Abstract
Background: Infection with Escherichia coli O157:H7 rarely leads to bloody diarrhea and causes hemolytic uremic syndrome with renal failure that can be deadly dangerous. Intimin, translocated Intimin receptor (Tir), and enterohemorrhagic E. coli (EHEC) secreted protein A (EspA) proteins are the virulence factors expressed by locus of enterocyte effacement locus of EHEC. This bacterium needs EspA as a conduit for Tir delivery into the host cell and the surface arrayed Intimin, which docks the bacterium to the translocated Tir. Methods: Here we used triplet synthetic gene (eit) which was designed from three genes: espA coding EspA 120 lacking 36 amino acids from the N-terminal of the protein, eae coding Intimin constructed of 282 amino acids from the C-terminal and tir coding Tir 103, residues 258-361 which interacts with Intimin. The multimeric gene was cloned in two eukaryotic vectors pAAV-multiple cloning site-green fluorescent protein and pCI-neo. The pAAV was used for gene expression assay in cell line 293T and pCI-neo-EIT (EspA, Intimin, Tir) was used as DNA vaccine in mice. Test groups were injected intramuscularly with pCI-neo-EIT four times and mice control group was injected under the same conditions with PBS or pCI-neo vector. Results: The titration of serums showed that BALB/c mice were successfully immunized with DNA vaccine compared to control groups and also they were protected against challenges of live oral using E. coli O157:H7. Conclusion: The results suggest that the DNA vaccine could induce protective immunity either alone or in combination with purified antigens to reduce EHEC infection.
Key Words: DNA vaccines, Escherichia coli O157:H7, Virulence factors
Introduction
Escherichia coli O157:H7 is an important zoonotic pathogen of human, which causes gastrointestinal diseases, including hemorrhagic colitis, hemolytic uremic syndrome and thrombotic thrombocytopenic purpura [1]. Enterohemorrhagic E. coli (EHEC) can form characteristic attaching and effacing lesions. eae encoding Intimin, an outer membrane adhesion molecule, is the first gene to be associated with attaching and effacing activity. Intimin is essential for intimate bacterial attachment to eukaryotic host cells. eae is a part of a pathogenicity island called the locus of enterocyte effacement [2-4]. The LEE encodes a type III secretion system, which includes a translocated Intimin receptor (Tir) [5] and three secreted proteins EHEC secreted protein A (EspA), EspB, and EspD. These proteins are required for signal transduction in mammalian host cells and attaching and effacing lesion formation [6]. During the early stage of attaching and effacing lesion formation, EspA interacts with the host cell [7]. Secretion of Tir through the EspA filament leads to intimate attachment of the bacterium through Intimin-Tir interaction [8]. It has been proven that EspA, Intimin and Tir (EIT) proteins are individually immunogenic. A combined triple EspA, Intimin, Tir antigens in a single recombinant protein recombinant EIT (rEIT) not only replaces separate production of the antigens but also makes its production easier and cost-effective [2, 4]. espA, eae and tir genes were used in a new recombinant construction coding EspA 120 amino acids from the C-terminal of the protein, Intimin, 282 amino acids from the C-terminal, and 103 residues (258-361) of Tir, which interacts with Intimin. The immunogenicity of a trivalent recombinant protein (EIT) coded by this synthetic gene has already been proved [2, 4, 9]. DNA-based vaccines beside having benefits of conventional vaccines possess additional advantages, including ease of construction, stability, low cost, the long-lived responses, safer than live attenuated bacteria [10], ability to induce both humoral and cellular immune responses [11], and have no problems as exist in recombinant protein vaccines [12-14]. Plasmid DNA was successfully used as vaccine when injected intramuscularly in mouse without need for a special delivery system [11, 14-16]. However, researches work on DNA vaccine optimization and an appropriate choice of plasmid vector still remains an important strategy in this field [15].
Several approaches have been designed to improve DNA vaccines against E. coli disease. Alves et al. [17] generated a protective immune response against enterotoxigenic E. coli by producing antibodies to block the colonization factor antigen/I-mediated binding of entero-toxigenic E. coli. They showed that DNA vaccine could prevent attachment of colonization factor antigen/I fimbriae bacteria [17]. A DNA vaccine was tested in BALB/c mice to induce antibodies against the non-immunogenic heat-stable enterotoxin heat-stable toxin from E. coli. heat-stable toxin specific antibodies could be detected after plasmid injections, but it is capable of inducing low levels of neutralizing antibodies [18]. Immunization trials were carried out against Stx2 co-expressed with granulocyte-macrophage colony-stimulating factor. The data showed systemic Stx-specific antibody responses targeting both A and B subunits of the native Stx2 [19].
