Abstract
Background: Testosterone and its metabolites have important roles in learning and memory. The current study has conducted to assess the effect of pre-training, post-training and pre-probe trial intrahippocampal CA1 administration of 3α-anderostanediol (one of the metabolites of testosterone) and indomethacin (as 3α-hydroxysteroid dehydrogenase enzyme blocker) on acquisition, consolidation and retrieval in Morris water maze (MWM) task. Methods: Adult male rats were bilaterally cannulated into CA1 region of hippocampus and then received 3α-diol (0.2, 1, 3 and 6 μg/0.5 μl/side), indomethacin (1.5, 3 and 6 μg/0.5 μl/side), indomethacin (3 μg/0.5 μl/side) + 3α-diol (1 μg/0.5 μl/side), 25-35 min before training, immediately after training and 25-35 min before probe trial in MWM task. Results: Our results showed that injection of 3α-diol and indomethacin significantly increased the escape latency and traveled distance to find hidden platform in acquisition and consolidation stage, but did not have any effect on retrieval of spatial learning as compared with the control group. Conclusion: It is concluded that intra-CA1 administration of 3α-diol and indomethacin could impair spatial learning and memory in acquisition and consolidation stage. Also, intrahippocampal injection of indomethacin + 3α-diol could not change spatial learning and memory impairment effect of indomethacin or 3α-diol in MWM task.
Key Words: Androgens, Spatial memory, Indomethacin, 3α-diol, GABAA receptor
Introduction
Steroid hormones are synthesized in the gonads and reach the brain via the blood circulation [1, 2]. In addition, the local endogenous synthesis of estrogens and androgens from cholesterol occurs in glial cells, astrocytes and neurons in the central and peripheral nervous system by the mediation of cytochrome p450, but not p450 enzymes [1-3]. Steroids not only affect the sexually behavioral responses, but also affect the ability of the brain to process, store and retrieve sensory information. Neuromodulatory function of steroid hormones has been investigated in the hippocampus, because the hippocampus is attractive as a center of learning and memory [2]. Androgens can enhance neural excit-ability in the hippocampus of male rats and increase dendritic spine density in the CA1 and CA3 regions of the dorsal hippocampus [4]. Changes in gonadal steroid levels over the lifetime, in addition to inducing cognitive deficits which causes the variations in cognitive function, make some types of neuro-degenerative disorders such as Alzheimer’s disease [5, 6].
Androgen receptors are densely expressed in rat hippocampal cells [1, 7] and much of the work on steroid-induced learning has focused on the effects of androgens in hippocampal spatial memory. The literature of androgen effects on spatial memory is complex and contradictory [1, 4, 7]. The evidence suggests that androgens can impair memory in animals [8]. It has been shown that injection of testosterone in the CA1 region of hippocampus impaired spatial memory in adult male rats [1, 7, 9]. A report also indicated that chronic treatment with androgens impaired spatial learning and retention of spatial information in young and middle-aged animals [10]. In contrast, some studies showed a positive correlation between testosterone and its metabolites and spatial ability [11, 12]. For instance, men with lower levels of testosterone due to hypogonadism or aging demonstrate poorer cognitive performance, and these deficits can be reduced through androgen-replacement therapy [13]. Also in animal models, it has been reported that gonadectomized or removal of the primary source of male androgens, the testes, decreases cognitive performance in adult male rats [1, 4, 7, 14].
One of the complexities in understanding the effects of androgens is the broad spectrum of steroid activity. One reason is that androgens represent substrates for the synthesis of several biologically active metabolites [5].
Our previous studies showed that testosterone impaired learning and memory [1, 4, 7]. An important question is how testosterone may have its effects on spatial memory. Indeed, testosterones, other routes of metabolism, should be more closely examined to determine its effects and mechanisms on spatial memory. In brain, testosterone is readily metabolized by the 5α-reductase enzyme to dihydrotestosterone (DHT), which is subsequently converted by the 3α-hydroxysteroid dehydrogenase enzyme (3α-HSD) to non-aromatizable metabolite 5α-androstane-3α, 17β-diol (3α-diol) [11, 15]. Systemically administration of 3α-diol to gonadectomized rats enhances cognitive performance in the inhibitory avoidance, place learning and object recognition tasks [15], but there is no information about the effect of direct intrahippocampal injection of 3α-diol on spatial learning and memory.
Indomethacin is a poorly water-soluble, non-steroidal anti-inflammatory drug [16] and a prostaglandin blocker that powerfully inhibits 3α-HSD reduction [17]. Indomethacin can act as a 3α-HSD inhibitor, block testosterone and DHT metabolism to 3α-diol and affect memory process. Some Studies showed that indomethacin as a 3α-HSD blocker may affect testosterone and 3α-diol metabolism in spatial learning and memory procedure [11, 15]. In addition, indomethacin is known as a non-selective COX (cyclooxygenase) inhibitor. COX enzymes catalyze the first two committed steps in the biosynthesis of prostanoids [17, 18]. Indomethacin as a non-selective COX inhibitor can affect learning and memory. Several lines of evidence indicate a potential role for COX in the physiological mechanisms underlying memory function [16, 17]. For example, non-specific COX inhibitors such as indomethacin impair passive avoidance memory in chick and prevent the learning-induced increase in prostaglandin release, which occurs 2 h after training [18].
Regarding the literature, the study was designed to assess the effect of intrahippocampal administration of 3α-diol, indomethacin and indomethacin +3α-diol on acquisition, consolidation and retrieval stage of spatial learning and memory in Morris water maze (MWM) performance.
