Abstract
The development of highly sensitive and specific molecular probes for cancer imaging still remains a daunting challenge. Recently, interdisciplinary research at the interface of imaging sciences and bionanoconjugation chemistry has generated novel activatable imaging probes that can provide high-resolution imaging with ultra-low background signals. Activatable imaging probes are designed to amplify output imaging signals in response to specific biomolecular recognition or environmental changes in real time. This review introduces and highlights the unique design strategies and applications of various activatable imaging probes in cancer imaging.
INTRODUCTION
Molecular imaging techniques have made great progress in modern diagnostics and have enabled the detection and visualization of specific molecular events that play important roles in disease processes [1, 2]. Several imaging modalities have been successfully utilized for cancer molecular imaging. However, conventional imaging techniques still suffer from insufficient resolution due to poor sensitivity and specificity of the imaging agents. Progress in the field of molecular imaging is becoming more dependent on the development of novel and customized imaging probes. To date, there has been considerable interest in investigating the design of highly specific and sensitive molecularly targeted probes for cancer imaging. A number of imaging probes have been designed by combining various imaging moieties (radionuclides, fluorophores, and nanoparticles) and targeted ligands (receptor-specific small molecules, peptides and peptide hormones, and antibodies), and these efforts have significantly improved the performance of imaging systems. Several comprehensive review articles have summarized the recent development and applications of molecular imaging probes [3–6].
An ideal imaging probe would have a high specificity for the target of interest and a high signal-to-background (S/B) ratio. From a practical standpoint, one particular class of molecular imaging probes comprising an “activatable” design strategy has generated great attention in the imaging community. Activatable imaging probes are designed to amplify or boost imaging signals in response to specific biomolecular recognition or interaction [6, 7]. The design of these probes is generally based on fluorescent signal activation methods, and the probes are predominantly limited to optical imaging applications. When the fluorophore (donor) and quencher (acceptor) are arranged in close proximity, fluorescence quenching can be induced by several physical energy transfer processes, including fluorescence resonance energy transfer (FRET), dark-quenching mechanisms, and nanoparticle-based surface energy transfer (NSET) [8–10]. The quenching process is strongly dependent on the distance between the donor and acceptor. Because quenched fluorescence can be efficiently restored by increasing the physical distance between the donor and acceptor, this principle has been employed in the design of activatable imaging probes. Sometimes referred to as molecular beacons, activatable probes are optically silent (quenched) in their native state. These probes are activated in the presence of specific biomolecules or chemical stimuli, which generate an amplified fluorescence signal. With the combination of advanced in vivo optical imaging and sophisticated chemistry for near-infrared (NIR) fluorophores, peptides, polymers, and inorganic nanoparticles, various kinds of activatable imaging probes for different biological targets have been developed and validated in preclinical investigations.
The most prominent activatable probes used for cancer imaging are protease-activatable imaging probes. Proteases are enzymes that hydrolyze specific peptide substrates and are overexpressed in a number of pathologies, including cancer. Imaging probes that detect protease activity represent valuable tools for cancer imaging because many protease activities are upregulated in tumor cells. Conventional methods for imaging proteases rely on direct labeling of antibodies or small molecules. Although they are specific for their enzyme targets, they generally provide low S/B ratios and do not give information about enzyme activity. In contrast, activatable probes can provide significantly improved S/B ratios with activity information in real time. To date, various activatable probes have been developed to detect and image representative cancer-related proteases, such as matrix metalloproteinases (MMPs), cathepsins, and caspases. In the following review, an overview of the recent reports of activatable imaging probes designed for cancer imaging is provided. Their unique platforms, physicochemical properties, and applications will be discussed.
PEPTIDE-BASED ACTIVATABLE PROBES
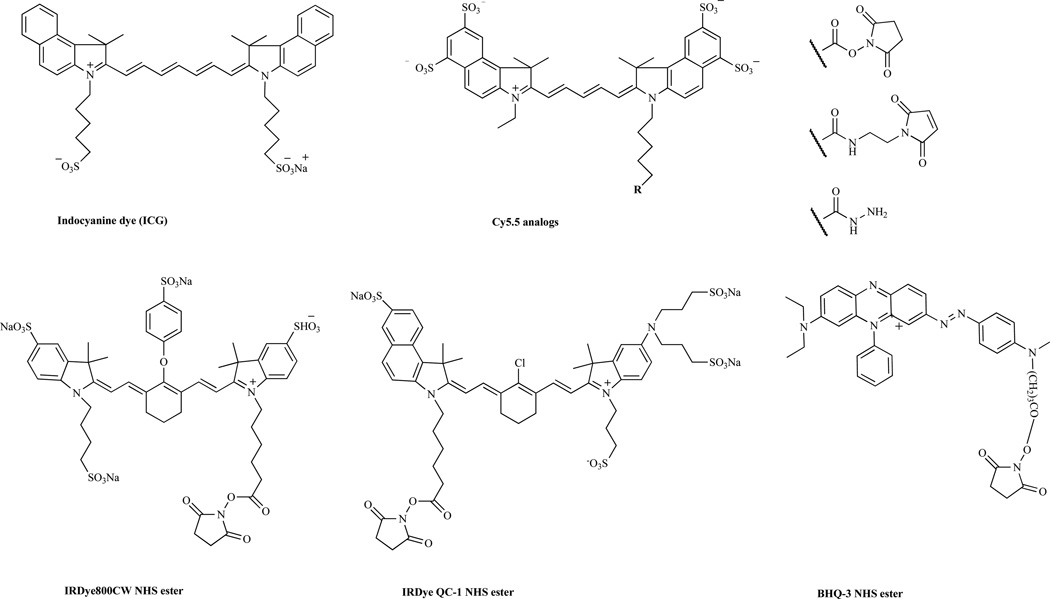
The simplest peptide-based activatable probes consist of a fluorophore and a quencher attached to opposite ends of a peptide linker. The peptide linkers are possible protease substrates, and any peptide substrate can be applied. In addition, fluorophores that operate in the NIR spectrum (650 nm to 900 nm) are preferable for in vivo imaging applications because NIR light has better tissue penetration with low background signals compared to visible light [11]. Most NIR fluorophores are created based on polymethines and are similar to indocyanine green (ICG), a tricarbocyanine dye that is FDA-approved for clinical use [12]. A number of NIR fluorophores have been synthesized, and a few of them are commercially available: Cy dyes (GE Healthcare), Alexa Fluor (Invitrogen), and IRdye dyes (Li-COR Bioscience). Two different kinds of peptide-based activatable probes are available depending on the type of quencher used. The first type relies on a dye-dye self-quenching mechanism, in which the donor and acceptor are the same or similar fluorophores. The latter type uses an acceptor molecule that is distinct from the donor to induce more efficient quenching. These probes are optically quenched in the native state; however, the probes become highly fluorescent following enzyme degradation of the peptide linker by the target proteases. Selected chemical structures of representative NIR dyes and quenchers are displayed in (Fig. 1).
