Abstract
Background
Rhodococcus equi is associated with pyogranulomatous infections, especially in foals, and this bacterium has also emerged as a pathogen for humans, particularly immunocompromised patients. R. equi infections in pigs, wild boar (Sus scrofa) and humans are mainly due to strains carrying the intermediate virulence (VapB) plasmid. In Brazil, R. equi carrying the VapB type 8 plasmid is the most common type recovered from humans co-infected with the human immunodeficiency virus (HIV). R. equi infection in pigs and wild boar is restricted predominantly to the lymphatic system, without any reports of pulmonary manifestations.
Findings
This report describes the microbiological and histopathological findings, and molecular characterization of R. equi in two bronchopneumonia cases in wild boar using PCR and plasmid profile analysis by digestion with restriction endonucleases. The histological findings were suggestive of pyogranulomatous infection, and the plasmid profile of both R. equi isolates enabled the characterization of the strains as VapB type 8.
Conclusions
This is the first report of bronchopneumonia in wild boar due to R. equi. The detection of the VapB type 8 plasmid in R. equi isolates emphasize that wild boar may be a potential source of pathogenic R. equi strains for humans.
Keywords: Rhodococcus equi, Wild boar (Sus scrofa), VapB plasmid, Bronchopneumonia
Findings
Background
Rhodococcus equi is a well-recognized gram-positive facultative intracellular pathogen. It primarily infects domestic animals, particularly foals. R. equi has also emerged as an opportunistic pathogen of humans, particularly immunocompromised patients [1,2]. The bacterium has been isolated from the feces and intestines of various herbivorous and omnivorous species, including cattle, sheep, horses, deer, goats, and pigs [3,4]. The most common manifestation of rhodococcosis in humans and animals is a progressive pyogranulomatous bronchopneumonia, characterized by purulent abscesses and cavitation [3].
The virulence of R. equi is attributed to several factors, including a capsule, cholesterol oxidase and, in particular, plasmid-encoded virulence-associated proteins (Vaps) [1]. These plasmids determine the pathogenicity of R. equi, as they are associated with the survival of the bacterium inside macrophages [3]. Three levels of virulence of R. equi are recognized: virulent (VapA), intermediately virulent (VapB) and avirulent. Virulent R. equi strains are predominantly found in horses, whereas intermediate virulence isolates have been more often observed in pigs [5], HIV-positive human patients [6], and, recently, in wild boar with and without lymphadenitis [7,8]. The avirulent R. equi strains do not express VapA or VapB [9]. The VapB plasmids contains vapB and other vap genes (vapJ, vapK1, vapK2, and vapM) [10,11].
In Brazil, the first wild boar (Sus scrofa) bred for commercial purposes were introduced into the state of Rio Grande do Sul from Europe approximately in 1980 [12]. They were subsequently introduced into other Brazilian states as the commercialization of wild boar meat production increased. This report describes the first case of bronchopneumonia in wild boar caused by R. equi carrying a VapB type 8 plasmid.
Materials and methods
Ethical statement
The present study was approved by Ethical Committee of Animals (number 192/09-CEUA), of School of Veterinary Medicine and Animal Science – UNESP, Botucatu, SP, Brazil.
Animals
Two 70 to 80-day-old wild boar from a breeding farm in Distrito Federal (DF), Brazil, showed clinical signs of pulmonary infection. The animals had exhibited delayed physical developmental, decreased appetite, lethargy, persistent coughing, difficulty breathing, body temperature approximately of 40°C and râles of moderate intensity on thoracic auscultation. Samples of the lungs and pulmonary lymph nodes were collected at necropsy and subjected to microbiological and histopathological analyses.
