Abstract
Background: Galantamine is a drug used for the treatment of Alzheimer’s disease and some other cognitive disorders. It is an inhibitor of acetylcholinesterase; however, interaction with nicotinic acetylcholine receptors has also been reported. Owing to the significant role of cholinergic anti-inflammatory pathways in neuro-immunomodulation, we decided to examine the effect of galantamine on tularemia-infected BALB/c mice. Methods: Animals were infected with Francisella tularensis LVS and treated with galantamine (0.1 mg/kg of body weight). Total mortality over the course of tularemia infection was assessed and interleukin 6 (IL-6) and interferon gamma (IFN-) in plasma samples were measured by enzyme-linked immunosorbent assays. Apart from the cytokine assays, biochemical markers such as inorganic phosphate, uric acid, lactate dehydrogenase, gamma glutamyltransferase, creatinine phosphokinase and amylase were assayed. Results: The modulation of immunity by galantamine depended on two opposing processes: up-regulation of IFN- and down-regulation of IL-6. Tularemia infection resulted in significant nephropathy, as hyperphosphataemia and hyperuricaemia occurred in infected animals. In addition, galantamine resulted in the mitigation of nephropathy, and markers of kidney dysfunction were modulated. Alterations in mortality were also found in this study. Conclusions: Galantamine can significantly influence the immune response via the cholinergic anti-inflammatory pathway. Despite the decrease in IL-6 levels, galantamine treatment enhanced protection against the intracellular pathogen F. tularensis, resulting in the remission of some pathology and reduced mortality.
Key Words: Acetylcholinesterase, Immunity, Tularemia, Inflammation
Introduction
Galantamine, also known as Nivalin, is an inhibitor of the enzyme acetylcholinesterase (AChE, EC 3.1.1.7). It was recognized by Mashkovsky and Kruglikova-Lvova [1] as a secondary metabolite in Caucasian snowdrops Galanthus woronowii (Amaryllidaceae) in the early 1950s. The inhibition of AChE is based on an interaction with the anionic site of AChE, and interaction with the aromatic gorge of AChE has also been reported [2]. Moreover, galantamine also causes allosteric potentiation of nicotinic acetylcholine receptors (nAChR) with well-reported potentiation of the 7 subtype [3]. Currently, galantamine is a drug of choice for treatment of Alzheimer’s disease and vascular dementia [4, 5].
Regulation of the cholinergic system can be used as a method to improve infectious disease pathogenesis. Apart from the other functions of the cholinergic system, the cholinergic anti-inflammatory pathway found in the blood is associated with the 7 nAChR [6]. Galantamine has been reported to be able to modify tumour necrosis factor alpha (TNF-α) levels through stimulation of the cholinergic anti-inflammatory pathway [7]. The resolution of tularemia infection is mediated by the production of interferon gamma (IFN-) and the activation of macrophages, resulting in an increase in inducible nitric oxide synthase and killing of the pathogen [8]. Inflammation and TNF-α production are necessary to resolve the disease [9]. Given the pathogenesis of tularemia, galantamine may be effective in resolving infection as it is reportedly implicated in nAChR modulation. However, no experimental evidence of such effects exists. As shown in this study, galantamine is potent enough to modulate immunity; however, it has not been extensively researched. The aim of the present study, therefore, was to investigate the ability of galantamine to modulate tularemia, an infectious model caused by the intracellular pathogen Francisella tularensis. The both innate and adaptive immunity are needed for the disease to be resolved. This knowledge could be useful for a novel application or contraindication of the currently available drug galantamine.
MATERIALS AND METHODS
Microorganism and animal experiments. F. tularensis LVS (American type collection code 29684) was cultivated on McLeod agar supplemented with bovine haemoglobin and Iso VitaleX (Becton-Dickinson, San Jose, CA, USA). After two days, cells were harvested, suspended in a saline solution and centrifuged at 2,000 ×g for 10 minutes. The number of F. tularensis colony forming units (CFU) in the solution was confirmed by re-cultivation.
In total, 126 female BALB/c mice (BioTest, Konarovice, Czech Republic) weighing 24 ± 2 g were used in the experiment. Mice were kept in air-conditioned temperature controlled room (22 ± 2C) with 50 ± 10% humidity and a light period from 7 a.m. to 7 p.m. Animals had free access to food and water. The experiment was supervised and approved by the Institutional Ethical Committee, Centre of Biological Defence (Techonin, Czech Republic).
