Abstract
Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) is considered an attractive anticancer agent due to its tumor cell–specific cytotoxicity. However, its low stability, solubility, unexpected side effects, and weak pharmacokinetic profiles restrict its successful clinical application. To develop efficient TRAIL-based anticancer biotherapeutics, a new version of trimeric TRAIL was constructed by incorporating trimer-forming zipper sequences (HZ-TRAIL), and then NH2-terminal–specific PEGylation was done to produce PEGylated TRAIL (PEG-HZ-TRAIL). The biological, physicochemical, and pharmaceutical characteristics of PEG-HZ-TRAIL were then investigated using various in vitro and in vivo experiments, including a cell-based cytotoxicity test, a solubility test, pharmacokinetic analysis, and antitumor efficacy evaluations. Although slight activity loss occurred after PEGylation, PEG-HZ-TRAIL showed excellent tumor cell–specific cytotoxic effects via apoptotic pathways with negligible normal cell toxicity. The stability and pharmacokinetic problems of HZ-TRAIL were successfully overcome by PEGylation. Furthermore, in vivo antitumor tests revealed that PEG-HZ-TRAIL treatment enhanced therapeutic potentials compared with HZ-TRAIL in tumor xenograft animal models, and these enhancements were attributed to its better pharmacokinetic properties and tumor-targeting performance. These findings show that PEG-HZ-TRAIL administration provides an effective antitumor treatment, which exhibits superior tumor targeting and better inhibits tumor growth, and suggest that PEG-HZ-TRAIL should be considered a potential candidate for antitumor biotherapy.
Introduction
Of the various biological and molecular targets for successful cancer therapy, tumor cell–specific apoptosis is considered a promising approach for development of efficient anticancer therapeutics (1, 2). Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a member of the TNF cytokine superfamily (members of which promote general cell death through extrinsic apoptosis pathways), is considered an optimal candidate for cancer therapy due to its tumor cell specificity and negligible normal cell cytotoxicity (3–8). Among the five TRAIL receptors discovered to date, death receptors 4 (DR4) and 5 (DR5) are connected with the cytoplasmic death domain, and this binding is responsible for transducing the cell death signaling (4–6). TRAIL to DR4 or DR5 bindings leads to recruitment of an adaptor molecule, Fas-associated death domain, which then signals tumor cell death via caspase-dependent apoptotic pathways (3–6). Therefore, numerous researchers have focused on the use of TRAIL for tumor therapy alone or in combination with conventional anticancer strategies, such as chemotherapies and radiotherapies (2, 9). Besides its characteristic antitumor activity, the TRAIL also acts as a negative regulator of erythropoiesis and an anti-inflammatory modulator in vascular biology and physiopathology (10–12).
Although TRAIL treatment has shown excellent therapeutic potential on a broad spectrum of human cancers, recombinant soluble TRAIL has several severe limitations, such as its hepatotoxicity (13–15), weak pharmacokinetic characteristics, such as a short biological half-life and rapid renal elimination (16, 17), its instability in physiologic environments, and its inability to form a homotrimeric structure, which is known to substantially augment its antitumor effect (18–20). To address these obstacles, several researches have focused on the development of TRAIL derivatives containing trimer-forming zipper sequences to facilitate active homotrimer formation (18, 19) or to enhance its tumor-targeting characteristics by introducing tumor-recognizing motifs (21–23). Other strategies, such as enhanced death receptor selectivity (DR4 and/or DR5) to increase antitumor specificity, have also been tried to develop novel proapoptotic receptor agonists, such as DR5-specific TRAIL variants by site-directed mutagenesis or death receptor–specific monoclonal antibodies (24, 25). However, although these investigations have shown excellent in vitro and in vivo antitumor activities, there still remains a strong demand for pharmaceutically improved TRAIL derivatives with better physicochemical and pharmacokinetic characteristics.
We considered that the PEGylation of TRAIL or TRAIL variants might provide an effective means of enhancing its pharmaceutical properties. PEGylation is a process involving the covalent attachment of polyethylene glycol (PEG) polymer chains to various biomolecules, such as peptides, proteins, and antibody fragments, and the resulting PEGylated analogues generally show better pharmaceutical and therapeutic efficiencies (26, 27). In addition, site-specific PEGylation of bioactive proteins or peptides achieves many desirable characteristics, such as enhanced therapeutic potential, uniform product compositions, and high production yields with minimal activity loss (28–31). Therefore, in the present study, we constructed a new TRAIL derivative using site-specific NH2-terminal PEGylation of a TRAIL variant possessing trimer-forming zipper sequences. In addition, we did physicochemical, pharmaceutical, and pharmacokinetic characterizations and evaluated its biological and tumoricidal activities, tumor-targeting efficacy, clearance kinetics, and biodistribution in vitro and in vivo.
Materials and Methods
Purification of active TRAIL
An active TRAIL variant possessing an NH2-terminal histidine tag and trimer-forming zipper sequences [6xHis-ILZ-hTRAIL(114–281), hereafter referred to as HZ-TRAIL] was produced in Escherichia coli using pET23dw-His-ILZ-hTRAIL expression vector, as previously described (32, 33). Briefly, after amplification in E. coli, HZ-TRAIL expression was induced using isopropyl-L-thio-B-D-galactopyranoside (1 mmol/L, 7 h at 27°C). Harvested cells were lysed and soluble HZ-TRAIL was purified by Ni-affinity chromatography (by sequentially washing with 50 and 100 mmol/L imidazole and eluting with 500 mmol/L imidazole). Finally, active trimeric HZ-TRAIL was obtained by gel filtration chromatography.