Fig. 1.
Selected near-infrared (NIR) dyes and quenchers.
MMPs are a family of zinc-dependent endopeptidases that play key roles in tumor invasiveness, metastasis, and angiogenesis [13]. Because of its significant role in cancer biology, MMP has been studied as an important target for cancer imaging and therapy. A dual-labeled MMP-7 activatable peptide probe was designed for potential use in imaging MMP-7 activity in tumors [14]. The probe was synthesized by attaching a quencher, NIRQ820, to the N-terminus of an MMP-7 substrate, GVPLSLTMGC-NH2, by Fmoc solid-phase peptide synthesis (SPSS). NIRQ820 is a water-soluble cyclohepta polymethine NIR fluorophore (ex/em, 790/820 nm) and is an efficient quencher for Cy5.5 [15]. The thiol-reactive Cy5.5 mono maleimide was further incorporated into the peptide sequence via the C-terminal cysteine residue to produce an MMP-7 probe, NIRQ820-GVPLSLTMGC(Cy5.5). In vitro enzyme studies showed that the probe was specifically cleaved by MMP-7, and the fluorescence signal was increased by seven-fold. Although FRET quenching is very efficient in many systems, it suffers from a number of drawbacks, including poor spectral overlap between quencher and reporter fluorescence or inherent fluorescence of the quencher, resulting in a relatively low signal-to-background ratio. Dark quenchers, dyes with no native fluorescence, offer advantages over conventional FRET-based probes because they do not occupy the emission spectra [9]. Black Hole Quencher (BHQ) dyes are one of the commercially available dark quenchers and are able to permit efficient quenching across the visible spectrum from 480 nm into the NIR through a combination of FRET and static quenching mechanisms. BHQ dyes have a polyaromatic-azo backbone, which makes the dyes nonfluorescent [16]. Dark-quenched, dual-labeled peptide probes have been designed by using a NIR fluorophore and a dark quencher for imaging protease activity. An MMP-13 activatable peptide probe was designed using a combination of the known MMP-13 substrate GPLGMRGLGK and Cy5.5 and BHQ-3 [17]. BHQ-3 has maximal absorption in the 620- to 730-nm range, which can efficiently quench NIR fluorophores such as Cy5.5. The MMP-13 probe Cy5.5-GPLGMRGLGK(BHQ-3) showed a 32-fold increased enhancement of NIR fluorescence signals after incubation with MMP-13 in vitro, and the fluorescent recovery of the probe was strongly inhibited in the presence of an MMP-13 inhibitor.
Imaging applications that target extracellular proteases, such as MMPs, have the potential disadvantage of gradually leaking from the tumor into the general circulation, which would contribute to reduced signals. To solve this problem, Tsien and colleagues developed an activatable cell-penetrating peptide (ACPP) system that can be selectively activated by proteases near cancer cells and transported into the cells only after protease-specific activation [18]. The ACPPs were composed of two motifs, a dye-conjugated polyarginine-based CPP and a polyanion. In this strategy, cellular uptake of dye-CPPs can be effectively blocked by fusing them to polyanionic sequences by means of peptide substrate linkers, neutralizing the polycations by forming intramolecular hairpins. Once the linker is cleaved by the target protease, the negatively charged inhibitory domain drifts away, and the cationic CPP is free to carry its cargo into the cells. An MMP-targeted ACCP has been designed by fusing Cy5-labeled polyarginine and polyglutamic acid to an MMP-cleavable substrate, GPLGAG. Association with MMP-positive cells typically results in more than a ten-fold increase of cellular uptake in vitro. In addition, ACPP has shown in vivo contrast ratios that are three-fold greater for standard uptake values for tumors relative to normal tissue in mice xenografted with MMP-positive HT-1080 tumor cells and in a transgenic model of spontaneous breast cancer [18, 19].
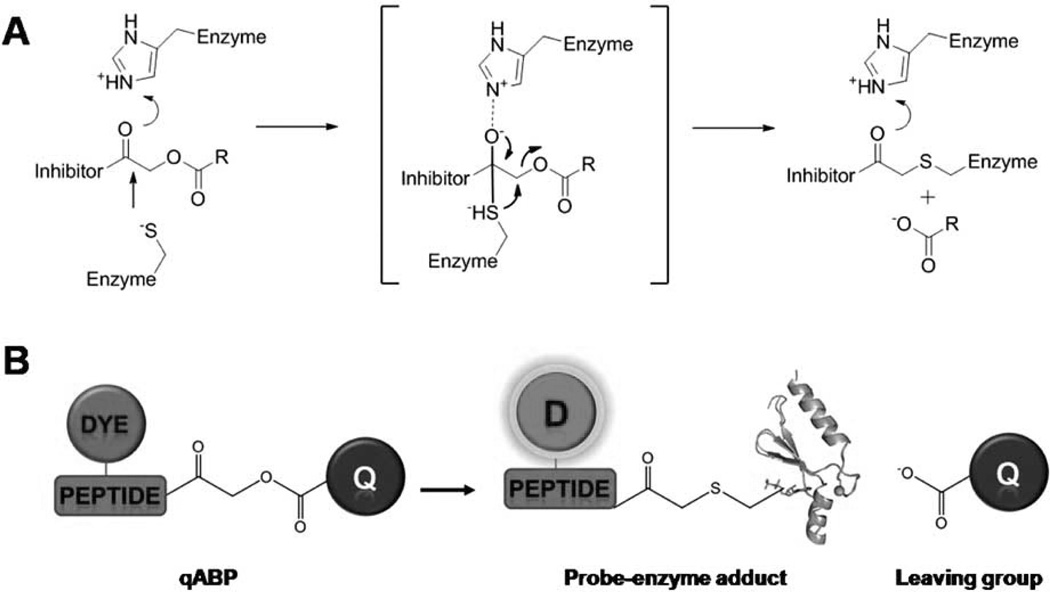
Beside using a quenched peptide substrate that becomes bright after cleavage of the peptide by target proteases, Boygo and colleagues developed activatable, quenched activity-based probes (qABPs) that become fluorescent only after chemical labeling by a target protease through activity-dependent covalent modification [20, 21]. Firstly, they developed nonquenched ABPs that covalently bind to the catalytic site of specific target enzymes in cells and in vivo. A typical ABP consists of i) a reactive functional group that covalently reacts with the target enzyme, ii) a peptide linker region that confers specificity, and iii) a fluorophore used for direct visualization of the probe-labeled enzymes. A series of fluorophore-labeled ABPs that target proteases, such as cathepsins and caspases, has been reported [22, 23]. In a follow-up study, a newly designed quenched ABP (qABP) was reported for cysteine cathepsins by attaching a quencher (QSY21) to NIR fluorophore (Cy5)-labeled acyloxymethyl-ketone analogs (Fig. 2) [21]. This cell-permeable and quenched probe shows reduced nonspecific fluorescent background in vitro and has been used to image cathepsin activity in several tumor cells such as NIH-3Tc and human MCF-10A breast cancer cells. Furthermore, activatable NIR fluorophore-labeled qABPs have produced spatially resolvable fluorescence in the tumor tissues of live mice and can achieve their maximum S/B ratios much more rapidly than nonquenched ABPs.