In the first part of the experiment, 96 animals were divided into four groups, with 24 animals in each group. Galantamine (Sigma-Aldrich, Saint Louis, Missouri, USA) was suspended in saline and administered subcutaneously at a dose of 0.1 mg/kg (as recommended for humans) [3, 10]. F. tularensis was dissolved in saline and the bacterial mass was adjusted to 106 CFU/ml. The animals were divided into the following groups: 1) Controls given 200 µl of saline solution only; 2) Galantamine group given 100 µl of the galantamine solution and 100 µl of saline (on days 1 and 2); 3) Tularemia infection group given 100 µl of the F. tularensis suspension and 100 µl of saline; and 4) Tularemia and galantamine group given 100 µl of the F. tularensis suspension and 100 µl of the galantamine solution (galantamine application was repeated on the second day).
From each group, six animals were sacrificed two hours after the first administration. Euthanasia of another six animals was performed three, five and seven days after the initiation of the experiment. Euthanasia was performed in carbon dioxide narcosis by cutting the carotid. Blood was collected from the carotid artery and kept in heparinised tubes. Plasma was prepared by centrifugation of the blood at 3000 ×g for 15 minutes.
A mortality test was performed on another 30 animals. Galantamine was applied at the dose reported above. The F. tularensis stock solution was prepared as a saline solution containing 107 CFU/ml. Animals were divided into three groups: 1) Galantamine group: 100 µl of galantamine and 100 µl of saline were administered and the administration was repeated on the second day of the experiment; 2) Tularemia infection group: 100 µl of the F. tularensis suspension and 100 µl of saline were given, and application of the saline solution was repeated on the second day; and 3) Tularemia and galantamine group: 100 µl of the F. tularensis suspension and 100 µl of the galantamine solution were given and galantamine and saline administration was repeated on the second day of the experiment. Animals were kept until complete healing of the surviving individuals.
Ex vivo assays. In the plasma, interleukin 6 (IL-6) and IFN- were assessed using Mouse IL-6 Eli-pair and Mouse IFN- Eli-pair kits (Abcam, Cambridge, MA, USA) in compliance with the manufacturer’s protocols. Standard 96-well plates and an MRX absorbance reader (Dynatech Laboratories, Chantilly, VA, USA) were used for the assay purposes. Biochemical analysis of plasma was performed using an automated analyzer (SPOTCHEMTM EZ SP-4430, ARKRAY, Japan). Glucose, total cholesterol, blood urea nitrogen, total bilirubin, alanine aminotransferase, alkaline phosphatase, creatinine, total protein, albumin, calcium, inorganic phosphate, magnesium, uric acid, lactate dehydrogenase, gamma glutamyltransferase, creatinine phosphokinase and amylase were assayed using the automated analyzer.
Statistical analysis. Origin 8E (OriginLab Corporation, Northampton, MA, USA) was used for data processing. Significance was tested using one-way analysis of variance with the Scheffe's test. Significance was calculated for two probability levels at P = 0.05 and P = 0.01.
Results
Mortality test. No mice died when only galantamine was administered. The first mouse died in the course of tularemia four days after infection. Mortality in the infected mice was 40%, and the last mouse died seven days after the initiation of the infection. The mice treated with galantamine and infected with F. tularensis showed 30% mortality from five to eight days after the initiation of infection (Fig. 1).
Fig. 1.
Diagram representing the mortality test.
Biochemical tests. In the interval from three to seven days after initiation of the experiment, significant (P = 0.05) hyperphosphataemia (3.64 ± 0.35 mmol/l, controls: 2.34 ± 0.21 mmol/l) and hyperuricaemia (191 ± 18 µmol/l, controls: 105 ± 20 µmol/l) were observed in the animals with tularemia. The animals treated with galantamine and those infected with tularemia and treated with galantamine were not significantly different in terms of the levels of uric acid and inorganic phosphate compared to controls. The animals infected with tularemia and treated with galantamine had significant (P = 0.05) hyperlactataemia (31.3 ± 2.4 µkat/l, controls: 10.1 ± 2.5 µkat/l) and elevated alanine aminotransferase levels (0.62 ± 0.18 µkat/l, controls: 0.31 ± 0.09 µkat/l). Treatment with galantamine had no effect on plasma levels of lactate dehydrogenase and alanine aminotransferase.