Preparation and characterizations of PEG-HZ-TRAIL
The site-specific PEGylation of the NH2 terminus of HZ-TRAIL with mPEG-ALD (molecular weight, 5,000 Da; NOF) was carried out by reductive amination in the presence of 20 mmol/L sodium cyanoborohydride (NaCNBH3) in 50 mmol/L acetate buffer at pH 5.0 (I = 0.15). Owing to the relatively lower pKa values of the α-amino group (NH2-terminal amine) than that of ε-amino group of internal lysine residues, the eductive amination reactions can show the excellent selectivity of NH2-terminal–specific PEGylation at cold acidic environment (34). Initially, reaction conditions were optimized for PEG-HZ-TRAIL/HZ-TRAIL molar ratio and reaction time by size exclusion chromatography monitoring. PEG-HZ-TRAIL was prepared using these optimized conditions (PEG-HZ-TRAIL/HZ-TRAIL molar ratio of 7.5:1, reaction time of 12 h at 4°C). In addition, the random PEGylation of HZ-TRAIL at lysine residues (ε-NH2, ran-PEG-HZ-TRAIL) was carried out using conventional PEGylation methods using NHS-activated PEG (mPEG-SPA; molecular weight, 5,000; Nektar) at a PEG-HZ-TRAIL/HZ-TRAIL molar ratio of 7.5:1 (in 20 mmol/L PBS, pH 7.5). The PEGylated HZ-TRAIL so produced was recovered by gel filtration chromatography. Finally, the PEGylated HZ-TRAIL fraction was collected, concentrated by ultrafiltration, and stored at −20°C until required.
PEGylation procedures were confirmed by size exclusion chromatography. The molecular weights of HZ-TRAIL, PEG-HZ-TRAIL, and the bovine serum albumin (BSA) standard were determined using a Superose 12 HR 10/30 column (GE Healthcare), equilibrated with 20 mmol/L acetate buffer (with 100 mmol/L NaCl, pH 5.0), and eluted at a constant flow rate of 0.75 mL/min. SDS-PAGE and Western blot were done to identify PEGylated HZ-TRAIL. After Western blotting, immunoreactive bands were quantified by scanning and using the Scion densitometry program (Scion Images for Windows Release Beta 4.02). NH2-terminal–specific PEGylation was identified by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry after proteolytic digestion. Briefly, after purification of HZ-TRAIL or PEG-HZ-TRAIL monomers from SDS-PAGE separations (22- and 32-dlaton (Da) position bands, respectively), the protein solutions (100 μg/mL in PBS, 100 μL) were incubated with 5 μL of endoproteinase Lys-C solution (100 μg/ mL in PBS) at 37°C for 120 minutes. Then, the respective Lys-C–digested samples were characterized by MALDI-TOF by using α-CHCA as an ionization matrix, as previously described (31).
In vitro biological activities of HZ-TRAIL and PEG-HZ-TRAIL
The biological activities of HZ-TRAIL and PEG-HZ-TRAIL were investigated in human tumor cells [HCT116, HeLa, and PC3 cells; American Type Culture Collection (ATCC)], normal fibroblasts [CCD-986sk; Korean Cell Line Bank (KCLB)], and primary human hepatocytes (In Vitro Technologies). The cell lines were characterized by ATCC or KCLB following the recommended guideline (ATCC Technical Bulletin No. 8, 2008). Tumor and CCD-986sk cells were maintained in DMEM supplemented with 10% (v/v) fetal bovine serum containing 1% penicillin/ streptomycin. For dose-dependent cytotoxicity assays, cells were seeded in 96-well plates at 1 × 104 per well and preincubated for 24 hours. Human hepatocytes were dispensed in a collagen-coated 96-well plate at 5 × 104 cells per well in InVitroGRO CP medium (In Vitro Technologies). Media were then replaced with fresh serum–free DMEM or InVitroGRO HI (hepatocytes), and predetermined amounts of HZ-TRAIL or PEG-HZ-TRAIL were added to final concentrations of 0 to 1,000 ng/mL. The in vitro cytotoxicities of the HZ-TRAIL and PEG-HZ-TRAIL produced were determined using MTT assays after treating cells for 24 hours.
To determine the apoptotic activities of HZ-TRAIL and PEG-HZ-TRAIL on tumor cells (HCT116), cell deaths were quantified using Annexin-V-FLUOS staining kits. Briefly, 1 × 106 cells were seeded into six-well plates and incubated for 24 hours to ensure cell adhesion. Cells were then treated with 100 ng/mL HZ-TRAIL or 300 ng/mL PEG-HZ-TRAIL for 0, 3, 6, 12, and 24 hours, respectively. After treatment, cells were washed with PBS and stained with 100 μL of an Annexin V and propidium iodide labeling solution for 15 minutes. Finally, apoptotic and necrotic cells were analyzed by fluorescence microscopy (green for apoptosis, and yellow or red for necrotic cell).
In vitro stabilities of HZ-TRAIL and PEG-HZ-TRAIL
The in vitro stabilities of HZ-TRAIL and PEG-HZ-TRAIL in PBS were investigated by measuring solubility changes in isotonic buffer solution. After preparing HZ-TRAIL and PEG-HZ-TRAIL solutions [100 μg/mL in PBS (pH 7.4), I = 0.15), they were incubated at 37°C, and at 0, 10, and 30 minutes and 1, 2, 3, 6, 12, 24, 48, and 72 hours, 100 μL aliquots were centrifuged at 10,000 rpm for 20 minutes to remove denatured protein aggregates. Protein concentrations in supernatants were determined using bicinchoninic acid protein assays using BSA as a standard.
