce negative disease impact and ongoing risk behavior, and increase treatment adherence [50]. In fact, unlike many problems facing people living with HIV, traumatic stress is modifiable. Effective behavioral interventions exist, and some pharmaceutical agents show promise for reducing traumatic stress.
The prevalence of comorbid chronic medical conditions and poor HRQL in this sample highlight the complex health challenges of individuals living with HIV. The percentage of participants with physician diagnosed chronic medical conditions in the current sample is remarkable and concerning. Further, the comorbid conditions examined in the current study were intentionally not opportunistic diseases or HIV related diseases; rather they are chronic medical conditions that exist outside of the HIV disease process and are generally found in aging populations. Findings support previous research that highlights the burden of chronic diseases in individuals with HIV [4–6, 16]. The medical management of comorbid chronic medical conditions in individuals with HIV has emerged as a result of many factors, including longer life expectancies [51] and negative side effects that result from ART [16]. Further, both trauma and ART are associated with the development of a number of chronic comorbid conditions including CAD [16, 35], diabetes and renal diseases [34], pulmonary diseases [16, 34], and chronic pain conditions [32, 52]. Our findings support previous research suggesting individuals living with HIV have poor health status, beyond that directly related to HIV, based on multiple indicators of health including physician diagnosed conditions and poor HRQL [2, 16]. We suggest that these findings may at least to some degree be due to the impact of trauma on health [33].
Findings should be understood within the limitations of the study. First, although there is a conceptual temporal sequence to the variables in our model, these data were collected cross-sectionally. Thus, we are prevented from determining causal and temporal relationships among traumatic stressors, trauma symptoms, comorbid chronic medical conditions, and HRQL. Prospective longitudinal studies are necessary to assess temporal relationships between these variables. Second, the retrospective recall of the emotional responses involved in receiving an HIV diagnosis is a major limitation. Unknown factors present during the acute aftermath of diagnosis and/or during study participation may have influenced recall. Further, time since diagnosis varied in this sample, potentially influencing recall and possibly limiting interpretation of results. Third, the sample size is small for the analysis utilized. Although significant findings are considered reliable, the study may have lacked power to identify small but significant relationships between variables. Additionally, there may have been insufficient power to reject the null hypothesis when considering model fit (i.e., non significant chi-square value). Therefore, our use of multiple fit indices was necessary to support goodness of fit. Fourth, individuals living with HIV are likely to have future-oriented concerns involving the trajectory of the disease process [23, 53]. Therefore, standard tools for assessing trauma symptoms in individuals with HIV may not assess trauma related symptoms accurately; specifically, these instruments were not developed to assess ongoing traumatic stressors such as HIV [54]. The development of instruments designed to assess ongoing traumatic stressors and the ability to assess future oriented trauma symptoms is necessary for a comprehensive understanding of the traumatic impact of living with HIV. Fifth, although participants were instructed to anchor all Impact of Event items in the index trauma of “receiving an HIV-positive diagnosis,” due to the ongoing nature of the living with HIV and the high number of prior severe traumatic stressors endorsed, they may have generalized their responses to the experience of living with HIV and/or other traumatic stressors. Sixth, the study sample was fairly homogenous (73% male and 89% African American), thus findings may not be generalizable to the larger HIV positive population.
Conclusion
In spite of these limitations, our findings established a relationship among traumatic stressors, HIV-related trauma symptoms, and indicators of health; and as such, have clinical implications. The high prevalence rate of both trauma, HIV-related trauma symptoms and inflammatory related comorbid chronic medical conditions (i.e. CAD, chronic pain conditions) in individuals living with HIV likely increases risk for mortality [31, 35]. Because these psychological and physiological conditions may have shared pathways, future research is necessary to determine the presence of and implications of dual inflammatory processes related to both trauma and comorbid chronic medical conditions. Given how common histories of trauma exposure are among people living with or at risk for HIV, it is imperative that clinicians and researchers keep in mind the potential for traumatic stress reactions when testing for or treating HIV infection.