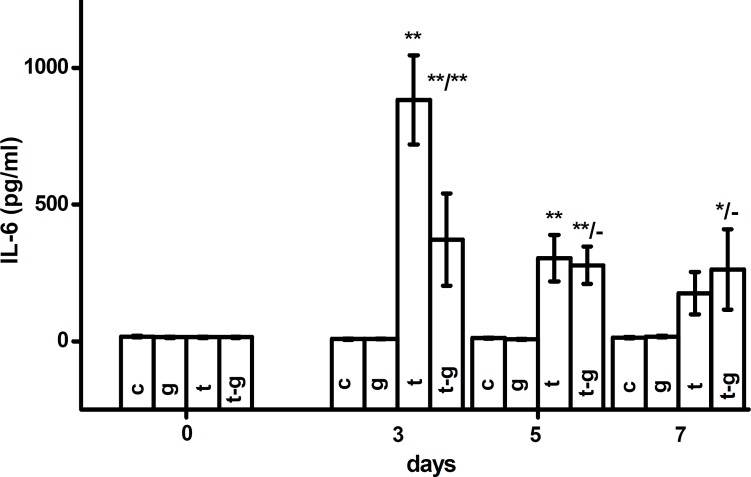
IL-6 and IFN- assay. Levels of IL-6, depicted in Figure 2, were significantly altered in the animals infected with F. tularensis. No significant alterations were found in IL-6 levels in animals treated with galantamine and not infected with F. tularensis. IL-6 was elevated (P = 0.01) three and five days after infection in both galantamine-treated and untreated groups. On the third day after infection, the increase in IL-6 was significantly (P = 0.01) lower when the animals were treated with galantamine. From the fifth to seventh days after infection, levels of IL-6 were not different in tularemia-infected and galantamine-treated animals. IL-6 remained elevated (P=0.01) both in tularemia-infected animals. Seven days after initiation of the experiment, co-administration of galantamine resulted in higher levels of IL-6 compared to tularemia-infected animals. The difference between the two groups was, however, not significant.
Fig 2.
IL-6 levels in controls (c), galantamine (g), tularemia (t) or tularemia-exposed animals given galantamine (t-g). Error bars indicate standard deviation. * and ** indicate significance vs. control at the probability levels of 0.05 and 0.01, respectively. In fraction, asterisks in numerator mean significance against control (c) and asterisks in denominator correspond to significance against animals infected with tularemia (t). In the statistical test, animals euthanized in the same interval were compared.
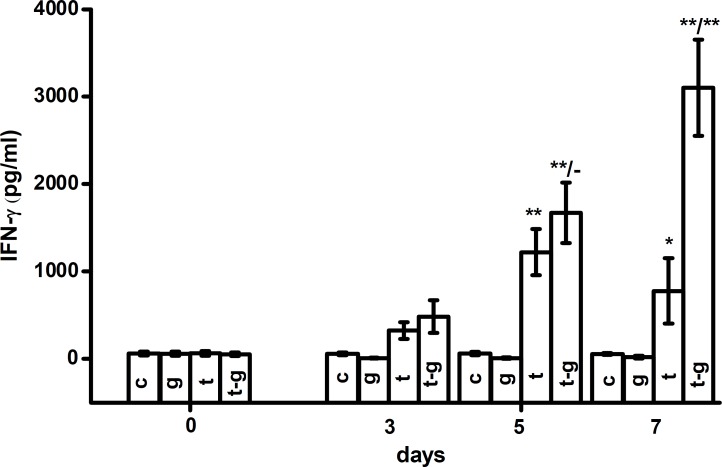
IFN- levels were increased in the course of tularemia five and seven days after the initiation of infection. This increase was more extensive when galantamine was co-administered. Five days after exposure, both groups demonstrated elevated IFN- levels on the probability level of 0.01. Seven days after exposure, tularemia-infected animals had plasmatic levels of IFN- (777 ± 374 pg/ml). Animals infected with tularemia and treated with galantamine had IFN- levels of 3102 ± 550 pg/ml. The difference in IFN- levels between the two groups was statistically significant (P<0.01) on the seventh day after initiation of the experiment. The experimental data are depicted in Figure 3. When compared to controls on the seventh day, tularemia caused an increase in IFN- levels on the probability level of 0.05 and tularemia with galantamine treatment on the probability level of 0.01.
Fig. 3.
IFN- levels in the plasma of experimental animals: controls (c), galantamine (g), tularemia (t) or tularemia-exposed animals given galantamine (t-g). Error bars indicate standard deviation. * and ** indicate significance vs. control at the probability levels of 0.05 and 0.01, respectively. Meaning of fractions is the same as in the Figure 2.