The biological stabilities of HZ-TRAIL and PEG-HZ-TRAIL were also investigated by measuring their cytotoxic effects on HCT116 colon tumor cells. Briefly, after preparing HZ-TRAIL and PEG-HZ-TRAIL solutions (10 μg/mL) in 50% rat plasma, samples were incubated at 37°C, and at 0, 15, and 30 minutes and 1, 3, 6, 12, and 24 hours, 100 μL aliquots were centrifuged at 10,000 rpm for 20 minutes to remove residual debris. HCT116 cells in 96-well cell culture plates were then treated with supernatants for 24 hours. Cell viabilities were determined using MTT assays.
In vivo pharmacokinetics of HZ-TRAIL and PEG-HZ-TRAIL
The pharmacokinetic characteristics of HZ-TRAIL and PEG-HZ-TRAIL were investigated in rats using an i.p. administration route. For these experiments, male Sprague-Dawley rats (200 g body weight) were cannulated in a jugular vein a day before the experiments. Animals were randomly divided to HZ-TRAIL and PEG-HZ-TRAIL groups (n = 4), and HZ-TRAIL (20 mmol/L acetate buffer, pH 5.0, 10 μg per rat) or the molar equivalent of PEG-HZ-TRAIL in PBS (pH 6.0) was administered i.p. Plasma samples were obtained at different times after injection by centrifugation and stored at −70°C until required for assay. Concentrations of HZ-TRAIL in rat plasma samples were measured using commercial TRAIL ELISA kits (BioSource International, Inc.). Pharmacokinetic parameters were calculated from plasma concentration profiles by using noncompartment model analysis.
In vivo antitumor activities of HZ-TRAIL and PEG-HZ-TRAIL
The antitumor effect of PEG-HZ-TRAIL was investigated in HCT116 tumor-bearing mice. Briefly, freshly harvested HCT116 cells (3 × 106 per mouse) were inoculated s.c. into the haunch of BALB/c athymic mice. Two days after inoculation, mice were treated with HZ-TRAIL (150 μg per mouse per day, i.p.) or PEG-HZ-TRAIL (150 μg per mouse per day, protein base, i.p.) for 10 days. Tumor volumes were continuously monitored. To evaluate dose-dependent antitumor activities, similar independent experiments at different PEG-HZ-TRAIL dosages (50, 150, and 500 μg per mouse per day, protein base, i.p.) were done using HCT116 colon tumor-bearing BALB/c athymic mice. Subsequently, tumor volumes were monitored for ~21 days after tumor cell inoculation. Tumor volumes were calculated using longitudinal (L) and transverse (W) tumor diameters using the formula V = (L × W2)/2, and tumor growth inhibition (TGI) values were calculated using the formula TGI = (1 − TVsample/TVcontrol) × 100, where TV is the tumor volume.
Acute liver or kidney toxicities and in vivo tumor cell apoptosis were also investigated in HCT116 tumor-bearing BALB/c athymic mice. After tumor volumes had reached 50 to 75 mm3 (~5 d after tumor cell inoculation), HZ-TRAIL or PEG-HZ-TRAIL (150 μg per mouse, 300 μL injection) was administered i.p. At 24 hours after drug injection, liver, kidney, and tumor tissues and blood samples were recovered from euthanized animals for histochemical evaluations and hepatic side effect investigations, respectively. Tissue sections (5 μm) were then cut from 10% neutral buffered formalin-fixed and paraffin-embedded tissue blocks and stained with H&E for routine histology. Apoptotic cell death in liver and tumor tissues was visualized using terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) assays (In Situ Cell Death Detection kits, Roche Applied Science). After TUNEL staining, sections were counterstained with H&E under a light microscope.
In vivo tumor targeting and the biodistributions of HZ-TRAIL and PEG-HZ-TRAIL
To evaluate in vivo tumor targeting, time-dependent excretion, and biodistribution, HZ-TRAIL and PEG-HZ-TRAIL were labeled with Cy5.5 [excitation/emission = 675 nm/695 nm, a near-IR (NIR) fluorophore; ref. 35]. Labeling was done by mixing HZ-TRAIL or PEG-HZ-TRAIL solutions (100 μg/mL in PBS, pH 7.4) with NHS-activated Cy5.5 and allowed to react overnight at 4°C. Reaction mixtures were concentrated by ultrafiltration and finally purified by gel filtration chromatography.
The Cy5.5-labeled HZ-TRAIL or PEG-HZ-TRAIL was injected i.v. into HCT116 tumor-bearing athymic BALB/c mice harboring tumors (average diameter, 5 mm) via a lateral tail vein (20 μg/mL, Cy5.5 based). Noninvasive NIR fluorescence images of mice were obtained using the eXplore Optix system (Advance Research Technologies, Inc.). Laser power and time settings were optimized at 25 μW and 0.3 second per point. To compare the time-dependent excretion profiles of HZ-TRAIL and PEG-HZ-TRAIL, total NIR fluorescence intensities per selected region (1,000 mm2) in whole body were calculated as a function of time. Their time-dependent excretion profiles were determined by calculating the ratios of whole-body fluorescence intensities at different times to whole-body fluorescence intensity at 1 hour after injection. The tumor-targeting abilities of HZ-TRAIL and PEG-HZ-TRAIL were also evaluated by calculating the ratios of fluorescence intensities at tumor sites at different times versus fluorescence intensities at tumor sites at 1 hour after injection.
The biodistributions of HZ-TRAIL and PEG-HZ-TRAIL were determined using the NIR fluorescence images of dissected organs (96 h after injection; liver, lung, kidney, spleen, and heart), and tumors were obtained using a 12-bit charge-coupled device camera (Kodak Image Station 4000 MM) equipped with a C-mount lens and a Cy5.5 bandpass emission filter (680–720 nm; Omega Optical). Then, the obtained fluorescence images were further analyzed by NIH image software.