MATERIALS AND METHODS
Subject. Male Albino Wistar rats (200-250 g) were obtained from Pasteur Institute of Iran and were housed five per big cage before surgery and individually in small cages after surgery. The animals were maintained at room temperature of 23 ± 2ºC under standard 12:12 h light-dark cycle with lights on at 07:00 A.M. Food and water were available ad libitum. Experimental procedures were carried out in accordance with recommendations from the declaration of Helsinki and internationally accepted principles for the use of experimental animals.
Surgery. Rats were anesthetized with a mixture of ketamine hydrochloride (Sigma, Germany) and xylazine (100 and 25 mg/kg i.p., respectively) and mounted in a stereotaxic frame (Stoelting, USA). Bilateral guide cannulae were inserted into the right and left CA1 and attached to the skull surface using dental cement and jeweler’s screws. Stereotaxic coordinates based on Paxinos and Watson’s Atlas of the rat brain [19] was: anterior-posterior, -3.8 mm from bregma; medial-lateral, ±2.2 mm from midline; and dorsal-ventral, -2.7 mm from the skull surface. After surgery, at least a seven-day recovery period was supposed before any other intervention.
Behavioral assessment:
Apparatus. The MWM is one of the most frequently used laboratory tools in behavioral neuroscience [20] that relies on distal cues to navigate from start locations around the perimeter of an open swimming area to locate a submerged escape platform [21]. The water maze is a black circular tank 140 cm in diameter and 60 cm in height. The tank was filled with water (21 ± 1ºC) to a depth of 25 cm. The maze was located in a room containing many extra maze cues (e.g. bookshelves, refrigerator and posters). The maze was divided geographically into four quadrants (northeast, northwest, southeast, southwest) and starting positions (north, south, east, west) that were equally spaced around the perimeter of the tank. A hidden circular platform (diameter: 10 cm) was located in the center of the southwest quadrant, submerged 1 cm below the surface of the water. A video camera above the water maze was connected to a video monitor and computer software (EthoVision XT, version 7) was used to track the animals' path and to calculate the escape latency, traveled distance and swimming speed.
Procedure. Each subject received four trials of one block per day in 4 consecutive days. Each rat was placed in the water facing the wall of the tank at one of the four designated starting points and allowed to swim and find the hidden platform located in southwest quadrant of the maze on every trial. Starting points were varied in a quasi-random fashion so that in each trial, the subject started from each location once and never started from the same place on any day. During each trial, each rat was given 90 s to find the hidden platform. If rat found the platform, it was allowed to remain on it for 20 s. If rat failed to find the platform within 90 s, it was placed on platform for another 20 s. During the first 4 days, the platform position remained constant. After completion of training, to test probe trial on day 5, the hidden platform was removed and the animal was released from the north location and allowed to swim freely for 60 s. After the probe trial, the platform was elevated above the water surface and placed in different positions (southeast quadrant). This stage assessed Visio-motor coordination toward a visible platform. All testing began at 8:00 A.M.
Microinjection procedure. Intrahippocampal injections were administered through a guide cannula (23 gauges) using injection needles (30 gauges) connected by polyethylene tubing to 0.5-μl Hamilton microsyringes. The injection needle was inserted 0.5 mm beyond the tip of the cannula. DMSO (0.5 μl) and different doses of 3α-diol (Sigma, USA) and indomethacin (Darou Pakhsh Manufacturing Co., Iran) were injected into each side of CA1 region during 2 min and the needles were left in place for an additional 60 s to allow for diffusion of solution away from the needle tip. In all experiments, DMSO was used as vehicle that has no significant effect on learning and memory in MWM [1, 7]. In acquisition stage, drugs and DMSO were administrated 25-35 min before training in four consecutive days. In consolidation stage, rats were trained with one block of four trials within 4 days. Immediately after the final trial in each day, microinjection was performed. Memory retrieval for the location of the platform was tested 24 h later using a 60-s free-swim probe trial. In retrieval stage, rats were trained within 4 consecutive days. On day 5, 25-35 min before probe trial, they received intra-CA1 injection of drugs and DMSO.
Histology. Following behavioral testing, animals were sacrificed by decapitation and brains were removed. For histological examination of cannulae and needle placement in CA1 region, 100-μm thick sections were taken, mounted on slides, stained with cresyl violet and the cannulae track was examined for each rat. Only those animals whose cannulae were exactly placed in the CA1 region, were used for data analysis.
Experimental protocol:
Experiment 1. The first experimental group consisting of intact (without cannula) and sham-operated rats was received 0.5 μl vehicle (DMSO or 0.5 μl DMSO + 0.5 μl DMSO) into CA1 region of hippocampus. The aim of this experiment was to compare the effects of intrahippocampal vehicle injection with intact group of rats on MWM performance. A total of 27 rats were divided into three groups as mentioned above.
Experiment 2. The aim of this experiment was to determine the effect of bilateral pre-training, post-training and pre-probe trial administration of 3α-diol into CA1 region, respectively on acquisition, consolidation and retrieval stages of spatial memory. A total of 130 rats were used, randomly divided into five groups in each stage and received different doses of 3α-diol (0.2, 1, 3 and 6 μg/0.5 μl/side) or DMSO as vehicle.
Experiment 3. The aim of this experiment was to determine the effect of bilateral pre-training, post-training and pre-probe trial administration of indomethacin into CA1 region, respectively on acquisition, consolidation and retrieval stages of spatial memory. A total of 105 rats were used, divided into four groups in each stage and received different doses of indomethacin (1.5, 3 and 6 μg/0.5 μl/side) or DMSO as vehicle.