Bouzari et al. [20] applied the DNA immunization approach to elucidate the possible protective role of the adhesions factors of diarrheagenic E. coli. They immunized BALB/c mice with three different modes of vaccination, i.e. DNA/DNA, DNA/Protein, or protein/ protein of aggregative adherence factors, AAF/I or AAF/II of enteroaggregative E. coli, respectively. AAF/I and AAF/II in DNA/DNA mode could not induce the immune response. However, the DNA/protein and protein/protein approach immune-zation of AAF/I significantly induced total IgG level [20].
We have already reported the humeral immune-genicity of a trivalent recombinant protein, the virulence factors of E. coli O157:H7 [4]. The present work was designed to assess gene expression of the virulence factors as a trivalent recombinant DNA on mammalian cells growing in vitro. The humeral immunogenicity playing a major role in protection and challenge of the construct as a DNA vaccine was also assayed in mice.
MATERIALS AND METHODS
Bacterial strains, plasmids and media. E. coli O157:H7 was obtained from reference laboratory, Booali Hospital (ATCC 35218), Tehran. Plasmid pCI-neo and pAAV-MCS (multiple cloning site) were obtained from Novagen and Invitrogen (both from USA), respectively. E. coli DH5α was procured from Pasteur Institute of Iran. All E. coli strains were grown in Luria Bertani (LB) broth at 37C. Medium was supplemented with Ampicillin (Sigma, 80 µg/ml) when required. E. coli isolates were stored at -70C in 20% glycerol.
Synthesis of chimeric gene and construction of DNA vector. The amino acid sequences of EspA, Intimin and Tir were back-translated using the codon preference of eukaryotic cells. The resulting artificial open reading frame were assembled with fusion linker and its mRNA and 3D structure were analyzed as described previously [9]. The EcoRI and SalI sites were introduced at the 5´ ends of forward, 5-TCTAAGAATTCGCCACCATGGCTGAT ATG-3, and reverse primer 5-ATATCTGTCGACAAG CTCATCCTTTCCAG-3, respectively. pCI-neo and pAAV-MCS-GFP (green fluorescent protein) plasmids were digested with EcoRI and SalI. The DNA fragment was ligated to both vectors and transformed to E. coli DH5α [21]. After cloning, the sequencing was carried out and the original sequence was confirmed. Large-scale purification of cloned plasmids for immunization trials was carried out by Plasmid Midi Extraction Kit (Bioneer, Korea).
Expression and purification of recombinant EIT protein. The synthetic eit gene was expressed in E. coli BL21DE3 with the 6× His tag in the N-terminal and the purification was performed under native condition. SDS-PAGE analysis verified the presence of 61.6 kDa rEIT protein as previously reported [4].
In vitro gene transfection and expression. Human embryonic kidney cell line (293T, obtained from Cell line bank, Pasture Institute of Iran) was grown in DMEM. Media (2 ml) consisting of 200,000 cells was poured in each plate and was incubated for 24 h. Transfection was performed by the lipofection method using Lipofectamine (Invitrogen, USA). Briefly, 4 µg DNA plus 250 µl DMEM serum medium and 10 µl Lipofectamine plus 250 µl DMEM serum medium were incubated in different tubes at room temperature for 5 min. The tubes were then mixed and incubated at room temperature for 20 min. The mixed solution was added to 6-well plates and cultured at 37ºC for 6 h. RPMI was then added and transfected cells were cultured at 37C for an additional 72 h.
Fluorescent microscopy. The expression of GFP fused to EIT was observed under a fluorescent microscope (Inverso TC100, UK). 6-well plates were placed under the microscope and 293T cell line was viewed through G-filter for GFP fluorescence. Images were captured by a digital camera (Sony, cyber-shot DSC-H50, Japan).