Acknowledgment
This research was partly funded by a dissertation grant awarded to the first author from the Institute of Psychology at Illinois Institute of Technology and was supported by grants T32-MH020031, P30 MH62294 (Center for Interdisciplinary Research on AIDS) and K23-MH076671 from the National Institute of Mental Health. The authors gratefully acknowledge our community collaborations with the HIV Care Program formally located within Michael Reese Hospital in Chicago, IL, especially Anita McGruder-Johnson, Arthur Moswin, Norma Rolfsen, Billie Strauss, and Maryanne Williams as well as the research assistance of Lissa Baur and Kristin Neil.
Contributor Information
Vienna R. Nightingale, Email: vienna.nightingale@yale.edu, Center for Interdisciplinary Research on AIDS, Yale University, 135 College Street, Suite 200, New Haven, CT 06510, USA.
Tamara G. Sher, Institute of Psychology, Illinois Institute of Technology, Chicago, IL, USA
Melissa Mattson, Institute of Psychology, Illinois Institute of Technology, Chicago, IL, USA.
Sarah Thilges, Institute of Psychology, Illinois Institute of Technology, Chicago, IL, USA.
Nathan B. Hansen, Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA
References
- 1.Hariri S, McKenna MT. Epidemiology of human immunodeficiency virus in the United States. Clin Microbiol Rev. 2007;20:478–488. doi: 10.1128/CMR.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen NB, Vaughan EL, Cavanaugh CE, Connell CM, Sikkema KJ. Health-related quality of life in bereaved HIV-positive adults: relationships between HIV symptoms, grief, social support, and Axis II indication. Health Psychol. 2009;13:375–384. doi: 10.1037/a0013168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez IR, Bano JR, Lopez Ruz MA, et al. Health-related quality of life of patients with HIV: impact of sociodemographic, clinical, and psychosocial factors. Qual Life Res. 2005;14:1301–1310. doi: 10.1007/s11136-004-4715-x. [DOI] [PubMed] [Google Scholar]
- 4.Adeyemi O, Vibhakar S, Max B. Are we meeting the American Diabetes Association goals for HIV-infected patients with diabetes mellitus? Clin Infect Dis. 2009;49(5):799–802. doi: 10.1086/605286. [DOI] [PubMed] [Google Scholar]
- 5.Campbell LJ, Ibrahim F, Fisher M, Holt SG, Hendry BM, Post FA. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10(6):329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 6.John L, Sotirios T, Ignatios I, et al. HIV-positive patients treated with protease inhibitors have vascular changes resembling those observed in atherosclerotic cardiovascular disease. Clin Sci. 2008;115(6):189–196. doi: 10.1042/CS20070353. [DOI] [PubMed] [Google Scholar]
- 7.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston: Health Institute; 1993. [Google Scholar]
- 8.der Kolk IM, Sprangers MAG, Prins JM, Smit C, de Wolf F, Nieuwkerk PT. Health-related quality of life and survival among HIV-infected patients receiving highly active antiretroviral therapy: a study of patients in the AIDS therapy evaluation in the Netherlands (ATHENA) cohort. Clin Infect Dis. 2010;50(2):255–263. doi: 10.1086/649216. [DOI] [PubMed] [Google Scholar]
- 9.Vidrine DJ, Amick BC, Gritz ER, Arduino RA. Assessing a conceptual framework of health-related quality of life in a HIV/AIDS population. Qual Life Res. 2005;14(4):923–933. doi: 10.1007/s11136-004-2148-1. [DOI] [PubMed] [Google Scholar]
- 10.Hays RD, Cunningham WE, Sherbourne CD, et al. Health related quality of life in patients with human immunodeficiency virus infection in the United States. Results from the HIV cost and service utilization study. Am J Med. 2000;108(9):714–722. doi: 10.1016/s0002-9343(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 11.Schnurr PP, Lunney CA, Bovin MJ, Marx BP. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):727–735. doi: 10.1016/j.cpr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Cavanaugh CE, Hansen NB, Sullivan TP. HIV sexual risk behavior among low-income women experiencing intimate partner violence: the role of posttraumatic stress disorder. AIDS Behav. 2010;14:318–327. doi: 10.1007/s10461-009-9623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pence BW, Reif S, Whetten K, et al. Minorities, the poor, and survivors of abuse: HIV-infected patients in the US deep south. South Med J. 2007;100(11):1114–1122. doi: 10.1097/01.smj.0000286756.54607.9f. [DOI] [PubMed] [Google Scholar]