Diagnosis methods
For histopathology, the specimens were fixed in 10% neutral buffered formalin and, embedded in paraffin, and the sections were stained with hematoxylin and eosin (HE). The lung samples of both animals were cultured, and bacterial identification was based on the observed morphological, staining and biochemical characteristics [13]. The results were confirmed using an R. equi-specific polymerase chain reaction (PCR), as previously described [14]. Bacterial DNA was obtained from a pure colony suspended in 100 μL of Milli-Q water, boiled for 7 minutes and centrifuged at 60,000 × g for 4 minutes [15]. A second PCR was used to identify the virulence-associated genes of the isolates, as described previously [16]. Primer 1 (5′-ACAAGACGGTTTCTAAGGCG-3′) and primer 2 (5′-TTGTGCCAGCTACCAGAGCC-3′) were used to detect the virulent (vapA gene) strains by amplifying a 550-bp product. Primer 3 (5′-GAATTCGAAAGCGCAAAGGT-3′) and primer 4 (5′-TTCCGTGAACATCGTACTGC -3′) were used to amplify a 650-bp product from intermediately virulent (vapB gene) isolates. The virulent strain ATCC 33701p and a human isolate previously characterized as vapB-positive were used as the positive controls in all the PCR reactions.
The plasmid types were determined by digestion with restriction endonucleases. R. equi plasmid DNA was isolated using a modified alkaline lysis method [17], as described previously [18]. The Plasmid DNA was digested with the restriction endonucleases EcoRI, EcoT22I and HindIII[19]. The fragments were fractionated on 1.0% agarose gels, stained with ethidium bromide and examined under ultraviolet light.
Results
Pathology
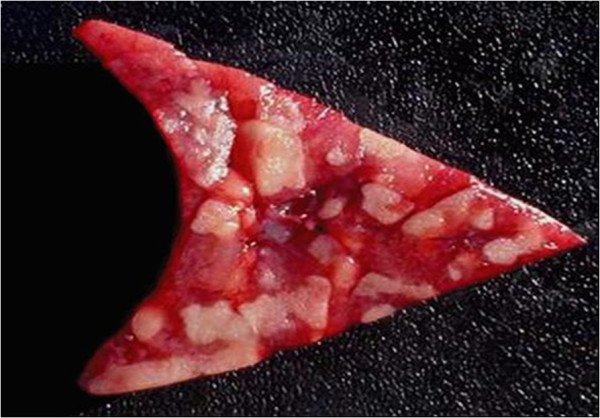
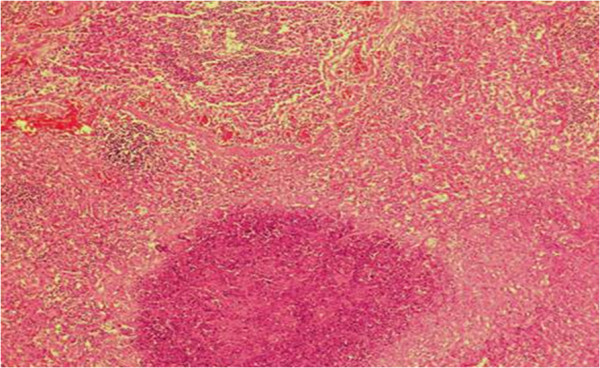
At necropsy, the lungs were found to have multifocal to coalescent areas and granulomatous lesions, with dark-red consolidated areas in the cranial lobes and the ventral portion of the caudal lobes (Figure 1), and mucupurulent exudates in the bronchial lumina. The histological examinations revealed marked neutrophil infiltration into the bronchial and bronchiolar lumina. Large areas of necrosis, with bacterial colonies surrounded by degenerate neutrophils, epithelioid macrophages were also observed in the cranial lung lobes (Figure 2). Numerous foamy macrophages and neutrophils within the alveoli and chronic marked bronchopneumonia were also observed. The histological changes observed in the lungs and pulmonary lymph nodes were suggestive of R. equi infection [20].
Figure 1.
Pneumonia in lung lobe of wild boar caused by R. equi characterized by necrotic multifocal to coalescent lesions, and dark-red consolidated areas.
Figure 2.