Results
Purifications of HZ-TRAIL and PEG-HZ-TRAIL
HZ-TRAIL was expressed in E. coli and purified by Ni-affinity chromatography. The HZ-TRAIL was successfully purified after sequential washing and finally 500 mmol/L imidazole elution from Ni-NTA column (Supplementary Fig. S1). HZ-TRAIL was obtained at high purity (>90%) without detectable impurities by SDS-PAGE and with a molecular size of~66 Da, which suggested trimer formation by three monomeric units (22 Da; Supplementary Fig. S1).
NH2-terminal–specific PEGylation of HZ-TRAIL was done under acidic aqueous conditions (pH 5.0; Supplementary Fig. S2). PEG-HZ-TRAIL was obtained by selective fractionation using gel filtration chromatography and analyzed by size exclusion chromatography using BSA (67 Da) and HZ-TRAIL (66 Da) controls. PEG-HZ-TRAIL eluted from the column with an estimated average molecular weight of 100 Da.
TRAIL PEGylations were examined in more detail by SDA-PAGE and Western blot. Due to denaturation during sample preparations, HZ-TRAIL produced only one band at 22 Da (monomer) by Coomassie blue–stained SDS-PAGE and by Western blotting (Fig. 1B). However, NH2-terminal–specific PEGylated HZ-TRAIL (PEG-HZ-TRAIL) produced two distinct bands corresponding to HZ-TRAIL monomer at 22 Da and NH2-terminal–PE-Gylated HZ-TRAIL monomer at 32 Da. The PEGylated 32 Da is higher than the expected weight of 27 Da due to the larger hydrodynamic radius of PEG molecules in aqueous environment than that of protein molecules. Quantitative Western blot analysis revealed that PEG-HZ-TRAIL was composed of two HZ-TRAIL monomers and one PEGylated HZ-TRAIL monomer, presumably due to PEGylation of an available NH2 terminus, because the introduction of the zipper sequences on NH2 termini of TRAIL and resulting trimer formation might prevent the further PEGylations (Supplementary Fig. S3). Unlike the NH2-terminal PEGylation, the lack of selectivity of lysine residue random PEGylation resulted in a complex PEGylation pattern and the formations of multiple PEGylated TRAIL monomers. For more detailed confirmation of NH2-terminal–specific PEGylation, HZ-TRAIL or PEG-HZ-TRAIL monomers extracted after SDS-PAGE separations were subjected to MALDI-TOF mapping analysis followed by Lys-C digestion. Owing to the lysine-specific digestion, the NH2 terminus containing Lys-C–digested fraction of HZ-TRAIL (m/z 1,539; Supplementary Fig. S4, bottom) disappeared after PEGylation, and instead, a PEGylated NH2-terminal fraction (m/z of Mn = 6,700; Supplementary Fig. S4, top) was detected in the PEG-HZ-TRAIL digestion mixture.
Figure 1.

Preparation and characterization of PEG-HZ-TRAIL. A, schematic diagram of the NH2-terminal–specific PEGylation of HZ-TRAIL. B, SDS-PAGE and Western blot patterns of HZ-TRAIL and PEG-HZ-TRAIL obtained by NH2-terminal PEGylation and random PEGylation.
In vitro biological activities of HZ-TRAIL and PEG-HZ-TRAIL
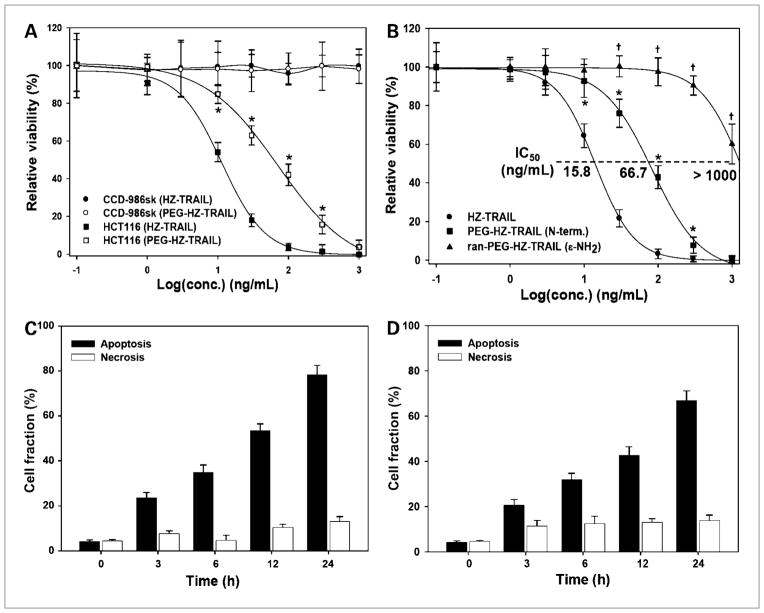
The biological activities, especially the tumor cell–specific cytotoxicities of HZ-TRAIL and PEG-HZ-TRAIL, were evaluated using normal fibroblast cells (CCD-986sk) or HCT116 tumor cells. As shown in Fig. 2A, HZ-TRAIL and PEG-HZ-TRAIL did not have a cytotoxic effect on normal cell lines (CCD-986sk). However, both HZ-TRAIL and PEG-HZ-TRAIL had a concentration-dependent cytotoxic effect on HCT116 human colon tumor cells. Similar to HCT116, the TRAIL-sensitive HeLa cervical cancer cells also showed dose-dependent cytotoxic effects on HZ-TRAIL or PEG-HZ-TRAIL treatments (IC50 values of 3.35 and 14.83 ng/mL, respectively). In contrast, the PC3 prostate tumor cells showed mild TRAIL resistance with >75% of cell viability after 1,000 ng/mL HZ-TRAIL or PEG-HZ-TRAIL treatment (Supplementary Fig. S5). Irrespective to the TRAIL sensitivity, the in vitro cytotoxic effects of HZ-TRAIL were slightly reduced by the PEGylation. Because some preparations of recombinant TRAIL were reported to induce cell death in human hepatocytes, we investigated the hepatocellular toxicity of HZ-TRAIL and PEG-HZ-TRAIL using primary human hepatocytes in vitro. HZ-TRAIL induced cell death in hepatocytes; however, PEG-HZ-TRAIL had little, if any, hepatotoxicity (Supplementary Fig. S5).