Fig. 2.
(A) Mechanism of covalent inhibition of a cystein protease by an acyloxymethyl ketone. (B) Schematic diagram of quenched activity-based probe (qABP). Covalent modification of the target results in loss of the quenching group, resulting in production of a fluorescently labeled target enzyme. Modified with permission from ref. 20. Copyright 2005, Macmillan Publishers Limited.
Apoptosis is a programmed cell death process that occurs in multicellular organisms and plays a key role in the pathologies of many disorders, including cancers and tumor responses to chemotherapy or radiotherapy [24, 25]. Because most anticancer therapies involve apoptosis, noninvasive methods for monitoring the apoptosis process could clinically assist in determining anticancer therapeutic efficacy. One common approach is to target caspases, a family of intracellular cysteine proteases that is known as a specific mediator of the apoptotic process [26]. Several caspase-activatable peptide probes have been produced by directly anchoring a fluorophore and a quencher to a caspase substrate. For example, the caspase-3 substrate GDEVD GSGC was conjugated to a nonfluorescent azulene quencher at the N-terminus and Alexa Fluorophore 680 at the C-terminus cysteine residue [27]. A similar dual-labeled probe was designed using polymethine carbocyanine dyes, cybate and Me2N-cypate, sequenced with Ac-GK(Me2N-cypate) DEVDAPK(cybate) [28]. These probes show a substantial increase in the fluorescent signal upon incubation with caspase-3; however, their use is limited due to low resolution and cell membrane impermeability. To efficiently image apoptosis in cell cultures and in vivo, another example of cell-penetratable activatable peptide probes has been designed. Piwnica-Worms et al. developed a cell-permeable, caspase-activatable probe consisting of a cell-penetrating peptide, Tat peptide (Ac-rkkrrorrr) or KKKRKV, conjugated to a caspase-3/7 substrate, DEVD) (Fig. 3) [29, 30]. The addition of a fluorophore/quencher pair (Alexa Fluor 647 and QSY 21, respectively) strongly quenches the cleavable caspase-specific substrate. Following uptake mediated by the CPP, cleavage of the quenched substrate results in activation of the fluorophore. In vitro enzyme assays showed that the probe was preferentially activated by the effector caspases 3 and 7 over the initiator caspases. Analysis of apoptosis in cells treated with the anticancer drug doxorubicin demonstrated amplified fluorescence signals in apoptotic cells. In addition, the probe was able to detect apoptosis in animal models, including parasite-induced apoptosis in human colon xenografts and N-methyl-D-aspartate-induced apoptosis in an in vivo model of glaucoma [29–31].
Fig. 3.
Schematic diagram of cell-permeable and caspase-activatable probe. The caspase-3 substrate DEVD is flanked by a quencher (QSY 21) and fluorophore (Alexa Fluor 647), which silence the reporter. The cell-penetrating peptide sequence enables transport into the cell interior. Upon activation by caspase, the fluorophore is released inside target cells. Modified with permission from ref. 30. Copyright 2009, American Chemical Society.
Peptide-based probes can easily be synthesized and manipulated for target proteases based on an optimal peptide substrate sequence. Well-established peptide chemistry permits a custom synthesis of constructs with accurate structures and also provides scale-up synthesis and easy handling. Yet peptide-based probes often lack cell-permeability, have short half-lives in physiological conditions, and are not easily targeted to tumors. However, these drawbacks can be significantly and efficiently overcome by modifying peptide-based probes with various materials such as cell-penetrating peptides, macromolecules, and functional nanoparticles. The next section summarizes the different strategies used in activatable imaging probes.
MACROMOLECULE-BASED ACTIVATABLE PROBES
Many of the reported peptide-based probes have demonstrated promising results both in vitro and in vivo; however, low-molecular-weight peptides are often nonspecific and unstable and generally lack functional groups for chemical modification. Moreover, chemical modification of the peptide may significantly reduce its biological activity. To date, a variety of biopolymers have been developed, including poly(amino acids), polysaccharide, dendrimers, grafts, and block-co-polymers [32, 33]. In addition, biological macromolecules such as monoclonal antibodies can also be applied in developing activatable imaging probes. Macromolecules generally have large surface areas and a number of functional groups for efficient modification with a wide range of imaging moieties. These macromolecule-based probes can provide improved stability, enhanced targeting, and reduced nonspecific binding compared to low-molecular-weight imaging probes. Combinations of polymer/protein chemistry and activatable probe concepts have yielded new strategies for designing macromolecule-based activatable probes that efficiently image biomarkers or diagnose diseases. Here, we discuss representative macromolecule-based activatable probes that cannot be generated by conventional peptide chemistry.