Experiment 4. The aim of this experiment was to determine the effect of bilateral pre-training, post-training and pre-probe trial administration of indomethacin + 3α-diol into CA1 region, respectively on acquisition, consolidation and retrieval stages of spatial memory. A total of 50 rats were used, randomly divided into two groups in each stage and received bilateral microinjections of 3 μg/0.5 μl/side indomethacin + 1 μg/0.5 μl/side 3α-diol or DMSO + DMSO as a vehicle with a similar volume.
Statistical analysis. Statistical evaluation was carried out by Kolmogorov-Smirnov test to examine the normal distribution. Data were analyzed by student's t-test for comparison between the two groups and one-way analysis of variance (ANOVA) was followed by Tukey's test for multiple comparisons. All results have been shown as means ± S.E.M. In all statistical comparisons, P<0.05 considered as significant difference.
Results
Experiment 1. The effects of vehicles. The results obtained from intact and vehicle (DMSO and DMSO + DMSO) indicate no significant difference in escape latency (F2,80 = 0.211, P = 0.810), traveled distance (F2,80 = 1.197, P = 0.307) and swimming speed (F2, 80 = 1.838, P = 0.166) among the groups. These results indicated that injection of DMSO or injection of DMSO + DMSO together had no effect on spatial learning and swimming ability in behavioral studies.
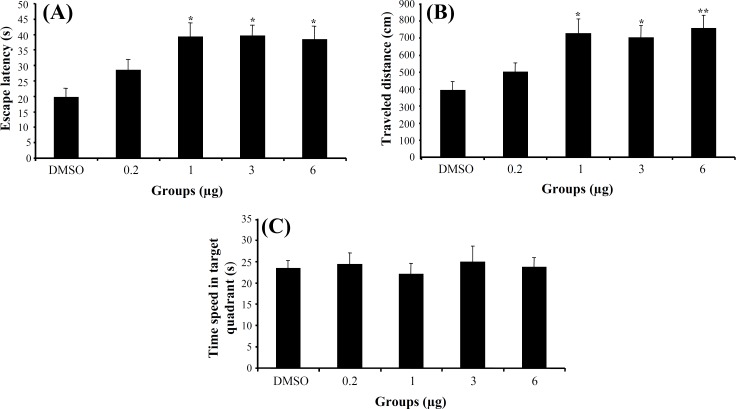
Experiment 2. The effect of 3α-diol on acquisition, consolidation and retrieval of MWM task. In acquisition stage, a significant difference was generally found in escape latency (F5,166 = 5.713, P<0.001) and traveled distance (F5,166 = 5.281, P<0.001) (Fig. 1A and 1B) among the groups in hidden platform trials (days 1-4), but no significant differences were found in swimming speed (F5,166 = 1.825, P>0.05). Post hoc multiple comparisons showed that 3α-diol at doses 1, 3 and 6 μg had significantly impaired acquisition of spatial learning compared to control group. One way ANOVA in probe test revealed no significant differences in escape latency (F5,41 = 0.753, P = 0.563) (Fig. 1C), traveled distance (F5,41 = 1.047, P = 0.397) and swimming speed (F5,41 = 2.16, P = 0.094) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (F5,41 = 0.741, P = 0.597) or for traveled distance (F5,41 = 1.173, P = 0.339).
Fig. 1.
The effect of intra-CA1 administration of 3α-diol and DMSO on acquisition of spatial memory in MWM task. The columns represent the mean ± S.E.M. Average escape latency (A) and traveled distance (B) across all training days and time spent in target quadrant (C) in probe trial. *P<0.05 and **P<0.01 indicate significant difference vs. DMSO group.
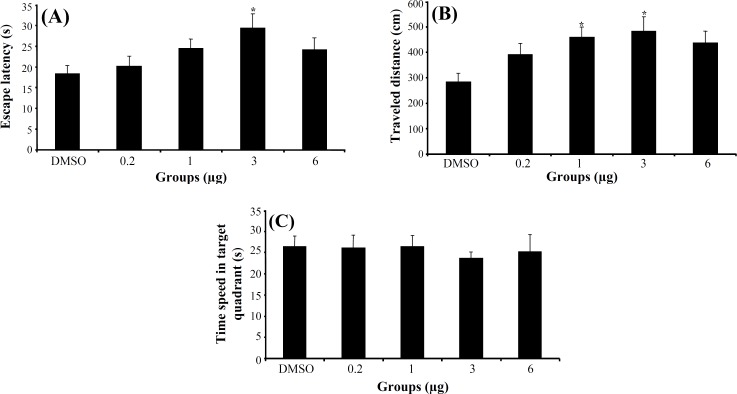
In consolidation stage, a significant difference was generally found in escape latency (F4,159 = 2.511, P<0.05) and in traveled distance (F4,159 = 3.196, P<0.05) (Fig. 2A and 2B) among the groups in hidden platform trials, but no significant differences were found in swimming speed (F4,159 = 2.29, P = 0.062). Post hoc multiple comparisons showed that 3α-diol at doses 1 and 3 μg had a significantly impaired consolidation stage in training trials compared to control group. One way ANOVA in probe test revealed no significant differences in escape latency (F4,37 = 0.169, P = 0.953) (Fig. 2C), traveled distance (F4,37 = 0.381, P = 0.821) and swimming speed (F4,37 = 1.85, P= 0.139) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (F4,37 = 0.988, P = 0.426) or for traveled distance (F4,37 = 2.22, P = 0.085).
Fig. 2.
The effect of 3α-diol and DMSO on consolidation. The columns represent the mean ± S.E.M. Average escape latency (A) and traveled distance (B) across all training days and time spent in target quadrant (C) in probe trial. *P<0.05 indicates significant difference vs. DMSO group.