Discussion
In agreement with the experimental hypothesis, galantamine is an agonist of the 7 nAChR as reported by several researchers [11, 12], and the data reported here indirectly confirm this agonism. It has been reported that galantamine can activate the 7 nAChR in patients suffering from Alzheimer and ameliorate the disease in this way [13]. Given the mechanism of the cholinergic anti-inflammatory pathway, a decrease in inflammatory markers is expected after galantamine application [6, 14] and modulation of tularemia pathogenesis can therefore be anticipated. We showed a reduction in IL-6 production when galantamine was administered to F. tularensis-infected mice. Our results are in agreement with a study that showed alterations of TNF- levels after galantamine application [7]. In another experiment, Pavlov and his co-workers [15] proved a link between galantamine and the cholinergic anti-inflammatory pathway in mice. They reported anti-inflammatory effects during endotoxaemia when galantamine was administered. Findings of Pavlov and co-workers [15] well correspond with our results on inflammation represented by IL-6. Moreover, the Pavlov´s team repeated the experiment and proved suitability of galantamine for the alleviation of inflammation associated with obesity in a mouse model [16]. Owing to our findings and evidence from the literature, we can plausibly confirm that galantamine could act as an anti-inflammatory drug and alleviate the inflammatory reaction in the course of tularemia.
Alterations in IFN- levels may reflect an anti-intracellular pathogen response in addition to inflammation. The decreased mortality seen in this study was probably a consequence of IFN- production and related processes. IFN- activation is necessary for the restriction of F. tularensis growth within infected cells [17]. The higher production of IFN- seen here may have contributed to the beneficial outcome of galantamine administration in mice with tularemia.
The hyperphosphataemia and hyperuricaemia demonstrated here confirm previous findings that tularemia causes nephropathy [18]. In humans, interstitial nephritis and glomerulonephritis are reported [19, 20]. The decrease in hyperphosphataemia and hyperuricaemia due to galantamine application may be attributed to the remission of tularemia-induced pathogenesis. Surprisingly, the effect of galantamine was not equal in different tissues, as hyperlactataemia was not ameliorated by galantamine. The overall reduction in mortality after galantamine administration can be inferred to the immuno-modulatory potency of galantamine.
As discussed above, galantamine activates the cholinergic anti-inflammatory pathway. For this reason, a short stimulus of the pathway can significantly modulate the response of the immune system [21]. In a recent experiment, we reported a beneficial effect of the compound HI-6 on tularemia-induced pathogenesis when administered concurrently with the pathogen [22, 23]. The potency of galantamine in ameliorating tularemia has not been previously tested. However, some authors have reported that galantamine has a strong immuno-modulatory effect [16, 25]. Effect of compounds including galantamine interacting with 7 nAChR was extensively reviewed previously [24]. In addition to the above mentioned papers [12, 13], galantamine has been shown to be capable of reducing microglial activation after stimulation with the human immunodeficiency virus type 1 coat glycoprotein, gp120 [25].
Galantamine is a drug used for the treatment of Alzheimer's disease and some other cognitive disorders. However, strong immuno-modulatory effects of this compound have also been shown. As a consequence, galantamine can cause the remission of some pathologic processes and a reduction in mortality when administered to infected mice. The mechanism of action is probably based on stimulation of the cholinergic anti-inflammatory pathway.
ACKNOWLEDGEMENTS
The Ministry of Education, Youth and Sports of the Czech Republic is kindly acknowledged for the project LH11023 and for “A long-term organization development plan 1011”. The funding agency had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mashkovsky MD, Kruglikova-Lvova RP. On the pharmacology of the new alkaloid galantamine. Farmakologia Toxicologia (Moscow) 1951;14:27–30. [Google Scholar]
- 2.Bartolucci C, Perola E, Pilger C, Fels G, Lamba D. Three dimensional structure of a complex of galanthamine (Nivalin) with acetylcholinesterase from Torpedo californica: implications for the design of new anti-Alzheimer drugs. Proteins. 2001 Feb;42(2):182–91. doi: 10.1002/1097-0134(20010201)42:2<182::aid-prot50>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007 Jan;32(1):43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- 4.Senanarogn V, Poungvarin N, Phanthumchinda K, Thavichachart N, Chankrachang S, Praditsuwan R, et al. Safety and tolerability of galantamine in possible Alzheimer’s disease with or without cerebrovascular dementia in Thai patients. J Med Assoc Thai. 2009 Mar;92(Suppl 2):S12–S8. [PubMed] [Google Scholar]