The first generation of polymer-based activatable probes was developed by Weissleder and colleagues. The type of probe consists of multiple Cy5.5 molecules attached to a methoxy-PEG-grafted poly-L-lysine copolymer (PGC) [34]. The presence of multiple Cy5.5 molecules on the PGC backbone results in NIR dye-dye self-quenching due to the relative proximities of the fluorophores. This probe utilizes unmodified lysine groups on the PGC as a substrate for proteases such as cathepsin B and trypsin, which are both specific for KK pairs. Furthermore, a high-molecular-weight PGC can also serve as a long-circulatory carrier that can accumulate in tumors by the enhanced permeability retention (EPR) effect [35]. Upon activation of the self-quenched PGC-based probe in vitro by target enzymes, a 12-fold increase in NIR fluorescence signal was observed. Optical imaging in tumor-bearing mice showed that this probe can detect overexpressed cathepsin B activity in breast cancer, and different expression levels of tumoral enzymes can be depicted [36]. To expand the range of applications, Cy5.5-peptide substrates have been conjugated to the lysine backbone. A PGC probe was modified to specifically target cathepsin D by introducing a cathepsin D substrate, GPICFFRLISKC, between the PGC backbone and Cy5.5 [37]. An in vitro analysis revealed a 350-fold increase in fluorescence upon incubation with cathepsin. When the probe was intravenously administered in a cathepsin D-positive tumor model, the probe provided a sufficient NIR fluorescence signal to be directly detectable in vivo compared with negative control tumors [38]. A similar strategy has been utilized on an MMP substrate, GPLGVRGC [39]. An MMP-activatable PGC probe successfully imaged the MMP-2 activity in MMP-2-positive HT1080 tumor-bearing mice. In addition, these PGC-based activatable probes demonstrated promising in vivo imaging results in several disease models including rheumatoid arthritis and atherosclerosis and an HIV-1 protease transduced transgenic model [40–42].
Dendrimers are repeatedly branched molecules with spheroid or globular nanostructures that can be engineered to carry molecules that are encapsulated or attached to surface functional groups [43]. The size, shape, and surface functionality of dendrimers can be precisely controlled, and their well-defined structures allow for custom design and modification. Several dendrimer-scaffold-based activatable probes have been developed for imaging applications. Scherer et al. demonstrated an MMP-7-specific poly (amidoamine) (PAMAM)-based activatable probe, PB-M7NIR [44]. PB-M7NIR is a PEGylated generation-4 PAMAM dendrimer core covalently conjugated to a Cy5.5-labeled MMP-7 substrate, Cy5.5-ahx-RPLALWRS(ahx)C, with AF750 as a quencher. PB-M7NIR exhibited a 5-fold increase in NIR fluorescence in vitro and showed 2.2-fold enhanced signals in MMP-7 SW480 tumors compared with MMP-7-negative tumors in vivo. To improve circulation half-lives and tumor specificity in vivo, the previously described ACPP peptide-based probe was conjugated to a generation-5 PAMAM (ACCPD) and was used to visualize tumors during surgery [45]. The complete removal of tumor in surgery is dependent on the surgeon’s ability to distinguish tumor cells from normal tissue; therefore, a way to objectively assess tumors in patients during surgery would be of great benefit. In tumor mouse models, ACPPD was shown to be superior to free ACPP for visualizing tumor tissue during surgery in living mice. Surgery guided by ACPPD resulted in fewer residual cancer cells left in the animal after surgery and demonstrated long-term tumor-free survival and overall survival compared to animals whose tumors were resected in the traditional way [45]. An alternative design for a self-quenched dendrimeric peptide probe was synthesized using the multiple antigenic peptide (MAP) system [46]. With this strategy, a tetravalent branched lysine core was extended to dendritic arms with cathepsin S dipeptide substrate (LR)-incorporated PEGs. The N-terminus of the peptide was conjugated to the NIR fluorophore CyTE777 to induce dye-dye self-quenching. With in vitro proteolytic activation with cathepsin S, the probe showed a greater than 70-fold increase and 95% fluorescence recovery.
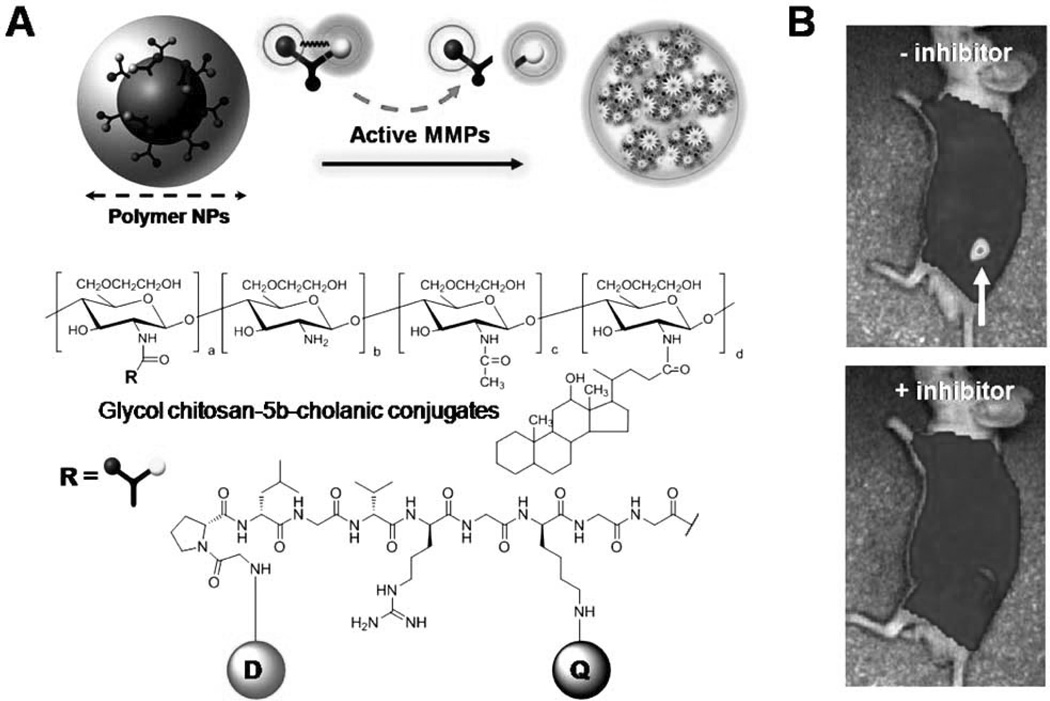
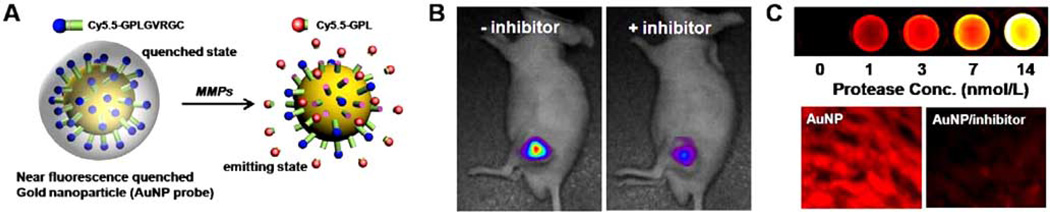
Polymeric nanoparticles (NPs) have also been applied to activatable imaging probes. The use of polymeric nano-particles as imaging agents has a number of advantages compared to small molecules or linear polymers: relative ease in modifying their surface chemistry, which permits the attachment of imaging moieties, reduced uptake by the RES due to hydrophilic coating, and easy transference into cells [47]. In addition, NPs preferentially accumulate at tumor sites due to the EPR effect, resulting in the localization of a greater amount of imaging probes. A polymeric NP-based activatable probe that detects early signs of apoptosis has been developed [48]. Specifically, a caspase-3-cleavale NIR dye-peptide substrate, Cy5.5-DEVDC, was conjugated to biocompatible, self-assembled polymeric NPs (PEI-DOCA) synthesized from hydrophilic branched poly(ethylenimine) (PEI) and a hydrophobic deoxycholic acid (DOCA) moiety. The close proximity of the Cy5.5 dyes on the surface of the NPs resulted in a self-quenched state. These polymeric NPs exhibited a ten-fold increase in NIR fluorescence in the presence of in vitro caspase-3 enzyme and could visualize caspase-dependent apoptosis in living HeLa cells treated with a potent apoptosis inducer, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). More recently, Lee et al. designed a tumor-homing activatable polymeric NP for in vivo protease imaging based on coupling dark-quenched MMP-activatable peptide probes with self-assembled chitosan NPs (Fig. 4) [49]. Hydrophobically modified glycol chitosan (HGC) NPs were prepared by conjugating hydrophilic glycol chitosan with hydrophobic bile acid analogs. The resulting HGC can form spherical NPs (200 to 400 nm in diameter) in aqueous conditions and shows specific tumor targeting ability in vivo [50]. To improve the tumor-specificity of the activatable peptide probe, the surfaces of these NPs were further chemically modified with a dark-quenched quenched MMP substrate, Cy5.5-GPLGVRGK (BHQ-3)GG. The probe showed a proportional relationship between activated NIR fluorescence signals and MMP concentrations in vitro. Furthermore, the probe demonstrated a higher specificity and provided improved sensitivity in MMP-positive tumor models, such as a SCC-7 xenograft tumor and a colon cancer mouse model, compared to a peptide-based activatable probe without NPs [49].