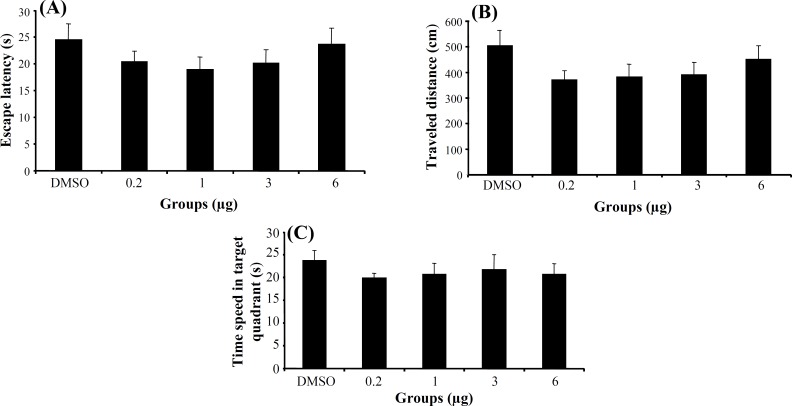
In retrieval stage, no significant difference was generally found in escape latency (F4,155 = 0.891, P = 0.471), traveled distance (F4,155 = 1.264, P = 0.287) (Fig. 3A and 3B) and swimming speed (F4,155=1.56, P = 0.188) among the groups in hidden platform trials. One way ANOVA of probe test revealed no significant differences in escape latency (F4, 36 = 0.382, P = 0.820)
Fig. 3.
The effect of 3α-diol and DMSO on retrieval. The columns represent the mean ± S.E.M. Average escape latency (A) and traveled distance (B) across all training days and time spent in target quadrant (C) in probe trial.
(Fig. 3C), traveled distance (F4,36 = 0.460, P = 0.764) and swimming speed (F4,36 = 0.871, P = 0.491) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (F4, 36 = 1.728, P = 0.165) or for traveled distance (F4, 36 = 0.691, P = 0.603).
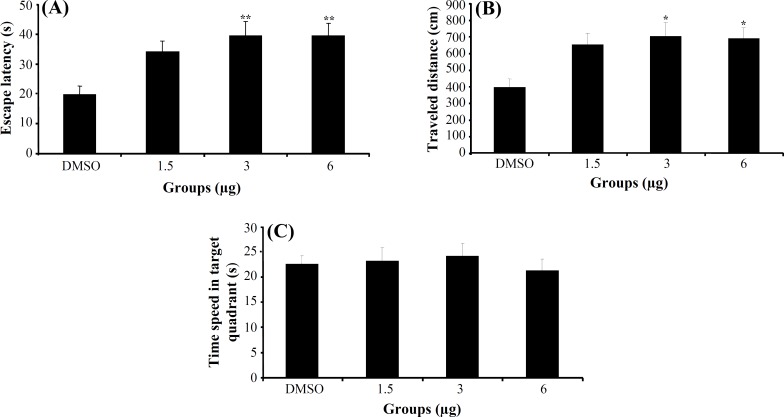
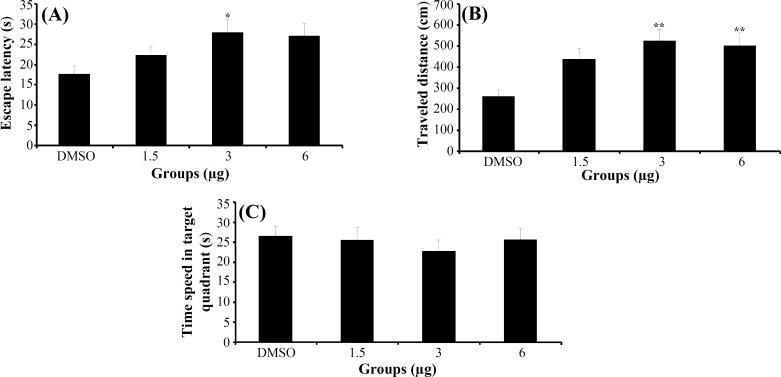
Experiment 3. The effect of indomethacin on acquisition, consolidation and retrieval of MWM task. In acquisition stage, a significant difference was generally found in escape latency (F3,112 = 4.974, P<0.01) and traveled distance (F3,112 = 3.761, P = 0.013) (Fig. 4A and 4B) among the groups in hidden
Fig. 4.
The effect of indomethacin and DMSO on acquisition. The columns represent the mean ± S.E.M. Average escape latency (A) and traveled distance (B) across all training days and time spent in target quadrant (C) in probe trial. *P<0.05 and **P<0.01 indicate significant difference vs. DMSO group.
platform trials (days 1-4), but no significant differences were found in swimming speed (F3,112 = 1.530, P = 0.211). Post hoc multiple comparisons showed that the rats treated with 3 and 6 μg indomethacin had significantly impaired acquisition of spatial learning compared to the control group. One way ANOVA of probe test revealed no significant differences in escape latency (F3,30 = 0.779, P = 0.515) (Fig. 4C), traveled distance (F3,30 = 0.329, P = 0.804) and swimming speed (F3,30 = 1.083, P = 0.371) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (F3,30 = 0.790, P = 0.510) or for traveled distance (F3,30 = 2.625, P = 0.075).