- 5.Pohanka M. Alzheimer’s disease and related neurodegenerative disorders: implication and counteracting of melatonin. J Appl Biomed. 2011 Oct;9(4):185–96. [Google Scholar]
- 6.Pohanka M. Acetylcholinesterase inhibitors; a patent review (2008 – present) Expert Opin Ther Patents. doi: 10.1517/13543776.2012.701620. doi: 10.1517/13543776.2012.701620. [DOI] [PubMed] [Google Scholar]
- 7.Liu ZH, Ma YF, Wu JS, Gan JX, Xu SW, Jiang GY. Effect of cholinesterase inhibitor galanthamine on circulating tumor necrosis factor alpha in rats with lipopolysaccharide-induced peritonitis. Chin Med J (Engl) 2010 Jul;123(13):1727–30. [PubMed] [Google Scholar]
- 8.Edwards JA, Rockx-Brouwer D, Nair V, Celli J. Restricted cytosolic growth of Francisella tularensis subsp tularensis by IFN-gamma activation of macrophages. Microbiology. 2010 Feb;156(Pt 2):327–39. doi: 10.1099/mic.0.031716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley SG, Goldberg MF, Elkins KL. The membrane form of tumor necrosis factor is sufficient to mediate partial innate immunity to Francisella tularensis live vaccine strain. J Infect Dis. 2008 Jul;198(2):284–92. doi: 10.1086/589620. [DOI] [PubMed] [Google Scholar]
- 10.Piotrovsky V, Van PeerA, Van OsselaerN, Armstrong M, Aerssens J. Galantamine population pharmacokinetics in patients with Alzheimer’s disease: modeling and simulations. J Clin Pharmacol. 2003 May;43(5):514–23. [PubMed] [Google Scholar]
- 11.Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, et al. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 2006 Sep;318(3):1108–17. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- 12.Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012 Jan;37(1):16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takata K, Kitamura Y, Saeki M, Terada M, Kagitani S, Kitamura R, et al. Galantamine-induced amyloid-beta clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem. 2010 Dec;285(51):40180–91. doi: 10.1074/jbc.M110.142356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oke SL, Tracey KJ. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann NY Acad Sci. 2009 Aug;1172(1):172–80. doi: 10.1196/annals.1393.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009 Jan;23(1):41–5. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satapathy SK, Ochrani M, Dancho M, Hudson LK, Rosas-Ballina M, Valdes-Ferrer SI, et al. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med. 2011 Jul;17(7-8):599–606. doi: 10.2119/molmed.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards JA, Rockx-Brouwer D, Nair V, Celli J. Restricted cytosolic growth of Francisella tularensis subsp. tularensis by IFN-gamma activation of macrophages. Microbiology. 2010 Feb;156(Pt 2):327–39. doi: 10.1099/mic.0.031716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandouchova H, Pohanka M, Vlckova K, Damkova V, Peckova L, Sedlackova J, et al. Biochemical responses and oxidative stress in Francisella tularensis infection: a European brown hair model. Acta Vet Scand. 2011 Jan;13:53–2. doi: 10.1186/1751-0147-53-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilley WS, Garman RW, Stone WJ. Tularemia complicated by acute renal failure. South Med J. 1983 Feb;76(2):273–4. doi: 10.1097/00007611-198302000-00041. [DOI] [PubMed] [Google Scholar]
- 20.Onuigbo M, Hise M, Ramos E, Traong N, Amelung P, Drachenberg C. Fatal granulomatous broncho-pneumonia complicated by acute renal failure. South Med J. 2002 Aug;95(8):947–9. doi: 10.1097/00007611-200208000-00039. [DOI] [PubMed] [Google Scholar]
- 21.Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009 Dec;12(6):355–64. [PubMed] [Google Scholar]
- 22.Pohanka M, Pavlis O, Pikula J, Treml F, Kuca K. Modulation of tularemia disease progress by the bisquaternary pyridinium oxime HI-6. Acta Vet Brno. 2010 Nov;79(3):443–8. [Google Scholar]
- 23.Bandouchova H, Sedlackova J, Pohanka M, Novotny L, Hubalek M, Treml F, et al. Tularemia induces different biochemical responses in BALB/c mice and common voles. BMC Infect Dis. 2009 Jun;26(9):101. doi: 10.1186/1471-2334-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohanka M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci. 2012 Feb;13(2):2219–38. doi: 10.3390/ijms13022219. Epub 2012 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giunta B, Ehrhart J, Townsend K, Sun N, Vendrame M, Shytle D, et al. Galantamine and nicotine have a synergistic effect on inhibition of microglial activation induced by HIV-1 gp120. Brain Res Bull. 2004 Aug;64(2):165–70. doi: 10.1016/j.brainresbull.2004.06.008. [DOI] [PubMed] [Google Scholar]