Fig. 4.
(A) Schematic diagram and chemical structures of polymeric nanoparticle-based MMPs activatable probe. (B) In vivo NIR fluorescence images of subcutaneous MMP-positive SCC7 tumor-bearing mice after intravenous injection of the probe with or without the MMP inhibitor. Only tumors injected with the probe without inhibitor were clearly visualized. D: Cy5.5, Q: BHQ-3.
Antibodies are well established and represent a rapidly growing class of targeted therapeutics, particularly in oncology. In addition, the exceptional specificity of antibodies to tumor cell surfaces has great potential for detecting and characterizing cancers. Several antibody-based imaging probes have been developed and applied in in vivo imaging [51, 52]. Antibody imaging can provide a sensitive, noninvasive means for characterizing cell surfaces; however, many antibody-based imaging techniques have demonstrated limited success, due to a low S/B ratio induced by slow background clearance in vivo. To maximize the sensitivity and specificity of antibody-based imaging, several types of activatable antibody probes have been reported. Kobayashi and colleagues developed optically quenched antibodies by conjugating indocyanine green (ICG) to a series of monoclonal antibodies [53]. ICG loses its fluorescence once bound to a protein; however, fluorescence can be strongly recovered upon intracellular protein catabolism [54]. ICG conjugated with FDA-approved monoclonal antibodies directed at cell surface markers overexpressed on tumor cells (anti-CD25, anti-EGFR1 (epidermal growth factor receptor 1), and anti-HER2 (human epidermal growth factor receptor 2)) has almost no fluorescence in the native state and shows activation in buffer contains dithiothreitol (up to 50-fold) and within the endolysosomes in target tumor cells. In vivo imaging in mice has demonstrated that tumors over-expressing specific markers can be successfully visualized and characterized. Another form of antibody-based activatable probes has been reported, which can be activated after cellular internalization by sensing pH changes in the lysosome (Fig. 5) [55]. The interior of the lysosome is acidic (pH 5.0 - 6.0) compared to the slightly alkaline cytosol (pH 7.2). By designing a probe that is specific to tumor cells and becomes activated in an acidic environment, the probe can yield a highly tumor-specific signal with a reduced background signal. In this study, acidic pH-activatable small molecules based on the boron-dipyrromethene (BODIPY) fluorophore were synthesized and then conjugated to the HER2-targeted monoclonal antibody trastuzumab. These pH-activatable probe-antibody conjugates showed the quenched state under neutral pH in buffer and on the surface of HER2-positive cells; however, the probe became highly fluorescent after selective uptake by tumor cells. Furthermore, the probe was highly specific for tumor tissue, with minimal background signals in a HER2-positive lung metastasis model but not in HER2-negative tumor-bearing mice.
Fig. 5.
(A) Scheme for the acidic pH-sensitive BODIPY-based probe. (B) Schematic strategy for selective imaging of target cancer cells with an acidic pH-sensitive small molecule conjugated with cell surface molecule targeted monoclonal antibodies. (C) In vivo tumor detection with targeted activatable probe in HER2-positive lung metastasis model mice. The pH-activatable probe produces a fluorescence signal only from tumors in the lung. However, the control always-on probe produces a fluorescent signal not only from tumors, but also from the background normal lung and heart. Modified with permission from ref. 55. Copyright 2009, Macmillan Publishers Limited.
Modern polymer and bioconjugation chemistry has produced an increasing number of defined biocompatible macromolecular structures. The wide variety of both synthetic and biological macromolecules has encouraged their use in targeted drug delivery systems and, more recently, has resulted in various innovative imaging probes that could not have been generated by conventional small molecule- or peptide-based approaches. Furthermore, hybrid structures that combine macromolecules with activatable imaging strategies will begin to make macromolecule-based imaging probes a credible option for targeted cancer imaging.
METAL-BASED ACTIVATABLE PROBES
Certain types of metal-based NPs such as quantum dots (QDs) and gold nanoparticles (AuNPs) are suitable candidates for optical imaging applications due to their unique characteristics, such as size, intense luminosity, high photostability, and fluorescence quenching efficacy. QDs offer an advantage over organic dyes because they do not suffer from photobleaching, low fluorescent signals, or environment sensitivity [56]. In addition, QDs can be used as excellent donors in FRET because of their narrow emission and broad excitation spectra [57]. AuNPs have the ability to strongly quench fluorescence by nanoparticle surface energy transfer (NSET) mechanisms [10, 58]. Compared to conventional FRET, NSET offers a low signal-to-noise ratio and a longer covering distance (approximately 20 nm). Based on their unique optical properties, metal-based NPs have been utilized in various activatable probes by combining fluorophores, peptides, proteins, and polymers. This section describes the applications of innovative metal-based activatable probes for cancer imaging.