Animal immunization. Four-week-old female BALB/c mice (Pasteur Institute of Iran) were divided into 3 test and 2 control groups. Test groups included: 1) pCI-neo-EIT, 2) combination of pCI-neo-EIT and purified recombinant protein (rEIT) [22] and 3) purified recombinant protein (rEIT) [4]. Each mouse in the 1st test group was injected intramuscularly with 50 µg of pCI-neo-EIT for four times at intervals of 0, 14, 21, 28 days without any adjuvant. The 2th test group was injected prime boost of purified EIT protein followed with 50 µg intramuscular injection of pCI-neo-EIT for three times without using any adjuvant. Mice in the 3rd group were injected subcutaneously with 5 µg of the recombinant protein (EIT) plus complete Freund’s adjuvant on day 0 and the same dose using incomplete Freund’s adjuvant on days 14 and 28 as the booster doses. Control groups were received PBS or pCI-neo vector only. Blood samples were collected from the mice after the third and fourth injection. The collected sera before third, fourth, and after fourth injections were assessed for rEIT-specific IgG by ELISA method and then stored at -20C for further studies.
Determination of serum IgG antibody responses against DNA Vaccine. Specific antibody responses were determined by ELISA. Polystyrene 96-well plates (Nunc, Denmark) were coated with 2 µg of rEIT protein [4] in coating buffer (1.9 g Na2CO3, 2.93 g NaHCO3, 0.2 g NaN3, up to 1 L H2O, pH 9.8) at 4C overnight. The plates were washed three times in PBS containing 0.05% Tween 20 (PBST) and the non-specific sites were blocked with 3% gelatin in PBST. After 1 h incubation at 37C and repeated washing, mouse serum samples of control and 1st test groups were serially diluted (from 1:10 to 1:1,280) in PBST and those of 2rd and 3th test groups were serially diluted (from 1:200 to 1:51,200) in PBST. They were then added to the ELISA plates and incubated at 37C for 45 min. The plates were washed three times in PBST and horseradish peroxidase goat anti-mouse IgG (1:2,000 in PBST, Sigma, USA) was added to the ELISA plates. Plates were incubated at 37C for 30 min and washed three times in PBST. The wells were added by 100 µl of O-phenylenediaminedihydro-chloride (Sigma, USA) and incubated at room temperature for 15 min. The reaction was stopped with 100 µl of 2 M H2SO4 and the OD492 was read on a microplate reader (Bio-Rad, USA).
Challenging the immunized mice. To determine that the EIT-specific antibodies in immunized mice sera could reduce or block the colonization and subsequent shedding of E. coli O157:H7, the challenge was carried out by feeding 1010 bacteria on mice. The mice were challenged two weeks after the last immunization. Prior to the challenges, all groups were given drinking water containing streptomycin sulfate (5 mg/ml) to reduce the normal bacterial flora of the gut [4]. Following one day of treatment with streptomycin, mice were fasted overnight, and were then fed with 1010 colony forming units (CFU) of E. coli O157:H7 (ATCC: 35218) suspended in 100 µl of LB broth. The individually housed animals had access to food and water. Every group was divided into two sets. The fecal samples from each set were collected at alternate days for two weeks. E. coli O157:H7 fecal shedding was monitored by adding approximately 0.1 g of feces to 1 ml of LB broth, followed by incubation at room temperature for 2-4 h to allow the fecal pellets to soften. The mixture was then vortexed until the pellets were no longer visible. Serial dilutions of the supernatants were plated onto sorbitol MacConkey agar plates containing tellurite. Plates were incubated at 37C overnight and E. coli O157:H7 colonies were enumerated.
Statistical analysis. All experiments were assayed triple independently and analyses performed using Microsoft EXCEL 2007. ANOVA test was used to compare antibody responses between immunized and control mice groups. Evaluation and diagnosis of differences in EHEC colonization inhibition on intestinal epithelium surface of mice were also performed with ANOVA test. P<0.05 was considered statistically significant.
Results
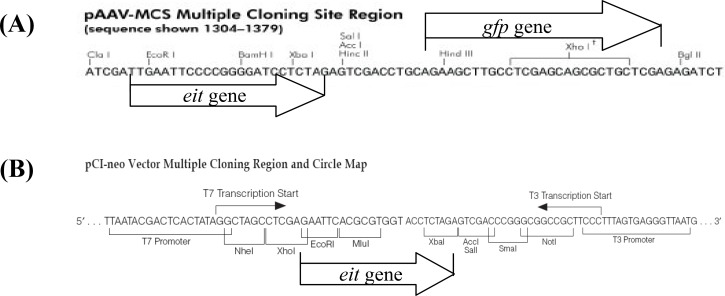
Subcloning of the eit synthetic gene in pCI-neo and pAAV-MCS-GFP. A number of 120 amino acids from C-terminal of EspA, the Tir-binding carboxy terminal of Intimin (282 amino acids) and Tir fragment interacting with Intimin (residues 258 to 361) were selected. The elicited sequence was analyzed by bioinformatics software described previously [9]. Finally, eit gene was synthesized and successfully inserted in MCS of the vectors between EcoRI and SalI restriction sites (Fig. 1).