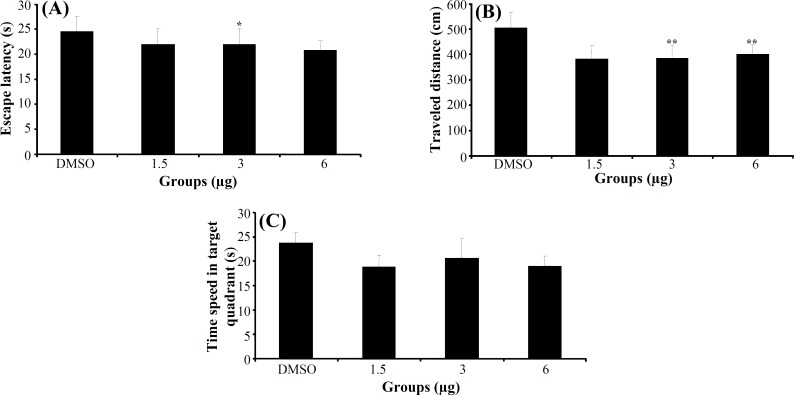
Fig. 5.
The effect of indomethacin and DMSO on consolidation. The columns represent the mean ± S.E.M. Average escape latency (A) and traveled distance (B) across all training days and time spent in the target quadrant (C) in probe trial. *P<0.05 and ** P<0.01 indicate significant difference vs. DMSO group.
In retrieval stage, no significant difference was generally found in escape latency (F3,120 = 0.301, P = 0.825), traveled distance (F3, 120 = 1.272, P = 0.287) (Fig. 6A and 6B) and swimming speed (F3,120 = 1.01, P = 0.391) among the groups in hidden platform trials. One way ANOVA of probe test revealed no significant differences in escape latency (F3,27 = 0.845, P = 0.482) (Fig. 6C), traveled distance (F3,27 = 1.187, P = 0.334) and swimming speed (F3, 27 = 0.644, P = 0.594) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (F3,27 = 1.323, P = 0.287) or for traveled distance (F3,27 = 0.1.678, P = 0.195).
Fig. 6.
The effect of indomethacin and DMSO on retrieval. The columns represent the mean ± S.E.M. Average escape latency (A) and traveled distance (B) across all training days and time spent in the target quadrant (C) in probe trial. *P<0.05 and ** P<0.01 indicate significant difference vs. DMSO group.
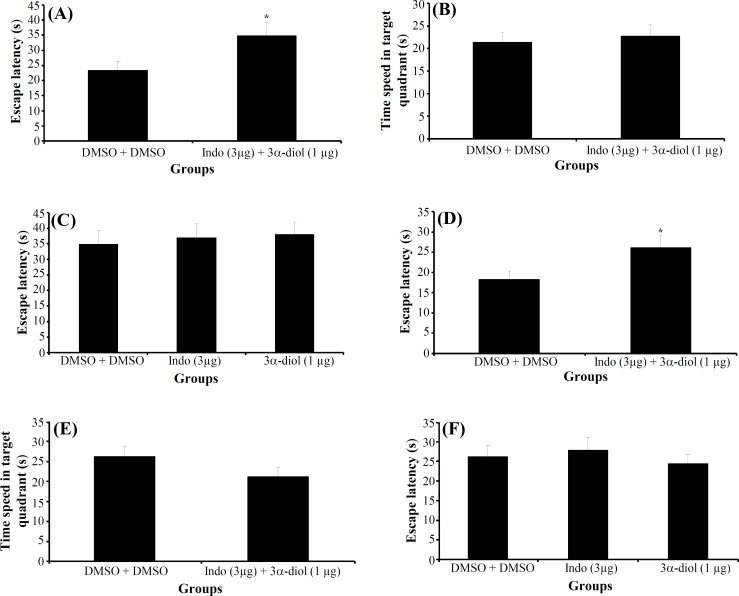
Experiment 4. The effect of indomethacin + 3α-diol on acquisition, consolidation and retrieval of MWM task. In acquisition stage, a significant difference was generally found in escape latency (T62 = 4.65, P = 0.029) (Fig. 7A) and traveled distance (T62 = 3.265, P = 0.02) among the groups in hidden platform trials (days 1-4), but no significant differences were found in swimming speed (T62 = 10.885, P = 0.521). T-test probe of trial revealed no significant differences in escape latency (T14 = 0.872, P = 0.686) (Fig. 7B), traveled distance (T14 = 0.537, P = 0.500) and swimming speed (T14 = 0.163, P = 0.790) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (T14 = 2.912, P = 0.697) or for traveled distance (T14 = 3.204, P = 0.421).
Fig. 7.
The effect of indomethacin + 3α-diol and DMSO + DMSO on acquisition and consolidation. The columns represent the mean ± S.E.M. Average escape latency (A) and time spent in the target quadrant (B) in acquisition and consolidation stage (D and E). One way ANOVA between indomethacin + 3α-diol, indomethacin and 3α-diol in acquisition and consolidation have been shown in the Figure (C and F, respectively). *P<0.05 indicates significant difference vs. DMSO group. Indo, indomethacin.
There were no significant differences in escape latency (F2,97 = 0.139, P = 0.870) (Fig. 7C), traveled distance (F2,97 = 0.170, P = 0.844) and swimming speed (F2,97 = 1.054, P = 0.353) between indomethacin 3 μg + 3α-diol 1 μg , indomethacin 3 μg and 3α-diol 1 μg group in acquisition stage. These data indicated that presence of indomethacin cannot inhibit impairing effect of 3α-diol on acquisition stage of spatial memory.