QDs are made from semiconductor materials and have several exceptional properties that promise advantages in imaging applications [3, 59]. By varying the size and composition, QDs can be tuned to emit light over a wide range of wavelengths, from the UV-visible to the NIR region, with narrow emission spectra. They also have broad absorption windows, which makes QDs well-suited for multi-colored imaging applications. These properties would facilitate effective separation between donor and acceptor fluorescence and therefore widen the selection of excitation wavelengths. This allows for the use of QDs as very attractive donors in FRET-based applications. Comprehensive review articles have summarized and discussed their applications in the field of biology [57, 60]. Medintz et al. developed activatable QD-based probes to detect proteolytic activity by FRET [61]. They designed a series of sensing assemblies consisting of QD- and fluorophore-labeled peptide substrates. Various fluorophore-labeled, His6-tag (HHHHHH)-incorporated peptide substrates (targeted to protease caspoase-1, thrombin, collagenase, and chymotrpsin) have been self-assembled onto CdSe-ZnS QDs by using metal-affinity-driven self-assembly [62]. Multiple fluorophore-peptides conjugated on a QD surface bring the acceptors in close proximity to the QD and induce a ratio-dependent quenching of the QD photoluminescence (PL). The increase in number of fluorophore-peptides on the QDs proportionally enhances the overlap integral between the QD and the fluorophore acceptor and can reduce their PL emission by 45% to 100%. The addition of enzymes can specifically cleave the peptide substrate and displace the fluorophore from the QD, resulting in a progressive recovery of the QD emission. Another example of a QD-fluorophore- FRET pair has been designed for MMP imaging [63]. A short MMP substrate, CDGR, was linked between QDs and an acceptor molecule, rhodamine, to observe enzyme activity. The probe was able to monitor the proteolytic activity of MMPs in buffer and could discriminate between normal and MMP-positive cancerous tissue.
As an alternative to using organic fluorophore acceptors, macromolecules such as modified fluorescent proteins and bioluminescent proteins have also been applied to QD-based activatable applications. Boeneman et al. demonstrated that the fluorescent protein mCherry modified to express a protease cleavage can be ratiometrically self-assembled onto QDs to create a sensitive and specific FRET-based protease sensor [64]. mCherry protein (excitation/emission, 587 nm/610 nm) that included a His6-tag was modified to introduce the caspase-3-recognized cleavage sequence SGDEVDSG. QDs assembled with fluorescent proteins engineered as peptide substrates provided sensitive, specific quantitative monitoring of in vitro caspase-3 activity at concentrations as low as 20 pM. Bioluminescence resonance energy transfer (BRET) uses bioluminescent proteins and transfers the generated nonradiative excitation energy to a proximal fluorescent acceptor [65]. Because BRET uses bioluminescent proteins such as Renilla reniformis luciferase (Rluc), it is perfectly suited for QDs, as it does not need an external light source for excitation. QD-BRET combines the advantages of QDs with the high sensitivity of bioluminescence imaging. The most representative in vivo BRET study using a QD-mutant Rluc conjugate was reported by Rao and colleagues and is summarized elsewhere [65, 66]. In an analogous approach, the same group developed a BRET-based QD probe for protease determination [67]. In this study, an MMP-2 substrate containing a His6-tag, GGPLG VRGGHHHHHH, was genetically fused to the C-terminus of the BRET donor, a mutant of Rluc (Luc8). The addition of MMP-2 to a pre-assembled QD-substrate-Luc8 reduced the BRET ratio and enabled the detection of MMP-2 activity with high sensitivity.
AuNPs have been widely used in many medical applications over the past decades [68]. In contrast to many other inorganic nanoparticles, AuNPs are generally regarded as nontoxic. Their distinctive surface plasmon absorption property provides excellent optical properties that can be applied for labeling, imaging, and sensing targeted biomolecules. AuNPs can be easily prepared and stabilized by a variety of stabilizers including biomolecules such as oligonucleotides, DNA, peptides, and proteins. In particular, AuNPs can be used as an ultra-sensitive fluorescence quencher. It has been reported that fluorophores in close proximity to AuNPs experience strong electron interactions with the surface of the AuNPs, producing a perfectly quenched state [10, 58]. Thus, several AuNP-based activatable probes have been designed for in vivo imaging. Lee et al. reported AuNP-quenched activatable probes for use in in vitro protease detection and in vivo cancer imaging (Fig. 6) [69]. In this construct, AuNPs (20 nm in diameter) were stabilized by an N-terminally Cy5.5-labeled MMP substrate, Cy5.5-GPLGVRGC, using a AuNP-thiol modification. It was hypothesized that the chemically conjugated NIR fluorophore-peptide on the surface of the AuNPs would effectively induce a stronger quenched state because AuNPs serve as a quencher of NIR fluorophore via NSET and because Cy5.5 can be self-quenched due to FRET. The resulting nanoparticles demonstrated strong quenching properties with minimal background signals, and an in vitro enzyme assay showed that the AuNP probe was able to recover strong NIR fluorescence signals upon incubation with MMPs. When the probe was administered to MMP-2- positive SCC-7 tumor-bearing mice, the AuNP probe produced strong NIR fluorescence signals only in tumor regions by targeting active MMP-2. Another example of an AuNP-quenched activatable probe has been developed for in vivo detection of reactive oxygen species (ROS) and hyaluronidase (HAdase) [70]. Highly metastatic tumors and rheumatoid arthritis are known to overexpress ROS and HAdase and induce progressive degradation of local hyaluronic acid (HA) in the extracellular matrix [71]. A probe was fabricated by attaching NIR fluorophore (HilteFluor 647)-labeled thiolated oligo HA onto the surface of AuNPs. When the surface-immobilized HA was degraded by ROS and HAdase, strong fluorescence recovery signals were obtained with extreme sensitivity. Upon injection of the probe into a human ovarian metastatic tumor (OVCAR-3) model, the HA-immobilized AuNP probe generated rapid and amplified NIR fluorescence signals in tumors as compared to any other organs.
Fig. 6.
(A) Schematic diagram of gold-quenched MMPs-activatable probe. (B) In vivo near-infrared imaging of MMPs-positive SCC7 tumor bearing mice after intratumoral injection of probes without (left) or with (right) MMPs inhibitor. (C) Optical fluorescence image containing the probe in the presence of various concentrations of MMP-2. (D) Fluorescence microscopy of excised SCC7 tumors.