- 14.Plotzker RE, Metzger DS, Holmes WC. Childhood sexual and physical abuse histories, PTSD, depression, and HIV risk outcomes in women injection drug users: a potential mediating pathway. Am J Addict. 2007;16:431–438. doi: 10.1080/10550490701643161. [DOI] [PubMed] [Google Scholar]
- 15.Whetten K, Leserman J, Lowe K, et al. Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. Am J Public Health. 2006;96(6):1028–1030. doi: 10.2105/AJPH.2005.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010;85(1):201–209. doi: 10.1016/j.antiviral.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Lesserman J, Whetten K, Lowe K, Stangl D, Swartz M, Thielman N. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 18.Cohen BE, Marmar CR, Neylan TC, Schiller NB, Alt S, Whooley MA. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease. Findings from the heart and soul study. Arch Gen Psychiatry. 2009;66:1214–1220. doi: 10.1001/archgenpsychiatry.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming CA, Christiansen D, Nunes D, et al. Health-related quality of life of patients with HIV disease: impact of hepatitis C coinfection. Clin Infect Dis. 2004;38(4):572–578. doi: 10.1086/381263. [DOI] [PubMed] [Google Scholar]
- 20.Kalichman SC, Gore-Felton C, Benotsch E, Cage M, Rompa D. Trauma symptoms, sexual behaviors, and substance abuse: correlates of childhood sexual abuse and HIV risks among men who have sex with men. J Child Sex Abus. 2004;13(1):1–15. doi: 10.1300/J070v13n01_01. [DOI] [PubMed] [Google Scholar]
- 21.Boarts JM, Buckley-Fischer BA, Armelie AP, Bogart LM, Delahanty DL. The impact of HIV diagnosis-related vs. non-diagnosis related trauma on PTSD, depression, medication adherence, and HIV disease markers. J Evid Based Soc Work. 2009;6(1):4–16. doi: 10.1080/15433710802633247. [DOI] [PubMed] [Google Scholar]
- 22.Kelly B, Raphael B, Judd F, Kernutt G, Burnett PC, Burrows GD. Posttraumatic stress disorder in response to HIV infection. Gen Hosp Psychiatry. 1998;20:345–352. doi: 10.1016/s0163-8343(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 23.Martin L, Kagee A. Lifetime and HIV-related PTSD among persons recently diagnosed with HIV. AIDS Behav. doi: 10.1007/s10461-008-9498-6. in press. [DOI] [PubMed] [Google Scholar]
- 24.Olley BO, Zeier MD, Seedat S, Stein DJ. Post-traumatic stress disorder among recently diagnosed patients with HIV/AID in South Africa. AIDS Care. 2005;17(5):550–557. doi: 10.1080/09540120412331319741. [DOI] [PubMed] [Google Scholar]
- 25.Catz SL, Kalichman SC, Benotsch EG, Miller J, Suarez T. Anticipated psychological impact of receiving medical feedback about HIV treatment outcomes. AIDS Care. 2001;13(5):631–635. doi: 10.1080/09540120120063250. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostatisis and allostatic load. Ann NY Acad Sci. 1999;893:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 27.Friedman MJC, Dennis S. Neurobiological and clinical consequences of stress: from normal adaptation to posttraumatic stress disorder. In: Friedman MJC, Schnurr PP, editors. The Relationship Between Trauma, Posttraumatic Stress Disorder and Physical Health. Philidelphia: Lippincott Williams & Wilkins; 1995. pp. 507–524. [Google Scholar]
- 28.Yehunda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 7):14–21. [PubMed] [Google Scholar]
- 29.Shrestha NM, Sharma B, Vanmmeren M, et al. Impact of torture on refugees displaced within the developing world: symptomatology among Bhutanese refugees in Nepal. JAMA. 1998;280:443–448. doi: 10.1001/jama.280.5.443. [DOI] [PubMed] [Google Scholar]
- 30.Mollica RF, McInnes K, Poole C, Tor S. Dose-effect relationships of trauma to symptoms of depression and post-traumatic stress disorder among Cambodian survivors of mass violence. Br J Psychiatry. 1998;173:482–488. doi: 10.1192/bjp.173.6.482. [DOI] [PubMed] [Google Scholar]