Necrotic bronchopneumonia caused by R. equi in the cranial lung lobe from a wild boar. Note area of necrosis containing micro-organisms surrounded by degenerate neutrophils, epithelioid cells and macrophages (Hematoxylin and eosin, 100X).
Bacterial isolation and molecular characterization
R. equi was isolated from the lungs of both boar, and no other bacteria were isolated. The R. equi isolates from both wild boar were vapB R. equi.
Plasmid characterization
The plasmid profile of both R. equi isolates were those of VapB type 8.
Discussion
R. equi infections in wild boar cause lesions that are similar to those observed in rhodococcosis in pigs, and are generally restricted to the lymphatic tissues, involving the cervical and submaxillary lymph nodes, and the tonsils [11,21]. R. equi infection in pigs is associated with immunosuppression and co-infections with other pathogens [4], although R. equi have been isolated from swine with and without lymphadenitis [8,22]. In the cases describe herein, R. equi was identified as a primary cause of pulmonary lesions in the wild boar. Intermediately virulent R. equi strains are commonly found in submandibular lymph nodes in pigs, immunosuppressed humans [5], and more recently, wild boar [7,8]. In Hungary, R. equi was isolated from 14% and 12.4% of the submaxillary lymph nodes of pigs and wild boar, respectively [7,21], and these studies found the vapB gene in 26.8% of the R. equi isolates from pigs [21] and 25.6% from wild boar [7]. In Japan, vapB-positive R. equi were isolated with high prevalence from 368 (93.9%) lymph nodes from pigs [23]. Another study in Japan, described recently isolation of 45 (52%) R. equi strains from submaxillary lymph nodes of 86 wild boar. From these strains were found vapB-positive in 21 (24.0%) strains, predominantly types 2, 1 and 4 plasmids, while vapA gene was found in 1 (1.0%) strain, and 23 (27.0%) remaining isolates were avirulent [24]. Currently, a Brazilian study of the virulence genes and plasmid profiles of the R. equi isolates from the lymph nodes of slaughtered wild boar found that 63.2% of the isolates contained the vapB gene, and the majority was identified as carrying type 8 plasmids [8]. Interestingly, this same plasmid profile (VapB type 8) is the one most frequently found in HIV-positive patients in Brazil [25], suggesting the zoonotic potential of R. equi isolates from wild boar. However, the human with rhodococcosis generally have no history of contact with pigs, wild boar or the environments of these species [25]. It is possible that the consumption of undercooked pig and wild boar products may be a route of infection with R. equi in humans in some countries [6,8,24,25].
Conclusions
This is the first report of R. equi causing bronchopneumonia in wild boar. Although the role of domestic animals and wildlife in the transmission of R. equi to humans remains unclear, the detection of R. equi carrying a VapB type 8 plasmid in wild boar emphasizes the possibility that this species may be a potential source of virulent R. equi in humans, due to this plasmid profile has been identified in R. equi isolates from humans.
Availability of supporting data
The data set supporting this short report are contain in paper.
Abbreviations
bp: Base pairs; DNA: Deoxyribonucleic acid; HE: Hematoxylin eosin; HIV: Human immunodeficiency virus; PCR: Polymerase chain reaction; Vap: Virulence-associated proteins
Competing interests
The authors declare that there are no conflicts of interest in this work.
Authors’ contributions
This case report was written by ACV, FM, LTG and SAB. It was reviewed by all the authors, particularly MGR and GHBL. Microbiological culture and phenotypic identification were performed by CCK, MMC and AML. Clinical and histopathological examination of animals were performed by RE. Determination of the plasmid types of the R. equi isolates was carried out by ST, FM and MGR. All authors read and approved the final version of the manuscript.
Contributor Information
Agueda Castagna de Vargas, Email: agueda.vargas@gmail.com.
Fernanda Monego, Email: fernandamonego@hotmail.com.
Letícia Trevisan Gressler, Email: letrevi@gmail.com.
Sônia de Avila Botton, Email: sabott20@gmail.com.
Andrea Maria Lazzari, Email: andrea01581@upis.br.