With their unique optical and photophysical characteristics, including intense luminosity, high photostability, and fluorophore-quenching efficacy, metal-based NPs such as QDs and AuNPs are one of the best candidates for multiplex imaging in both diagnostic and bioimaging applications. A number of activatable platforms have been designed by using metal-based NPs; however, most applications are limited to in vitro use, mainly due to their inherent instability under physiological conditions. Much effort is needed to maintain their desirable optical and photophysical properties in vivo and to optimize imaging methods. In addition, AuNPs appear to be much less toxic than other types of NPs, and several biocompatible QDs have been recently developed. However, potential in vivo toxicity and the metabolism of NPs must be carefully and precisely studied.
CONCLUSIONS
Molecular imaging is actively illuminating molecular and cellular aspects of disease in vivo and is rapidly moving into the clinical field. Molecular imaging already plays an important role in assessing biological events in clinical research and therapy and should be able to provide accurate disease identification in the near future. However, current imaging techniques are hampered by insufficient resolution at the target region due to poor sensitivity and specificity of the imaging probe. Therefore, a development of probes that provide more specific, high-resolution imaging in living cells and in vivo is daunting. The emergence of molecular-based activation technologies can overcome these limitations. The integration of imaging sciences and sophisticated bionano-conjugation chemistry has generated novel activatable imaging probes with ultra-low background signals. In this article, we have introduced and summarized various activatable probes with respect to their design strategies and applications. There are numerous unexplored possibilities for expanding the use of activatable design platforms. Although the activatable imaging probes discussed herein are limited to optical imaging applications, recent progress has led to the introduction of novel activatable probes for other modalities, such as bioluminescence, magnetic resonance imaging, and positron emission tomography. Undoubtedly, the application of these attractive technologies will highlight their potential as valuable clinical tools for future cancer imaging.
REFERENCES
- 1.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219(2):316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JM, Gambhir SS. Molecular imaging: the vision and opportunity for radiology in the future. Radiology. 2007;244(1):39–47. doi: 10.1148/radiol.2441060773. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3(11):1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Chen X. Dual-modality probes for in vivo molecular imaging. Mol. Imaging. 2009;8(2):87–100. [PubMed] [Google Scholar]
- 5.Lee S, Xie J, Chen X. Peptide-based probes for targeted molecular imaging. Biochemistry. 49(7):1364–1376. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem. Commun. (Camb) 2008;(36):4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 7.Elias DR, Thorek DL, Chen AK, Czupryna J, Tsourkas A. In vivo imaging of cancer biomarkers using activatable molecular probes. Cancer Biomark. 2008;4(6):287–305. doi: 10.3233/cbm-2008-4602. [DOI] [PubMed] [Google Scholar]
- 8.Sekar RB, Periasamy A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 2003;160(5):629–633. doi: 10.1083/jcb.200210140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson MK, Cook RM. Intramolecular dimers: a new design strategy for fluorescence-quenched probes. Chemistry. 2003;9(15):3466–71. doi: 10.1002/chem.200304941. [DOI] [PubMed] [Google Scholar]
- 10.Yun CS, Javier A, Jennings T, Fisher M, Hira S, Peterson S, Hopkins B, Reich NO, Strouse GF. Nanometal surface energy transfer in optical rulers, breaking the FRET barrier. J. Am. Chem. Soc. 2005;127(9):3115–3119. doi: 10.1021/ja043940i. [DOI] [PubMed] [Google Scholar]
- 11.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat. Med. 2003;9(1):123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 12.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem .Biol. 2003;7(5):626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 14.Pham W, Choi Y, Weissleder R, Tung CH. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjug. Chem. 2004;15(6):1403–1407. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- 15.Pham W, Lai WF, Weissleder R, Tung CH. High efficiency synthesis of a bioconjugatable near-infrared fluorochrome. Bioconjug Chem. 2003;14(5):1048–1051. doi: 10.1021/bc034070h. [DOI] [PubMed] [Google Scholar]
- 16.Johansson MK, Cook RM, Xu J, Raymond KN. Time gating improves sensitivity in energy transfer assays with terbium chelate/dark quencher oligonucleotide probes. J. Am. Chem. Soc. 2004;126(50):16451–16455. doi: 10.1021/ja0452368. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Park K, Lee SY, Ryu JH, Park JW, Ahn HJ, Kwon IC, Youn IC, Kim K, Choi K. Dark quenched matrix metalloproteinase fluorogenic probe for imaging osteoarthritis development in vivo. Bioconjug Chem. 2008;19(9):1743–1747. doi: 10.1021/bc800264z. [DOI] [PubMed] [Google Scholar]
- 18.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA. 2004;101(51):17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol. (Camb) 2009;1(5–6):382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum G, Mullins SR, Keren K, Fonovic M, Jedeszko C, Rice MJ, Sloane BF, Bogyo M. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat. Chem. Biol. 2005;1(4):203–209. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- 21.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem .Biol. 2007;3(10):668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 22.Fonovic M, Bogyo M. Activity based probes for proteases: applications to biomarker discovery, molecular imaging and drug screening. Curr. Pharm. Des. 2007;13(3):253–261. doi: 10.2174/138161207779313623. [DOI] [PubMed] [Google Scholar]
- 23.Edgington LE, Berger AB, Blum G, Albrow VE, Paulick MG, Lineberry N, Bogyo M. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 2009;15(8):967–973. doi: 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 25.Fischer U, Schulze-Osthoff K. New approaches and therapeutics targeting apoptosis in disease. Pharmacol. Rev. 2005;57(2):187–215. doi: 10.1124/pr.57.2.6. [DOI] [PubMed] [Google Scholar]
- 26.Grutter MG. Caspases: key players in programmed cell death. Curr. Opin. Struct. Biol. 2000;10(6):649–655. doi: 10.1016/s0959-440x(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 27.Pham W, Weissleder R, Tung CH. An azulene dimer as a near-infrared quencher. Angew. Chem. Int. Ed. Engl. 2002;41(19):3659–3962. 3519. doi: 10.1002/1521-3773(20021004)41:19<3659::AID-ANIE3659>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Fan J, Cheney PP, Berezin MY, Edwards WB, Akers WJ, Shen D, Liang K, Culver JP, Achilefu S. Activatable molecular systems using homologous near-infrared fluorescent probes for monitoring enzyme activities in vitro, in cellulo, and in vivo. Mol. Pharm. 2009;6(2):416–427. doi: 10.1021/mp800264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullok K, Piwnica-Worms D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J. Med. Chem. 2005;48(17):5404–5407. doi: 10.1021/jm050008p. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell D, Chang Q, Zhang X, Barnett EM, Piwnica-Worms D. An improved cell-penetrating, caspase-activatable, near-infrared fluorescent peptide for apoptosis imaging. Bioconjug. Chem. 2009;20(4):702–709. doi: 10.1021/bc800516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett EM, Zhang X, Maxwell D, Chang Q, Piwnica-Worms D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc. Natl. Acad. Sci. USA. 2009;106(23):9391–9396. doi: 10.1073/pnas.0812884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J-H, Park K, Nam HY, Lee S, Kim K, Kwon IC. Polymers for bioimaging. Prog. Polymer Sci. 32(8–9):1031–1053. [Google Scholar]