- 31.Dong M, Giles WH, Felititi VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways from ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 32.Lauterbach D, Vora R, Rakow M. The relationship between posttraumatic stress disorder and self-reported health problems. Psychosom Med. 2005;67:939–947. doi: 10.1097/01.psy.0000188572.91553.a5. [DOI] [PubMed] [Google Scholar]
- 33.Schnurr PP, Green BL. Trauma and Health: Physical Health Consequences of Exposure to Extreme Stress. Washington, DC: American Psychological Association; 2004. [Google Scholar]
- 34.Spitzer C, Barnow S, Volzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71:1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- 35.Gander MI, von Kanel R. Myocardial infarction and posttraumatic stress disorder: frequency, outcome, and atherosclerotic mechanisms. Eur J Cardiovasc Prev Rehabil. 2006;13:165–172. doi: 10.1097/01.hjr.0000214606.60995.46. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, IV. Washington, DC: America Psychiatric Association; 2000. [Google Scholar]
- 37.Weathers FW, Keane TM. The criterion A problem revisited: controversies and challenges in defining and measuring psychological trauma. J Trauma Stress. 2007;20:107–121. doi: 10.1002/jts.20210. [DOI] [PubMed] [Google Scholar]
- 38.Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41:209–217. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Gill JM, Szanton S, Taylor TJ, Page GG, Campbell JC. Medical conditions and symptoms associated with posttraumatic stress disorder in low-income urban women. J Womens Health. 2009;18(2):261–267. doi: 10.1089/jwh.2008.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustavsson-Lilius M, Julkunen J, Heitanen P. Quality of life in cancer patients: the role of optimism, hopelessness, and partner support. Qual Life Res. 2007;16:75–87. doi: 10.1007/s11136-006-9101-4. [DOI] [PubMed] [Google Scholar]
- 41.SPSS. Statistical package for the social sciences, version 18 (SPSS 18) for Windows. Chicago: SPSS Inc; 2010. [Google Scholar]
- 42.AMOS. Analysis of a moment structures, version 17 (AMOS 17) for Windows. Chicago: SPSS Inc; 2009. [Google Scholar]
- 43.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 44.Kline RB. Principles and Practice of Structural Equation Modeling. New York: Guilford Press; 1998. [Google Scholar]
- 45.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed. Boston: Allyn and Bacon; 2007. [Google Scholar]
- 46.Lix LM, Kesselman HJ. To trim or not to trim: tests of location equality under heteroscedasticity and nonnormality. Educ Psychol Meas. 1998;58:409–429. [Google Scholar]
- 47.Glover DA, Stuber M, Poland RE. Allostatic load in women with and without PTSD symptoms. Psychiatr Interpers Biol Process. 2006;69(3):191–203. doi: 10.1521/psyc.2006.69.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann NY Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 49.Morland L, Goebert D, Onove J, et al. Posttraumatic stress disorder and pregnancy health: preliminary update and implications. Psychosomatics. 2007;48(4):304–308. doi: 10.1176/appi.psy.48.4.304. [DOI] [PubMed] [Google Scholar]
- 50.Sikkema K. Watt Drabkin Meade Hansen Pence Mental health treatment to reduce HIV transmission risk behavior. AIDS Behav. 2010;14:252–262. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc. 2009;57(11):2129–2138. doi: 10.1111/j.1532-5415.2009.02494.x. [DOI] [PubMed] [Google Scholar]
- 52.Schnurr PP, Spiro A, Paris AH. Physician-diagnosed medical disorders in Relation to PTSD symptoms in older military veterans. Health Psychol. 2000;19:91–97. doi: 10.1037//0278-6133.19.1.91. [DOI] [PubMed] [Google Scholar]
- 53.Safren SA, Gershuny BS, Hendriksen E. Symptoms of posttraumatic stress and death anxiety in persons with HIV and medication adherence difficulties. AIDS Patient Care STDS. 2003;17(12):657–664. doi: 10.1089/108729103771928717. [DOI] [PubMed] [Google Scholar]
- 54.Kagee A. Application of the DSM-IV criteria to the experience of living with AIDS. J Health Psychol. 2008;13(8):1008–1011. doi: 10.1177/1359105308097964. [DOI] [PubMed] [Google Scholar]