Fig. 1.
Map of pAAV-MCS and pCI-neo vectors. In the pAAV-MCS, gfp gene was cloned between BglII and HindIII and the eit gene was inserted between EcoRI and SalI restriction sites (A). In the pCI-neo vector, the eit gene was inserted between EcoRI and SalI restriction enzymes (B).
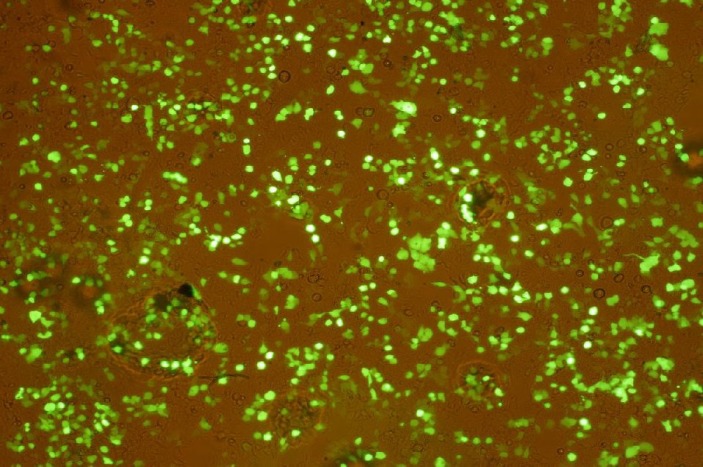
rEIT-GFP protein under Fluorescence microscope . For verification of eit gene expression in pAAV-EIT-GFP, the construct was transformed into 293T cell line. The expression of EIT-GFP in 293T cell line was observed under a fluorescence microscope indicating correct codon frame of cloned genes and evidence for eit gene expression in vector (Fig. 2).
Fig. 2.
Expression of rEIT-GFP in 293T cell line. Transfected cells with pAAV-EIT-GFP vector demonstrated green fluorescence light under a fluorescence microscope. Since eit is cloned upstream of GFP, the appearance of green fluorescence is evidence for expression of eit.
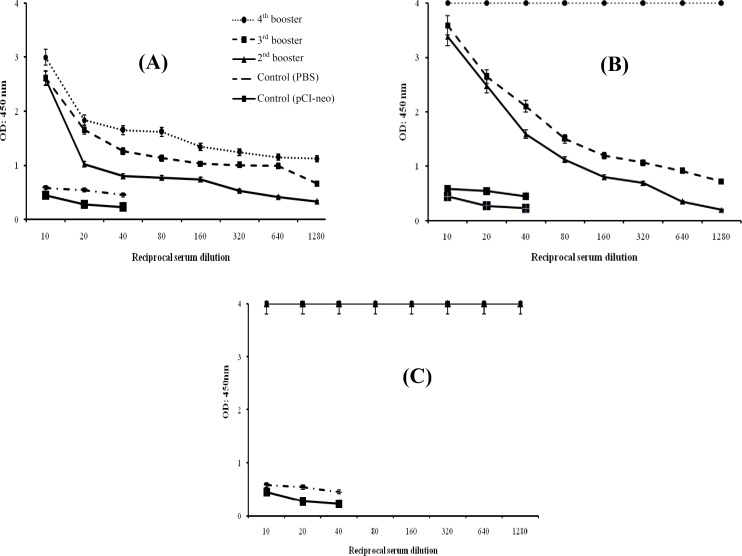
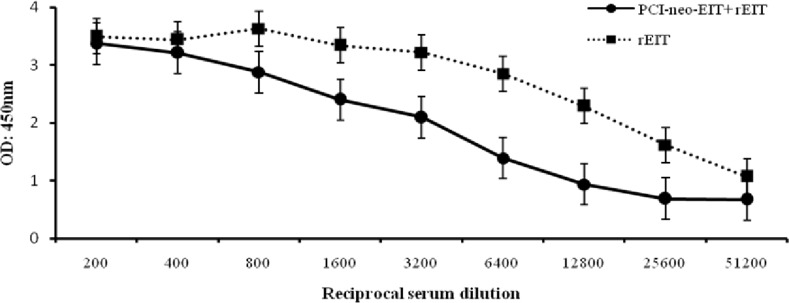
Mice immunization. For immunization procedure, three test groups were designed as: pCI- neo-EIT, combination of pCI-neo-EIT with purified rEIT and rEIT alone. Mice immunized intramuscularly with pCI-neo-EIT DNA vaccine showed significant EIT-specific IgG antibodies. The anti-EIT IgG antibody titer was significantly higher than the control groups up to 1:1,280 dilutions (Fig. 3). Immunization with pCI-neo-EIT DNA vaccine plus a booster of extracted protein rEIT showed efficient EIT-specific IgG antibodies and the anti-EIT IgG antibody titer was significant till 1:51,200 dilutions. Comparing to mice immunized subcutaneously with purified rEIT protein, which had a considerable anti-EIT IgG antibody titer (P<0.05), the results obtained here are significant (Fig. 4).