Mateus Matiuzzi da Costa, Email: mmatiuzzi@hotmail.com.
Roselene Ecco, Email: eccoro72@gmail.com.
Márcio Garcia Ribeiro, Email: marcio.ribeiro@pq.cnpq.br.
Gustavo Henrique Batista Lara, Email: gunnys7@gmail.com.
Shinji Takai, Email: takai@vmas.kitasato-u.ac.jp.
Acknowledgements
We thank the Cristina Krewer for analysis and interpretation of data and manuscript review. The authors acknowledge the financial support from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico from Brazil) for: Letícia Gressler Master of Science Scholarship (130023/2012-4); Agueda Castagna Vargas Research Productivity Grant (313599/2009-2) and for the financial support awarded from Universal Grant 14/2011 (481943/2011-0).
References
- Meijer WG, Prescott JP. Rhodococcus equi. Vet Res. 2004;35:383–396. doi: 10.1051/vetres:2004024. [DOI] [PubMed] [Google Scholar]
- Puthucheary SD, Sangkar V, Hafeez A, Karunakaran R, Raja NS, Hassan HH. Rhodococcus equi - an emerging human pathogen in immunocompromised hosts: a report of four cases from Malaysia, Southeast Asian. J Trop Med Public Health. 2006;37:157–161. [PubMed] [Google Scholar]
- Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4:20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondalus MK. Pathogenesis and virulence of Rhodococcus equi. Vet Microbiol. 1997;56:257–268. doi: 10.1016/S0378-1135(97)00094-1. [DOI] [PubMed] [Google Scholar]
- Takai S, Fukunga N, Ochiai S. Identification of intermediately virulent Rhodococcus equi isolates from pigs. J Clin Microbiol. 1996;34:1034–1037. doi: 10.1128/jcm.34.4.1034-1037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Tharavichitkul P, Takarn P, Khantawa B, Tamura M, Tsukamoto A, Takayama S, Yamatoda N, Kimura A, Sasaki Y, Kakuda T, Tsubaki S, Maneekarn N, Sirisanthana T, Kirikae T. Molecular epidemiology of Rhodococcus equi of intermediate virulence isolated from patients with and without acquired immune deficiency syndrome in Ching Mai, Thailand. J Infect Dis. 2003;188:1717–1723. doi: 10.1086/379739. [DOI] [PubMed] [Google Scholar]
- Makrai L, Kobayashi A, Matsuoka M, Sasaki Y, Kakuda T, Dénes B, Hajtós I, Révész I, Jánosi K, Fodor L, Varga J, Takai S. Isolation and characterization of Rhodococcus equi from submaxillary limph nodes of wild boar (Sus scrofa) Vet Microbiol. 2008;131:318–323. doi: 10.1016/j.vetmic.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Ribeiro MG, Takai S, Guazzelli A, Lara GHB, Silva AV, Fernandes MC, Condas LA, Siqueira AK, Salerno T. Virulence genes and plasmid profiles in Rhodococcus equi isolates from domestic pigs and wild boar (Sus scrofa) in Brazil. Res Vet Sci. 2011;91:478–481. doi: 10.1016/j.rvsc.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Ribeiro MG, Seki I, Yasuoka K, Kakuda T, Sasaki Y, Tsubaki S, Takai S. Molecular epidemiology of virulent Rhodococcus equi from foals in Brazil: virulence plasmids of 85–kb type I, 87–kb type I, and a new variant, 87–kb type III. Comp Immunol Microbiol Infect Dis. 2005;28:53–61. doi: 10.1016/j.cimid.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Letek M, Ocampo-Sosa AA, Sanders M, Fogarty U, Buckley T, Leadon DP, González P, Scortti M, Meijer WG, Parkhill J, Bentley S, Vázquez-Boland JA. Evolution of the Rhodococcus equi vap pathogenicity island seen through comparison of host-associated vapA and vapB virulence plasmids. J Bacteriol. 2008;190:5797–5805. doi: 10.1128/JB.00468-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bargeen K, Haas A. Molecular and infection biology of the horse pathogen Rhodococcus equi. FEMS Microbiol Rev. 2009;33:870–891. doi: 10.1111/j.1574-6976.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- Spitz F. The Atlas of European Mammals. London: T & AD Poyser Natural History; 1999. [Google Scholar]