- 33.Park JH, Lee S, Kim J-H, Park K, Kim K, Kwon IC. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008;33(1):113–137. [Google Scholar]
- 34.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 36.Bremer C, Tung CH, Bogdanov A, Jr, Weissleder R. Imaging of differential protease expression in breast cancers for detection of aggressive tumor phenotypes. Radiology. 2002;222(3):814–818. doi: 10.1148/radiol.2223010812. [DOI] [PubMed] [Google Scholar]
- 37.Tung CH, Bredow S, Mahmood U, Weissleder R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug. Chem. 1999;10(5):892–896. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 38.Tung CH, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60(17):4953–4958. [PubMed] [Google Scholar]
- 39.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001;7(6):743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 40.Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004;50(8):2459–2465. doi: 10.1002/art.20379. [DOI] [PubMed] [Google Scholar]
- 41.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114(1):55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 42.Shah K, Tung CH, Chang CH, Slootweg E, O'Loughlin T, Breakefield XO, Weissleder R. In vivo imaging of HIV protease activity in amplicon vector-transduced gliomas. Cancer Res. 2004;64(1):273–278. doi: 10.1158/0008-5472.can-03-1123. [DOI] [PubMed] [Google Scholar]
- 43.Samad A, Alam MI, Saxena K. Dendrimers: a class of polymers in the nanotechnology for the delivery of active pharmaceuticals. Curr. Pharm. Des. 2009;15(25):2958–2969. doi: 10.2174/138161209789058200. [DOI] [PubMed] [Google Scholar]
- 44.Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol. Imaging. 2008;7(3):118–1131. [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. USA. 2010;107(9):4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galande AK, Hilderbrand SA, Weissleder R, Tung CH. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J. Med. Chem. 2006;49(15):4715–4720. doi: 10.1021/jm051001a. [DOI] [PubMed] [Google Scholar]
- 47.Kyeongsoon P, Seulki L, Eunah K, Kwangmeyung K, Kuiwon C, Ick Chan K. New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv. Funct. Mater. 2009;19(10):1553–1566. [Google Scholar]
- 48.Kim K, Lee M, Park H, Kim JH, Kim S, Chung H, Choi K, Kim IS, Seong BL, Kwon IC. Cell-permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J. Am. Chem. Soc. 2006;128(11):3490–3491. doi: 10.1021/ja057712f. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Ryu JH, Park K, Lee A, Lee SY, Youn IC, Ahn CH, Yoon SM, Myung SJ, Moon DH, Chen X, Choi K, Kwon IC, Kim K. Polymeric nanoparticle-based activatable near-infrared nanosensor for protease determination in vivo. Nano Lett. 2009;9(12):4412–4416. doi: 10.1021/nl902709m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim IS, Choi K, Kim SY, Kwon IC. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J. Control. Release. 2007;122(3):305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008;14(3):191–197. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 52.Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS, Paik CH, Urano Y, Choyke PL, Kobayashi H. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin. Cancer Res. 2007;13(22 Pt 1):6639–6648. doi: 10.1158/1078-0432.CCR-07-1119. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69(4):1268–1272. doi: 10.1158/0008-5472.CAN-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tadatsu Y, Muguruma N, Ito S, Tadatsu M, Kusaka Y, Okamoto K, Imoto Y, Taue H, Sano S, Nagao Y. Optimal labeling condition of antibodies available for immunofluorescence endoscopy. J. Med. Invest. 2006;53(1–2):52–60. doi: 10.2152/jmi.53.52. [DOI] [PubMed] [Google Scholar]
- 55.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009;15(1):104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Longterm multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003;21(1):47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 57.Medintz IL, Mattoussi H. Quantum dot-based resonance energy transfer and its growing application in biology. Phys. Chem. Chem. Phys. 2009;11(1):17–45. doi: 10.1039/b813919a. [DOI] [PubMed] [Google Scholar]
- 58.Dubertret B, Calame M, Libchaber AJ. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol. 2001;19(4):365–370. doi: 10.1038/86762. [DOI] [PubMed] [Google Scholar]
- 59.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6(4):669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 60.Algar WR, Krull UJ. Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules. Anal. Bioanal. Chem. 2008;391(5):1609–1618. doi: 10.1007/s00216-007-1703-3. [DOI] [PubMed] [Google Scholar]
- 61.Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL, Deschamps JR, Dawson PE, Mattoussi H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006;5(7):581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 62.Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat. Mater. 2003;2(9):630–638. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 63.Shi L, De Paoli V, Rosenzweig N, Rosenzweig Z. Synthesis and application of quantum dots FRET-based protease sensors. J. Am. Chem. Soc. 2006;128(32):10378–10379. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 64.Boeneman K, Mei BC, Dennis AM, Bao G, Deschamps JR, Mattoussi H, Medintz IL. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J. Am. Chem. Soc. 2009;131(11):3828–3829. doi: 10.1021/ja809721j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Z, Rao J. Biosensing and imaging based on bioluminescence resonance energy transfer. Curr. Opin. Biotechnol. 2009;20(1):37–44. doi: 10.1016/j.copbio.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 2006;24(3):339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 67.Yao H, Zhang Y, Xiao F, Xia Z, Rao J. Quantum dot/ bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. Int. Ed. Engl. 2007;46(23):4346–4349. doi: 10.1002/anie.200700280. [DOI] [PubMed] [Google Scholar]
- 68.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38(6):1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Cha EJ, Park K, Lee SY, Hong JK, Sun IC, Kim SY, Choi K, Kwon IC, Kim K, Ahn CH. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. Engl. 2008;47(15):2804–2807. doi: 10.1002/anie.200705240. [DOI] [PubMed] [Google Scholar]
- 70.Lee H, Lee K, Kim IK, Park TG. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Biomaterials. 2008;29(35):4709–4718. doi: 10.1016/j.biomaterials.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 71.Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc. Natl. Acad. Sci. USA. 1996;93(15):7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]