Fig. 3.
EIT-specific serum IgG following intramuscular and subcutaneous immunization. Test mice were injected with pCI-neo-EIT (A), pCI-neo-EIT plus protein (B) and rEIT protein (C). Protein was injected using complete and incomplete Freund’s adjuvants, while the construct as DNA vaccine was administered without adjuvant. Immunizations were performed four times within eight weeks. The sera were collected before each injection and assessed for rEIT-specific IgG by ELISA method. Non-immunized mice sera injected with PBS and pCI-neo were used as control.
Fig.4.
Comparison of subcutaneous and intramuscular immunization of rEIT and pCI-neo-EIT DNA vaccine with rEIT booster, respectively. Anti-EIT IgG antibody titer was clearly significant (P<0.05) till 1:51,200 dilutions.
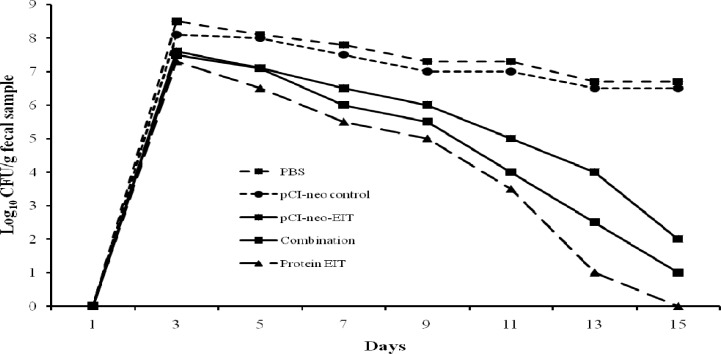
Challenging immunized mice with E. coli O157:H7. In order to determine that the EIT-specific antibodies in immunized mice sera could prevent the E. coli O157:H7 shedding in feces, three immunized and two control groups were infected orally with 1010 CFU of E. coli O157:H7. Shedding of the organism was monitored in feces as described under "Challenging the immunized mice" of methodology. Non-immunized control mice shed high levels of E. coli O157:H7 (108 CFU) over the two-week sampling period whereas shedding of immunized mice declined gradually and diminished completely after 14 days [4] (Fig. 5).
Fig. 5.
E. coli O157:H7 shedding following intramuscular administration in mice. Test groups were orally fed with 1010 E. coli O157:H7 and shedding was monitored every alternate day for two weeks. Differences were considered to be significant if P<0.05. The limit of detection for plating was 100 CFU/100 mg fecal samples (P<0.05).
Discussion
Researchers have actively been working to develop safe effective vaccines to lower the worldwide incidence of E. coli infection [1]. In 2007, the Canadian Biopharmaceutical Company Bioniche announced the development of a cattle-based vaccine (www.ctv.ca., www.cnxmarketlink.com) [23]. Gene cloning in a eukaryotic plasmid as a DNA vaccine is a new and promising approach that can induce both humoral and cellular immune responses [11]. In this
work, DNA-based vaccines were developed against E. coli O157:H7 infection. We attempted to evaluate immunogenicity of a DNA vaccine containing eit gene.