Figure 2.
In vitro biological activities of HZ-TRAIL and PEG-HZ-TRAIL. A, cytotoxic effects of HZ-TRAIL and PEG-HZ-TRAIL on CCD-986sk normal fibroblasts and on HCT116 tumor cells. B, effect of PEGylation methods on the cytotoxic effects of HCT116 human colon cancer cells. Lysine residue (random) PEGylation resulted in a significant reduction of tumor cell toxicity compared with that of NH2-terminal PEGylation. Time-dependent apoptotic tumor cell death induced by HZ-TRAIL (100 ng/mL; C) and PEG-HZ-TRAIL (300 ng/mL; D) as determined by Annexin V staining. HZ-TRAIL and PEG-HZ-TRAIL both induced HCT116 tumor cell death via apoptotic pathways. *, P < 0.05, Student’s t test, compared with HZ-TRAIL; †, P < 0.05, Student’s t test, compared with PEG-HZ-TRAIL.
Because HZ-TRAIL easily forms trimers due to the presence of the trimer-forming zipper sequence, HZ-TRAIL had an excellent apoptotic effect on HCT116 human tumor cells (IC50, 15.8 ng/mL; Fig. 2B), whereas PEG-HZ-TRAIL showed slightly less biological activity (IC50, 66.7 ng/mL). However, PEGylation at lysine residues caused serious activity loss (IC50, >1,000 ng/mL), presumably because this impaired TRAIL recognition by death receptors. Furthermore, both HZ-TRAIL and PEG-HZ-TRAIL had a time-dependent apoptotic effect on tumor cells, as determined by selective Annexin V staining. After incubating HCT116 cells for 24 hours with 100 ng/mL HZ-TRAIL or 300 ng/mL PEG-HZ-TRAIL, 78.2 ± 4.2% and 66.7 ± 4.4% of cells, respectively, showed typical apoptotic cell death (Fig. 2C and D; Supplementary Fig. S6).
In vitro physicochemical characterizations of PEG-HZ-TRAIL
The solution stabilities of HZ-TRAIL and PEG-HZ-TRAIL were investigated in physiologic PBS. As shown in Fig. 3A, HZ-TRAIL under physiologic conditions precipitated and aggregated rapidly. However, PEG-HZ-TRAIL showed excellent physiologic stability. Furthermore, although HZ-TRAIL did not aggregate at a concentration of 10 μg/mL, which is below its solubility limit (Fig. 3A), its biological activity continuously diminished, presumably due to proteolytic degradation and/or denaturation. On the other hand, PEG-HZ-TRAIL maintained 80% of its apoptotic activity on HCT116 tumor cells after 24 hours of incubation in rat plasma (Fig. 3B).
Figure 3.

Physicochemical characterizations of HZ-TRAIL and PEG-HZ-TRAIL. A, time-dependent solubility changes of HZ-TRAIL and PEG-HZ-TRAIL in PBS (pH 7.4) at 37°C. B, time-dependent activity changes of HZ-TRAIL and PEG-HZ-TRAIL in 50% rat plasma (1:1 mixture of plasma and PBS, 37°C for 24 h, activity test was conducted using HCT116 human colon cancer cells using MTT).
In vivo pharmacokinetics of PEG-HZ-TRAIL
Pharmacokinetic profiles showed that PEG-HZ-TRAIL retained it activity better than HZ-TRAIL in plasma. Pharmacokinetic evaluations revealed that the elimination half-life of TRAIL was significantly prolonged from 1.5 to 19.8 hours (Fig. 4) by PEGylation. Furthermore, drug bioavailability, as determined by area under the curve analysis, was dramatically enhanced (26-fold enhancement) by PEGylation (40.1 versus 1044.2 ng·h/min by HZ-TRAIL and PEG-HZ-TRAIL, respectively).
Figure 4.
Pharmacokinetic profiles of HZ-TRAIL and PEG-HZ-TRAIL. Sprague-Dawley rats were administered an i.p. injection of HZ-TRAIL or PEG-HZ-TRAIL (10 μg per rat), and blood concentrations were monitored by ELISA. Points, mean (n = 4); bars, SD.
In vivo antitumor activity of PEG-HZ-TRAIL
In vivo antitumor activity testing revealed that HCT116 cell growth was profoundly inhibited by PEG-HZ-TRAIL and not by vehicle alone (Fig. 5A and B). Both HZ-TRAIL and PEG-HZ-TRAIL efficiently suppressed mean tumor growth, with mean TGI values of 37.8% and 74.8%, respectively (Fig. 5A). Furthermore, TGI levels were maintained by PEG-HZ-TRAIL throughout the study period but were not maintained by HZ-TRAIL due to tumor rebound. Furthermore, the antitumor effect of PEG-HZ-TRAIL was superior to that of HZ-TRAIL. The absence of body weight loss revealed the absence of serious toxic side effects by HZ-TRAIL or PEG-HZ-TRAIL treatments (Supplementary Fig. S7). Independent experiments also showed that PEG-HZ-TRAIL dose dependently suppressed tumor growth (Fig. 5B).