In consolidation stage, a significant difference was generally found in escape latency (T54 = 2.42, P<0.05) (Fig. 7D) and traveled distance (T54 = 8.79, P<0.01) among the groups in hidden platform trials, but no significant differences were found in swimming speed (T54 = 0.002, P = 0.102). T-test probe trial revealed no significant differences in escape latency (T14 = 0.061, P = 0.146) (Fig. 7E), traveled distance (T14 = 0.070, P = 0.798) and swimming speed (T14 = 0.436, P = 0.098) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (T14 = 0.1, P = 0.591) or for traveled distance (T14 = 0.780, P = 0.280).
There were no significant differences in escape latency (F2,93= 0.413, P = 0.663) (Fig. 7F), traveled distance (F2,93 = 0.994, P = 0.374) and swimming speed (F2,93 = 1.628, P = 0.202) between indomethacin 3 μg + 3α-diol 1 μg , indomethacin 3 μg and 3α-diol 1 μg group. These data indicated that presence of indomethacin cannot inhibit impairing effect of 3α-diol on consolidation stage of spatial memory.
In retrieval stage, no significant difference was generally found in escape latency (T54 = 0.00, P = 0.734), traveled distance (T54 = 0.943, P = 0.255) and swimming speed (T54 = 1.84, P = 0.104) among the groups in hidden platform trials. T-test probe trial revealed no significant differences in escape latency (T15 = 0.951, P = 0.290), traveled distance (T15 = 2.28, P = 0.368) and swimming speed (T15 = 1.11, P = 0.817) among the groups. Also, there was no significant difference of performance among the groups on visible platform day for escape latency (T15 = 0.395, P = 0.657) or for traveled distance (T15 = 1.25, P = 0.586).
Discussion
In the present study, the role of 3α-diol and indomethacin on acquisition, consolidation and retrieval of learning and memory has been investigated. The findings of the present study includes: (1) Bilaterally pre-training microinjection of 1, 3 and 6 μg/0.5 μl 3α-diol and 3 and 6 μg/0.5 μl indomethacin into CA1 region of the adult male rats impaired acquisition and consolidation; however, they had no effect on retrieval in 5-day protocol of MWM task. (2) Bilaterally microinjection of 3 μg/0.5 μl indomethacin + 1 μg/0.5 μl 3α-diol impaired acquisition and consolidation, but not retrieval of spatial learning and memory in MWM task. (3) There were no differences in the swimming speed of different doses in treated groups. (4) Intrahippocampal microinjection of 3α-diol and indomethacin had no effect on escape latency, traveled distance and swimming speed to find the visible platform in non-spatial visual discrimination task.
DMSO was used as vehicle in other investigations with similar conditions [1, 7]. In this experiment, we can see double injections of vehicle (DMSO + DMSO) had no significant effect on learning and memory. This finding is in consistent with some other reports [1, 4, 7].
The swimming speed in DMSO and 3α-diol or indomethacin-treated groups indicates that micro-injection of 3α-diol or indomethacin in CA1 area has no effect on locomotors behavior. There were no significant differences between the control and experimental groups on the 5th day of training on visible platform. Therefore, it can be inferred that the observed changes could not be attributed to alterations of non-mnemonic factors, such as motivation, motor, or sensory processes induced by the treatments.
Our finding based on 3α-diol role in impairing memory processing, taken with other previous studies showing conflicting effects of androgens on cognition, suggests that cognitive-hormone interactions are quite complex. In several studies, androgens impaired spatial learning and memory [1, 4, 7, 9], but other studies reported that androgens such as testosterone enhanced spatial memory [11, 12].
Androgens exert these effects on memory through genomic and non-genomic pathways. The genomic pathway typically takes at least more than half an hour to be involved in long-term effects of androgens affecting gene expression via the intracellular androgen receptors. In addition to the classical genomic effects of steroids, many neurosteroids induce non-genomic effects, manifested within seconds to few minutes [22]. This pathway involve in modulation of neuro-transmitter receptors and ion channels, ranging from activation of G-protein coupled membrane receptors or sex hormone-binding globulin receptors, stimulation of different protein kinases to direct modulation of voltage and gated ion-channels and transporters [4, 7, 22, 23].
Our previous study revealed that testosterone via both genomic and non-genomic pathways impaired long-term memory [7].
There are several possible explanations for memory impairment effect of 3α-diol. The first possibility resulted from this fact that neurons and glia express enzymes able to convert testosterone to estradiol and several 5α-reduced androgens such as DHT. Furthermore, metabolism of DHT results in formation of 5α-androstane-3,17β-diol (3α-diol) as well as its 3-isomer (3β A-diol) [24], both of which are capable of eliciting estrogen receptor-dependent responses [25, 26]. Although testosterone and DHT bind with high affinity to androgen receptors, 3α-diol does not typically bind to androgen receptors. Instead, 3α-diol has some effects on estrogen receptor especially estrogen receptor β [4]. Previous studies have shown that intrahippocampal microinjection of estrogen impaired spatial memory in rats [27]. Also, activation of estrogen receptor β reduces the transcription process and impairs cognitive performance [27].