The chimeric gene was subcloned in pCI-neo vector which is suitable for DNA vaccine. Cloning of pAAV-MCS-GFP vector was verified by eit gene expression in 293 T cell line. Transfection of 293T cells by pAAV-EIT-GFP demonstrated that EIT-GFP was expressed (Fig. 2). When developing as monolayer, the cell line was transfected with pAAV-EIT-GFP. Mice serum showed IgG antibodies specific for EIT followed by induction with intramuscular injection. The IgG ratios obtained from pCI-neo-EIT DNA vaccine was higher than the control groups. Plasmid injection through intramuscular route predominantly leads to the transfection of myocytes. Dendritic cells likely take part in immune priming presumably by migrating to the site of DNA inoculation in response to post-vaccination inflammatory or chemotactic signals [10]. Minimal effect induced by DNA vaccine plasmids with different numbers of CpG motifs has been reported on the humoral immune response [24].
Therefore, contrary to the documented immune stimulatory effect of synthetic and single stranded DNA, more controversy is seen in the effect of plasmid DNA. In the present work, large amounts of DNA were used in intramuscular immunization with no prior treatment or adjuvant addition in order to achieve maximal immune response. The resulting effect of in vivo DNA vaccination measured was significantly high (Fig. 3). The results suggest possibility of DNA vaccine longevity and its functioning as an adjuvant. Our findings suggest that the combined treatment of recombinant DNA and protein could be used as an efficient vaccine strategy. Efficacy of prime boost in DNA vaccine optimization has been well documented [10, 25]. Serum titration results from mice injected with pCI-neo-EIT DNA alone without any adjuvant or prime booster were significantly different from those of control groups. The mice injected with combination of pCI-neo-EIT DNA and rEIT protein without adjuvant showed a significant antibody titer (Fig. 4).
Our data indicate that the different DNA vaccination methods results different specific antibody responses. A previous study also showed different antibody responses with different DNA vaccination methods [20]. Since DNA vaccine generates only modest immune responses [25], combination approaches were used to circumvent this limitation. Prime-boost regimens were promising in elicitation of greater immune response in humans which could be attributed to the exploitation of the ability of the immune system to generate a large number of secondary antigen-specific T cells [10, 25]. Considerable reduction in bacterial shedding in immunized groups has been shown in Figure 5. The results depicted that pCI-neo-EIT vaccine was less effective than the purified rEIT.
In conclusion, the results suggest an alternative protection system against E. coli O157:H7. The pCI-neo-EIT vaccine vectors expressed EIT component and induced humoral immune responses. Although the potency of the developed system is not as high as that of the recombinant EIT protein, it may still be advantageous with long-lived responses and adjuvant points of view [10].
ACKNOWLEDGEMENTS
This work was supported through a grant (No. 5724) by Basic Sciences Research Centre of Shahed University (Tehran, Iran).
References
- 1.Girard MP, Steele D, Chaignat CL, Kieny MP. A review of vaccine research and development: human enteric infections. Vaccine. 2006 Apr;24(15):2732–50. doi: 10.1016/j.vaccine.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Amani J, Mousavi SL, Rafati S, Salmanian AH. Immunogenicity of a plant-derived edible chimeric EspA, Intimin and Tir of Escherichia coli O157:H7 in mice. Plant Sci. 2011 Apr;180(4):620–7. doi: 10.1016/j.plantsci.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Moxley RA. Escherichia coli 0157:H7: an update on intestinal colonization and virulence mechanisms. Anim Health Res Rev. 2004 Jun;5(1):15–33. doi: 10.1079/ahr200463. [DOI] [PubMed] [Google Scholar]