Figure 5.
Antitumor activity and histologic evaluations of HZ-TRAIL and PEG-HZ-TRAIL in HCT116 human colon cancer cell–bearing BALB/c athymic mice. A, tumor growth suppressions by HZ-TRAIL and PEG-HZ-TRAIL (daily injection 1–10 d, 150 μg per mouse, protein base, i.p.). Statistical differences (P < 0.05, Student’s t test) of PEG-HZ-TRAIL group data were observed at days 5 to 21 and days 9 to 21 compared with that of control and HZ-TRAIL groups, respectively. B, dose-dependent antitumor activity of PEG-HZ-TRAIL. C, histologic investigations of livers after a single HZ-TRAIL or PEG-HZ-TRAIL injection (150 μg per mouse, protein base, i.p., tissue was harvested 24 h after injection, H&E and TUNEL staining). D, histologic investigations of tumor tissues after a single HZ-TRAIL or PEG-HZ-TRAIL injection (administered as in C).
Histologic examinations of liver tissues 24 hours after injecting 150 μg of HZ-TRAIL or an equimolar amount of PEG-HZ-TRAIL showed no detectable changes in H&E staining compared with the liver tissues of PBS-treated mice (Fig. 5C). However, TUNEL assays of liver tissues showed that HZ-TRAIL induced apoptosis, whereas PEG-HZ-TRAIL did not (Fig. 5C). Furthermore, TUNEL assays of tumor tissues from PEG-HZ-TRAIL–treated rats showed substantially more tumor cell destruction than those of HZ-TRAIL–treated rats (Fig. 5D). Unlike the histologic investigations where HZ-TRAIL showed liver apoptosis, the liver function–related several biomarker levels in serum were not changed by the HZ-TRAIL or PEG-HZ-TRAIL treatments (Supplementary Table S1). In addition, the histologic investigation (H&E staining) of kidney tissues also revealed the absence of acute renal toxicity by HZ-TRAIL or PEG-HZ-TRAIL injections (Supplementary Fig. S8).
In vivo noninvasive molecular imaging of PEG-HZ-TRAIL
Time-dependent NIR fluorescence imaging of HCT116 tumor-bearing mice followed by Cy5.5-labeled HZ-TRAIL or PEG-HZ-TRAIL injections produced interesting tumor targeting, clearance kinetics, and body distribution pattern results (Fig. 6A). As illustrated in Fig. 6B, the monotonous clearance profiles of HZ-TRAIL and PEG-HZ-TRAIL were obtained without absorption phases due to the i.v. drug administrations. As expected, PEG-HZ-TRAIL showed significantly reduced clearance kinetics with more than three times enlarged clearance half-life (half-life values of 13.3 and 43.8 h by HZ-TRAIL and PEG-HZ-TRAIL, respectively; Fig. 6B). In agreement with our pharmacokinetic evaluations, PEG-HZ-TRAIL showed long-acting characteristics by NIR molecular imaging. However, the bioimaging of tumor tissues showed different clearance characteristics for HZ-TRAIL and PEG-HZ-TRAIL. Unlike the continuous reduction (exponential decay pattern) in fluorescence intensity observed in tumor tissues after a single HZ-TRAIL injection, a single PEG-HZ-TRAIL injection resulted in a continuously elevated fluorescence intensity in tumor tissues for up to 48 hours and then slowly decreased (Fig. 6C). Together, these data indicate that PEG-HZ-TRAIL possesses unique tumor-targeting characteristics.
Figure 6.
Noninvasive molecular imaging and clearance kinetics of PEG-HZ-TRAIL in HCT116 human colon cancer cell–bearing BALB/c athymic mice. A, noninvasive molecular imaging of mice injected i.v. with Cy5.5-labeled HZ-TRAIL or PEG-HZ-TRAIL [HCT116 tumor-bearing athymic mice with mean tumor diameter of 10 mm, 200 μL of 20 μg/mL Cy5.5-labeled HZ-TRAIL or PEG-HZ-TRAIL solution (Cy5.5 based)]. Top, arrows and circles, tumor inoculation sites; bottom, tumor-focused fluorescence images. B and C, time-dependent retention profiles of HZ-TRAIL and PEG-HZ-TRAIL in whole bodies (B) and tumors (C). D, distributions of HZ-TRAIL and PEG-HZ-TRAIL based on NIR fluorescence images of tumors (top) and dissected organs (bottom) at 96 h after injection. Left, optical image; right, fluorescence image. Tu, tumor; Liv, liver; Lu, lung; Kid, kidney; Spl, spleen; He, heart. *, P < 0.05, Student’s t test, compared with same time point of HZ-TRAIL group.
In vivo distribution of PEG-HZ-TRAIL
The biodistributions of HZ-TRAIL and PEG-HZ-TRAIL were investigated using a 12-bit charge-coupled device camera. At 96 hours after injection, all mice were sacrificed, and tumors and major organs (liver, lung, kidney, and spleen) were excised. The NIR fluorescence images of dissected tissues (Fig. 6D) showed that tumor and liver tissues in PEG-HZ-TRAIL–treated mice exhibited more fluorescence than HZ-TRAIL–treated mice. The quantitative analysis of the fluorescence images also revealed the improved PEG-HZ-TRAIL accumulation in tumor tissues than other organs, such as lung, kidney, and spleen. However, the HZ-TRAIL injection resulted in the uniform distribution of HZ-TRAIL on tumor and other organs (Supplementary Fig. S9).