The second possibility is based on this fact that in addition to effect on estrogen receptor, 3α-diol also acts similar to the analogous metabolites of progesterone and corticosterone such as allo-pregnanolone to enhance GABA-benzodiazepine regulated chloride channel function [25, 26]. One of the best documented examples of non-genomic actions of steroids is the ability of these hormones to activate GABAA receptors. The GABAA receptor is a member of the ligand-gated ion channel family that α and β subunits of this receptor affect GABAA neuro-modulation by either positive or negative neurosteroid modulators [5]. Activation of the GABAA receptor complex by neuroactive steroids resulted in opening of its central Cl-conducting pore, which led to a hyperpolarization of the plasma membrane and inhibition of neuronal fires. The major groups of neuroactive steroids and their metabolites are pro-gesterone, dehydrocorticosterone and some metabolites of them. In general, they mediate their actions not only through classic steroid receptors, but also through other mechanisms such as ligand-gated ion-channels, including GABAA, glutamate or opioid receptors [23]. On the other hand, the biphasic effects of progesterone are consistent with a critical role for GABA-mediated responses. Progesterone such as DHT is rapidly converted in the brain to 5α-reduced metabolites, some of which potentiate GABA action on the GABA-benzodiazepine-chloride channel complex [5]. Therefore, the effects of androgens as 3α-diol could be mediated in much the same way as the rapid responses of progesterone, via enhancement of GABAergic neurotransmission.
Beside, some studies have shown that all hippocampal subregions are rich in GABAA receptors as well as some neurosteroids such as allopregnanolone can inhibit neural activity in the CA1 and dental gyrus areas of the hippocampus [3, 28]. Treatment with GABAA receptor active substance, benzodiazepine, can inhibit learning and memory in humans and animals [3]. Acute treatment with neurosteroids that have GABA-modulatory effects impairs learning and memory. In contrast, steroids that act as GABAA receptor antagonists enhance learning and memory [29, 30].
The third possibility is based on this fact that there is an interaction between steroids and the serotonin system in the hippocampus. Some evidence showed that steroids including estrogen and progesterone metabolites such as allopregnanolone can affect spatial learning and memory via the serotonin system [3]. Also, a direct interaction between the GABA and the serotonin systems in the hippocampus is proved, where serotonin neurons often end at inhibitory GABAergic interneurons [3, 31]. Many studies have shown that excitation of serotonergic neurotransmission impairs learning and memory, whereas reduction of serotonin activity can improve these processes [32]. Serotonin and GABA systems may therefore interact in the hippocampus, a region which is important for cognitive functions [3, 33]. Since 3α-diol act similar to the analogous metabolites of progesterone, probably induce impairment in learning and memory performance via this interaction with serotonin system.
Also, steroid hormones modulate the memory processes perhaps by their relationship with other neurotransmitter systems, such as acetylcholine, dopamine, noradrenaline and glutamate, and by their relationship with the cerebral regions that participate in these phenomena [4]. Based on the evidences, testosterone and its metabolites, 3α-diol, can reduce acetylcholine release in the hippocampus via positive modulating hippocampal GABAergic interneurons that is proven to induce memory impairment [4, 34]. Many studies have also shown that NMDA receptors are critical for synaptic plasticity and long-term memory [1, 5, 35]. By acting as non-selective sigma (σ) antagonist, testosterone may produce a tonic damping of the function of sigma receptors and consequently a decrease in NMDA receptor function [4]. Therefore, these facts may be a good and reliable proof for the effects of 3α-diol (as one metabolites of testosterone) on spatial memory.
We purpose in using of indomethacin resulted from the Frye et al. [11, 15] literature about this fact that indomethacin is the major 3α-HSD inhibitor. Furthermore, blocking testosterones or DHT metabolism to 3α-diol with indomethacin decreases cognitive performance and increases anxiety behavior of gonadally-intact and/or DHT-replaced rats. In experiments 2 and 3, we assayed the effect of 3α-diol and indomethacin alone on learning and memory. Our finding shows that both 3α-diol and indomethacin impair acquisition and consolidation learning and memory performance. In experiment 4, we used indomethacin as 3α-HSD inhibitor to prevent the effect of endogenous 3α-diol on learning and memory. Then, we studied the effect of exogenous 3α-diol on acquisition, consolidation and retrieval learning and memory. Our results show that exogenous 3α-diol has impairment effect on acquisition and consolidation memory and indomethacin could not prevent the impairment effect of 3α-diol. Besides, there is no significant difference between intra-CA1 adminis-tration of indomethacin + 3α-diol, indomethacin and 3α-diol on acquisition and consolidation stage using One way ANOVA. It is possible that 3α-diol cognitive impairment effects cannot be influenced by indo-methacin. This result indicates that another mechanism may be responsible for the observed effects. It is believed that indomethacin as a non-selective COX inhibitor [18] can affect learning and memory. Therefore, COX enzymes catalyze the first two committed steps in the biosynthesis of prostanoids. Several lines of evidence indicate a potential role for COX in physiological mechanisms underlying memory function and indomethacin as a COX inhibitor could impair these mechanisms [16-18]. For example, indomethacin as a COX inhibitor impairs passive avoidance memory in chick and prevents the learning-induced increase in prostaglandin release, which occurs 2 h after training [18].
In summary, it is concluded that intra-CA1 administration of 3α-diol and indomethacin could impair acquisition and consolidation, but not retrieval spatial learning and memory. Also, intrahippocampal injection of indomethacin + 3α-diol could not change impairment effect of indomethacin or 3α-diol alone in MWM task.
ACKNOWLEDGMENTS
We wish to thank Department of Physiology and Pharmacology of Pasteur Institute of Iran for supporting this research.