- 4.Amani J, Salmanian AH, Rafati S, Mousavi SL. Immunogenic properties of chimeric protein from espA, eae and tir genes of Escherichia coli O157:H7. Vaccine. 2010 Oct;28(42):6923–9. doi: 10.1016/j.vaccine.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 5.Clarke SC, Haigh RD, Freestone PP, Williams PH. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin Microbiol Rev. 2003 Jul;16(3):365–78. doi: 10.1128/CMR.16.3.365-378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, Lai LC, et al. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998 Apr;28(1):1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Knutton S, Rosenshine I, Pallen MJ, Nisan I, Neves BC, Bain C, et al. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998 Apr;17(8):2166–76. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprioli A, Morabito S, Brugere H, Oswald E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res. 2005 May-Jun;36(3):289–311. doi: 10.1051/vetres:2005002. [DOI] [PubMed] [Google Scholar]
- 9.Amani J, Mousavi SL, Rafati S, Salmanian AH. In silico analysis of chimeric espA, eae and tir fragments of Escherichia coli O157:H7 for oral immunogenic applications. Theor Biol Med Model. 2009 Dec;6:28. doi: 10.1186/1742-4682-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biomed Biotechnol. 2010;2010:174378. doi: 10.1155/2010/174378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno S, Timón M. DNA vaccination: an immunological perspective. Immunology. 2004;23(1):41–55. [Google Scholar]
- 12.Faurez F, Dory D, Le MoigneV, Gravier R, Jestin A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine. 2010 May;28(23):3888–95. doi: 10.1016/j.vaccine.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Garmory HS, Perkins SD, Phillpotts RJ, Titball RW. DNA vaccines for biodefence. Adv Drug Deliv Rev. 2005 Jun;57(9):1343–61. doi: 10.1016/j.addr.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Armengol G, Ruiz LM, Orduz S. The injection of plasmid DNA in mouse muscle results in lifelong persistence of DNA, gene expression, and humoral response. Mol Biotechnol. 2004 Jun;27(2):109–18. doi: 10.1385/MB:27:2:109. [DOI] [PubMed] [Google Scholar]
- 15.Garmory HS, Brown KA, Titball RW. DNA vaccines: improving expression of antigens. Genet Vaccines Ther. 2003 Sep;(1):2. doi: 10.1186/1479-0556-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Borges N, Brun A, Whitton JL, Parra B, Diaz-San SegundoF, Salguero FJ, et al. DNA vaccination can break immunological tolerance to PrP in wild-type mice and attenuates prion disease after intracerebral challenge. J Virol. 2006 Oct;80(20):9970–6. doi: 10.1128/JVI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves AM, Lásaro MO, Almeida DF, Ferreira LC. DNA immunisation against the CFA/I fimbriae of enterotoxigenic Escherichia coli (ETEC) Vaccine. 2000 Nov;19(7-8):788–95. doi: 10.1016/s0264-410x(00)00244-9. [DOI] [PubMed] [Google Scholar]
- 18.Ruth N, Mainil J, Roupie V, Frère JM, Galleni M, Huygen K. DNA vaccination for the priming of neutralizing antibodies against non-immunogenic STa enterotoxin from enterotoxigenic Escherichia coli. Vaccine. 2005 May;23(27):3618–27. doi: 10.1016/j.vaccine.2004.11.080. [DOI] [PubMed] [Google Scholar]
- 19.Bentancor LV, Bilen M, Brando RJ, Ramos MV, Ferreira LC, Ghiringhelli PD, et al. A DNA vaccine encoding the enterohemorragic Escherichia coli Shiga-like toxin 2 A2 and B subunits confers protective immunity to Shiga toxin challenge in the murine model. Clin Vaccine Immunol. 2009 May;16(5):712–8. doi: 10.1128/CVI.00328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouzari S, Dashti A, Jafari A, Oloomi M. Immune response against adhesins of enteroaggregative Escherichia coli immunized by three different vaccination strategies (DNA/DNA, Protein/Protein, and DNA/Protein) in mice. Comp Immunol Microbiol Infect Dis. 2010 May;33(3):215–25. doi: 10.1016/j.cimid.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Russell DW. Molecular cloning: a laboratory manual: Cold spring harbor laboratory press, USA, 2001. USA: Cold spring harbor laboratory press; 2001. [Google Scholar]
- 22.Ranasinghe C, Ramshaw IA. Genetic heterologous prime-boost vaccination strategies for improved systemic and mucosal immunity. Expert Rev Vaccines. 2009 Sep;8(9):1171–81. doi: 10.1586/erv.09.86. [DOI] [PubMed] [Google Scholar]
- 23.Pearson H. The dark side of E. coli. Nature. 2007 Jan;445(7123):8–9. doi: 10.1038/445008a. [DOI] [PubMed] [Google Scholar]
- 24.Zelenay S, Elias F, Flo J. Immunostimulatory effects of plasmid DNA and synthetic oligodeoxynucleotides. Eur J Immunol. 2003 May;33(5):1382–92. doi: 10.1002/eji.200323614. [DOI] [PubMed] [Google Scholar]
- 25.Huang CF, Monie A, Weng WH, Wu TC. DNA vaccines for cervical cancer. Am J Transl Res. 2010;2(1):75–87. [PMC free article] [PubMed] [Google Scholar]