Discussion
Although several biological issues related to TRAIL sensitivity and its cytotoxic effects on normal cells must be resolved (13–15, 36, 37), TRAIL is of considerable clinical interest for cancer therapy due to its unique tumor-directed apoptotic activities (2–5). Despite its clinical advantages, several pharmaceutical issues, such as the instability of TRAIL under physiologic conditions, its inability to form an active trimer, and its weak pharmacokinetic characteristics, hinder its use as a cancer treatment (16–20). To address these pharmaceutical obstacles, researchers have focused on the development of TRAIL derivatives with improved tumor specificities and enhanced trimer-forming abilities (18–25), although chemical modification offers an alternative approach. As occurs in natural systems, in which posttranslational modifications play important roles during the productions of new biological or functional entities, numerous strategies have been adopted to overcome the physicochemical and pharmaceutical shortcomings of therapeutic proteins. PEG conjugation, known as PEGylation, is one of the most successful of these strategies, and several PEGylated proteins are now used clinically (26, 27, 38).
In this study, we designated a new version of TRAIL derivatives that readily form homotrimers, by introducing zipper sequences on the NH2 terminus of TRAIL (114–281 sequence), and then PEGylated these derivatives using an NH2-terminal–specific PEGylation procedure to improve their pharmaceutical characteristics. The recombinant TRAIL variant with a 6× histidine tag and trimer-forming zipper sequences (HZ-TRAIL) showed greatest apoptotic activity on human tumor cell lines (HCT116 human colon tumor) and a negligible toxic effect on normal cells (Figs. 1 and 2). Furthermore, PEG-HZ-TRAIL was found to be composed of one NH2-terminal–PEGylated monomer and two non-PEGylated monomers and to induce tumor cell apoptosis specifically (Fig. 1B; Supplementary Fig. S3). However, random PEGylation on lysine residues (ran-PEG-HZ-TRAIL) resulted in heterogeneous PEGylation patterns with multiple PEGylations, and as was found for TNF PEGylation, where random PEGylation resulted in substantial activity loss (28, 39), these different PEGylation patterns significantly and detrimentally affected the biological activity of PEGylated HZ-TRAIL. As shown in Fig. 2B, PEG-HZ-TRAIL showed well-preserved apoptotic activity compared with that of HZ-TRAIL. However, ran-PEG-HZ-TRAIL showed remarkably reduced antitumor activity of <2%, compared with HZ-TRAIL, presumably due to disrupted cognate receptor binding and signaling because the COOH-terminal extracellular domain of natural TRAIL plays a crucial role in TRAIL-mediated apoptosis (2, 40). Furthermore, PEG-HZ-TRAIL did not induce cell death in human primary hepatocytes, whereas HZ-TRAIL induced cell death in hepatocytes. Although the mechanism of cytotoxicity in human hepatocytes by TRAIL is controversial, our results clearly indicate that PEGylation may improve the clinical usefulness of this reagent for treating cancer patients.
Perhaps the most promising characteristics of PEGylated proteins are their in vitro and in vivo stabilities. As shown in Fig. 3, the instability of HZ-TRAIL under physiologic conditions is a serious obstacle to its application. Its precipitation and the associated side effects of activity loss and cytotoxicity to normal cells are presumably due to the tagging sequences used for its purification and the zipper sequences (14, 41). However, the present study shows that these physiochemical limitations can be successfully overcome by PEGylation, and in particular, PEG-HZ-TRAIL showed superior solubility and stability under physiologic solutions (26, 27). The improved in vitro physicochemical characteristics of PEG-HZ-TRAIL also improved in vivo pharmacokinetic characteristics. Several researches have reported that TRAIL is rapidly eliminated and has a short half-life (~30 min in apes), mainly due to renal clearance (16, 17). However, the increased molecular weight and the molecular shielding afforded by PEG dramatically enhance the pharmacokinetic profiles of PEG-HZ-TRAIL, as illustrated in Fig. 4.
As an anticancer agent, PEG-HZ-TRAIL showed superior in vivo antitumor activity and caused massive tumor cell apoptosis compared with HZ-TRAIL (Fig. 5). Interestingly, although acute HZ-TRAIL treatment resulted in hepatotoxicity in athymic mice, PEG-HZ-TRAIL did not show any noticeable hepatotoxicity according to histologic findings (Fig. 5C). Considering the reduced in vivo hepatotoxic side effects and negligible in vitro cytotoxic effect on primary human hepatocytes of PEG-HZ-TRAIL, the PEGylation might be a successful approach to overcome the hepatotoxic side effect of TRAIL, presumably due to the PEG-mediated stabilization of HZ-TRAIL in physiologic condition. Furthermore, unlike that found in a previous study, in which TNF PEGylation did not induce a tumor-targeting effect (28), our noninvasive imaging experiments revealed that PEG-HZ-TRAIL targeted tumors, which we attribute to the effects of its improved solubility/stability and enhanced pharmacokinetic characteristics and to the intrinsic premature nature of tumor blood vessels (known as the enhanced permeation and retention effect; Fig. 6).
In this study, we applied NH2-terminal–specific PEGylation technology to develop pharmaceutically improved TRAIL derivatives with enhanced physicochemical, pharmacokinetic, and antitumor characteristics. PEG-HZ-TRAIL showed improved physiologic stability and a better pharmacokinetic profile than HZ-TRAIL with minimal activity loss. These improved pharmaceutical characteristics resulted in improved therapeutic effects in an animal colon tumor model without detectable side effects. Furthermore, PEG-HZ-TRAIL showed unique tumor accumulation characteristics, which is believed to be due to enhanced tumor targeting. These findings strongly suggest that the PEGylated TRAIL strategy has therapeutic potential for the treatment of cancers.
Supplementary Material
Acknowledgments
Grant Support
Korean Ministry of Education, Science and Technology, Korea.