References
- 1.Emamian S, Naghdi N, Sepehri H, Jahanshahi M, Sadeghi Y, Choopani S. Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav Brain Res. 2010 Mar;208(1):30–7. doi: 10.1016/j.bbr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda Metal. Estrogen synthesis in the brain-Role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008 Aug;290(1-2):31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Birzniece V, Bäckström T, Johansson IM, Lindblad C, Lundgren P, Löfgren M, et al. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res Rev. 2006 Aug;51(2):212–239. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Harooni HE, Naghdi N, Sepehri H, Rohani AH. Intra hippocampal injection of testosterone impaired acquisition, consolidation and retrieval of inhibitory avoidance learning and memory in adult male rats. Behav Brain Res. 2008 Mar;188(1):71–7. doi: 10.1016/j.bbr.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 5.MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138(3):957–65. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2007 Jan;62(2):188–93. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 7.Naghdi N, Asadollahi A. Genomic and nongenomic effects of intrahippocampal microinjection of testos-terone on long-term memory in male adult rats. Behav Brain Res. 2004 Aug;153(1):1–6. doi: 10.1016/j.bbr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Brosens JJ, Tullet J, Varshochi R, Lam EW. Steroid receptor action. Best Pract Res ClinObstet Gynaecol. 2004 Apr;18:265–83. doi: 10.1016/j.bpobgyn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Hampson E. Spatial cognition in humans: possible modulation by androgens and estrogens. J Psychiatry Neurosci. 1995 Nov;20(5):397–404. [PMC free article] [PubMed] [Google Scholar]
- 10.Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, et al. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008 Mar;57(2):363–75. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5α-reduced androgens may have actions in the hippo-campus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004 Sep;29(8):1019–27. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anexiety and cognitive-enhancing effects in male rats. Age (Dordr) 2009 Sep;31(3):191–8. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund BC, Bever-Stille KA, Perry PJ. Testosterone and andropause: the feasibility of testosterone replacement therapy in older men. Pharmacotherapy. 1999;8:951–6. doi: 10.1592/phco.19.11.951.31574. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez-Pereyara F, Rivas-Arancibia S, Loaeza-Del CastilloA, Schneider-Rivas S. Modulation of short term and long term memory by steroid sexual hormones. Life Sci. 1995;56(14):PL255–60. doi: 10.1016/0024-3205(95)00067-g. [DOI] [PubMed] [Google Scholar]
- 15.Frye CA, Edinger KL, Lephart ED, Walf AA. 3α-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front Aging Neurosci. 2010;2:15. doi: 10.3389/fnagi.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nokhodchi A, Javadzadeh Y, Siahi-Shadbad MR, Barzegar-Jalali M. The effect of type and concentration of vehicles on dissolution rate of a poorly soluble drug (indomethacin) from liquisolid compacts. J Pharm Pharm Sci. 2005 Jan;8:18–25. [PubMed] [Google Scholar]
- 17.Sanders BK. Sex, drugs and sports: prostaglandins, epitestosterone and sexual development. Med Hypotheses. 2007;69(4):829–35. doi: 10.1016/j.mehy.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 18.Teather LA, Packard MG, Bazan NG. Post-Training Cyclooxygenase-2 (COX-2) inhibition impairs memory Consolidation. Learn Mem. 2002 Jan-Feb;9(1):41–7. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. New York: Academic Press; [DOI] [PubMed] [Google Scholar]
- 20.D , Hooge R, De DeynPP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Rev. 2001 Aug;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 21.Paul CM, Magda G, Abel S. Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009 Nov;203(2):151–64. doi: 10.1016/j.bbr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009 Jun;63(2):142–8. doi: 10.1016/j.maturitas.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Michels G, hoppe , UC Rapid actions of androgens. Front Neuroendocrinol. 2008 May;29(2):182–98. doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α- androstanediol and 17β-estradiol. Neuroscience. 2004;129(1):195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Genazzani AR, Pluchino N, Freschi L, Ninni F, Luisi M. Androgens and the brain. Maturitas. 207 May;57(1):27–30. doi: 10.1016/j.maturitas.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Pettersson H, Lundqvist J, Oliw E, Norlin M. CYP7B1-mediated metabolism of 5-androstane-3,17-diol (3-Adiol): A novel pathway for potential regulation of the cellular levels of androgens and neurosteroids. BiochimBiophys Acta. 2009;1791:1206–15. doi: 10.1016/j.bbalip.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997 Sep;227(5331):1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 28.Landgren S, Wang MD, Backstrom T, Johansson S. Interaction between 3 alpha- hydroxy-5 alpha-pregnan-20-one and carbachol in the control of neuronal excitability in hippocampal slices of female rats in defined phases of the oestrus. ActaPhysiol Scand. 1998 Jan;162(1):77–88. doi: 10.1046/j.1365-201X.1998.0287f.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathews DB, Morrow AL, Tokunaga S, McDaniel JR. Acute ethanol administration and acute allopregnan-olone administration impair spatial memory in the Morris water task. Alcohol ClinExp Res. 2002 Nov;26(11):1747–51. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- 30.Vallee M, Mayo W, Koob GF, Le MoalM. Neurosteroids in learning and memory processes. Int Rev Neurobiol. 2001;46:273–320. doi: 10.1016/s0074-7742(01)46066-1. [DOI] [PubMed] [Google Scholar]
- 31.Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000 Feb;417(3):337–48. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Dubrovsky, B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol Biochem Behav. 2006 Aug;84(4):644–55. doi: 10.1016/j.pbb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the serotonin (1A) receptor in mice results in down regulation of major GABA (A) receptor alpha subunits, reduction of GABA (A) receptor binding, and benzodiazepine- resistant anxiety. J Neurosci. 2010 Jun;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moor E, DeBoer P, Westerink BH. GABA receptors and benzodiazepine binding sites modulate hippocampal acetylcholine release in vivo. Eur J Pharmacol. 1998 Oct;359(2-3):119–26. doi: 10.1016/s0014-2999(98)00642-6. [DOI] [PubMed] [Google Scholar]
- 35.Santos SD, Carvalho AL, Calderia MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009 Jan;158(1):105–25. doi: 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]