- Quinn PJ, Carter ME, Markey B, Carter GR. Clinical Veterinary Microbiology. London: Wolfe; 1994. [Google Scholar]
- Bell KS, Philp JC, Christofi N, Aw DW. Identification of Rhodococcus equi using the polymerase chain reaction. Lett Appl Microbiol. 1996;23:72–74. doi: 10.1111/j.1472-765X.1996.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Sambrook R, Russel DW. Molecular Cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Monego F, Maboni F, Krewer C, Vargas A, Costa M, Loreto E. Molecular characterization of Rhodococcus equi from horse-breeding farms by means of multiplex PCR for the vap gene family. Curr Microbiol. 2009;58:399–403. doi: 10.1007/s00284-009-9370-6. [DOI] [PubMed] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Ikeda T, Sasaki Y, Watanabe Y, Ozawa T, Tsubaki S, Sekizaki T. Identification of virulent Rhodococcus equi by amplification of gene coding for 15–17–kDa antigens. J Clin Microbiol. 1995;33:1624–1627. doi: 10.1128/jcm.33.6.1624-1627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Imai Y, Fukumaga N, Uchida Y, Kamisawa K, Sasaki Y, Tsubaki S, Sekizaki T. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J Infect Dis. 1995;172:1306–1311. doi: 10.1093/infdis/172.5.1306. [DOI] [PubMed] [Google Scholar]
- Madarame H, Takai S, Matsumoto C, Minamiyama K, Sasaki Y, Tsubaki S, Hasegawa Y, Nakane A. Virulent and avirulent Rhodococcus equi indection in T-cell deficient athymic nude mice: pathologic, bacteriologic and immunologic responses. FEMS Immunol Med Microbiol. 1997;17:251–262. doi: 10.1016/S0928-8244(97)00013-8. [DOI] [PubMed] [Google Scholar]
- Makrai L, Takayama S, Dénes B, Hajtós I, Sasaki Y, Kakuda T, Tsubaki S, Major A, Fodor L, Varga J, Takai S. Characterization of virulence plasmids and serotyping of Rhodococcus equi isolates from submaxillary lymph nodes of pigs in hungary. J Clin Microbiol. 2005;43:1246–1250. doi: 10.1128/JCM.43.3.1246-1250.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara GHB, Ribeiro MG, Leite CQF, Paes AC, Guazzelli A, Silva AV, Listoni FJP. Occurrence of Mycobacterium spp and other pathogens in lymph nodes of slaughtered swine and wild boar (Sus Scrofa) Res Vet Sci. 2011;90:185–188. doi: 10.1016/j.rvsc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Takai S, Tsubaki S. The incidence of Rhodococcus (Corynebacterium) equi in domestic animals and soil. J Vet Med Sci. 1985;47:1291–1293. doi: 10.1292/jvms1939.47.493. [DOI] [PubMed] [Google Scholar]
- Sakai M, Ohno R, Higuchi C, Sudo M, Suzuki K, Sato H, Maeda K, Sasaki Y, Kakuda T, Takai S. Isolation of Rhodococcus equi from wild boar (Sus scrofa) in Japan. J Wildelife Dis. 2012;48:815–817. doi: 10.7589/0090-3558-48.3.815. [DOI] [PubMed] [Google Scholar]
- Ribeiro MG, Takai S, Vargas AC, Mattos-Guaraldi AL, Camello TCF, Silva AV. Identification of virulence associated plasmids in Rhodococcus equi from humans with and without acquired immune deficiency syndrome in Brazil. Am J Trop Med Hyg. 2011;85:510–513. doi: 10.4269/ajtmh.2011.10-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]