Footnotes
Note: Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A. Directing cancer cells to self-destruct with proapoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–12. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–30. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–90. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 7.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 8.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 10.Zauli G, Secchiero P. The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev. 2006;17:245–57. doi: 10.1016/j.cytogfr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Zamai L, Secchiero P, Pierpaoli S, et al. TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesis. Blood. 2000;95:3716–24. [PubMed] [Google Scholar]
- 12.Secchiero P, Corallini F, di Iasio MG, Gonelli A, Barbarotto E, Zauli G. TRAIL counteracts the proadhesive activity of inflammatory cytokines in endothelial cells by down-modulating CCL8 and CXCL10 chemokine expression and release. Blood. 2005;105:3413–9. doi: 10.1182/blood-2004-10-4111. [DOI] [PubMed] [Google Scholar]
- 13.Jo M, Kim TH, Seol DW, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–7. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–5. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 15.Volkmann X, Fischer U, Bahr MJ, et al. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498–508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 16.Kelley SK, Harris LA, Xie D, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–8. [PubMed] [Google Scholar]
- 17.Xiang H, Nguyen CB, Kelley SK, Dybdal N, Escandon E. Tissue distribution, stability, and pharmacokinetics of Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in human colon carcinoma COLO205 tumor-bearing nude mice. Drug Metab Dispos. 2004;32:1230–8. doi: 10.1124/dmd.104.000323. [DOI] [PubMed] [Google Scholar]
- 18.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 19.Kim MH, Billiar TR, Seol DW. The secretable form of trimeric TRAIL, a potent inducer of apoptosis. Biochem Biophys Res Commun. 2004;321:930–5. doi: 10.1016/j.bbrc.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 20.Seol DW, Billiar TR. Cysteine 230 modulates tumor necrosis factor-related apoptosis-inducing ligand activity. Cancer Res. 2000;60:3152–4. [PubMed] [Google Scholar]
- 21.Cao L, Du P, Jiang SH, Jin GH, Huang QL, Hua ZC. Enhancement of antitumor properties of TRAIL by targeted delivery to the tumor neovasculature. Mol Cancer Ther. 2008;7:851–61. doi: 10.1158/1535-7163.MCT-07-0533. [DOI] [PubMed] [Google Scholar]
- 22.Tarrus M, van der Sloot AM, Temming K, et al. RGD-avidin-biotin pretargeting to αvβ3 integrin enhances the proapoptotic activity of TNFα related apoptosis inducing ligand (TRAIL) Apoptosis. 2008;13:225–35. doi: 10.1007/s10495-007-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremer E, Samplonius DF, Peipp M, et al. Target cell-restricted apoptosis induction of acute leukemic T cells by a recombinant tumor necrosis factor-related apoptosis-inducing ligand fusion protein with specificity for human CD7. Cancer Res. 2005;65:3380–8. doi: 10.1158/0008-5472.CAN-04-2756. [DOI] [PubMed] [Google Scholar]
- 24.van der Sloot AM, Tur V, Szegezdi E, et al. Designed tumor necrosis factor-related apoptosis-inducing ligand variants initiating apoptosis exclusively via the DR5 receptor. Proc Natl Acad Sci U S A. 2006;103:8634–9. doi: 10.1073/pnas.0510187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–60. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 26.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 27.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–8. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Tsutsumi Y, Yoshioka Y, et al. Site-specific PEGylation of a lysine-deficient TNF-α with full bioactivity. Nat Biotechnol. 2003;21:546–52. doi: 10.1038/nbt812. [DOI] [PubMed] [Google Scholar]
- 29.Shaunak S, Godwin A, Choi JW, et al. Site-specific PEGylation of native disulfide bonds in therapeutic proteins. Nat Chem Biol. 2006;2:312–3. doi: 10.1038/nchembio786. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Youn YS, Lee SH, Byun Y, Lee KC. PEGylated glucagon-like peptide-1 displays preserved effects on insulin release in isolated pancreatic islets and improved biological activity in db/db mice. Diabetologia. 2006;49:1608–11. doi: 10.1007/s00125-006-0234-3. [DOI] [PubMed] [Google Scholar]
- 31.Chae SY, Jin CH, Shin HJ, Youn YS, Lee S, Lee KC. Preparation, characterization, and application of biotinylated and biotin-PEGylated glucagon-like peptide-1 analogues for enhanced oral delivery. Bioconjug Chem. 2008;19:334–41. doi: 10.1021/bc700292v. [DOI] [PubMed] [Google Scholar]
- 32.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn YS, Shin MJ, Chae SY, Jin CH, Kim TH, Lee KC. Biological and physicochemical evaluation of the conformational stability of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Biotechnol Lett. 2007;29:713–21. doi: 10.1007/s10529-006-9300-7. [DOI] [PubMed] [Google Scholar]
- 34.Kinstler O, Molineux G, Treuheit M, Ladd D, Gegg C. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 2002;54:477–85. doi: 10.1016/s0169-409x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 35.Park K, Kim JH, Nam YS, et al. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J Control Release. 2007;122:305–14. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Ricci MS, Kim SH, Ogi K, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–23. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 38.Haag R, Kratz F. Polymer therapeutics: concepts and applications. Angew Chem Int Ed Engl. 2006;45:1198–215. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]
- 39.Shibata H, Yoshioka Y, Ikemizu S, et al. Functionalization of tumor necrosis factor-α using phage display technique and PEGylation improves its antitumor therapeutic window. Clin Cancer Res. 2004;10:8293–300. doi: 10.1158/1078-0432.CCR-04-0770. [DOI] [PubMed] [Google Scholar]
- 40.Hymowitz SG, Christinger HW, Fuh G, et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–71. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 41.Ganten TM, Koschny R, Sykora J, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006;12:2640–6. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.