Abstract
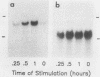
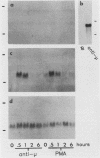
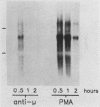
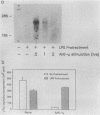
Egr-1 is a murine early growth factor-inducible gene that encodes a protein with zinc fingers and that is believed to be involved in transcriptional regulation. Expression of this gene was investigated in murine B lymphocytes stimulated through their receptor for antigen (surface immunoglobulin [sIg]) with antireceptor antibodies (anti-sIg). Rapid (by 15 min) up-regulation of Egr-1 mRNA expression was observed after sIg cross-linking at a dose of anti-sIg sufficient to drive the majority of cells into cell cycle. Interestingly, signaling through sIg on the murine B-lymphoma cell line WEHI-231 did not up-regulate Egr-1 expression even though similar signaling pathways, including up-regulation of c-fos expression, are associated with this receptor in these cells. Importantly, cell growth and proliferation of WEHI-231 cells were inhibited by anti-sIg stimulation, which suggested a relationship between Egr-1 expression and differential processing of receptor immunoglobulin signals with respect to cellular growth responses. This notion was further supported by the finding that murine B lymphomas whose proliferation was not inhibited by anti-sIg showed receptor immunoglobulin-coupled Egr-1 expression. In further support of this association are results showing that under conditions in which Egr-1 expression was induced in WEHI-231 cells in response to stimulation by anti-sIg, a concomitant change was observed in the growth response of these cells. These results, then, indicate a potential role for Egr-1 expression in the translation of receptor-generated signals into cellular activation or induced unresponsiveness.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Blumberg H., Eisen A., Sledziewski A., Bader D., Young E. T. Two zinc fingers of a yeast regulatory protein shown by genetic evidence to be essential for its function. 1987 Jul 30-Aug 5Nature. 328(6129):443–445. doi: 10.1038/328443a0. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. The regulation of growth and differentiation of a murine B cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. J Immunol. 1981 Jun;126(6):2466–2469. [PubMed] [Google Scholar]
- Cambier J. C., Monroe J. G. B cell activation. V. Differentiation signaling of B cell membrane depolarization, increased I-A expression, G0 to G1 transition, and thymidine uptake by anti-IgM and anti-IgD antibodies. J Immunol. 1984 Aug;133(2):576–581. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Gold M. R., Jakway J. P. B-lymphocyte signal transduction in response to anti-immunoglobulin and bacterial lipopolysaccharide. Immunol Rev. 1987 Feb;95:161–176. doi: 10.1111/j.1600-065x.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Edwards D. R., Parfett C. L. Gene expression during the mammalian cell cycle. Biochim Biophys Acta. 1986 Oct 28;865(2):83–125. doi: 10.1016/0304-419x(86)90024-7. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Hornbeck P., Paul W. E. Anti-immunoglobulin and phorbol ester induce phosphorylation of proteins associated with the plasma membrane and cytoskeleton in murine B lymphocytes. J Biol Chem. 1986 Nov 5;261(31):14817–14824. [PubMed] [Google Scholar]
- Hämmerling U., Chin A. F., Abbott J. Ontogeny of murine B lymphocytes: sequence of B-cell differentiation from surface-immunoglobulin-negative precursors to plasma cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2008–2012. doi: 10.1073/pnas.73.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C. Antiimmunoglobulin-treated B cells respond to a B cell differentiation factor for IgG1. J Exp Med. 1986 Jul 1;164(1):303–308. doi: 10.1084/jem.164.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakway J. P., Usinger W. R., Gold M. R., Mishell R. I., DeFranco A. L. Growth regulation of the B lymphoma cell line WEHI-231 by anti-immunoglobulin, lipopolysaccharide, and other bacterial products. J Immunol. 1986 Oct 1;137(7):2225–2231. [PubMed] [Google Scholar]
- Joseph L. J., Le Beau M. M., Jamieson G. A., Jr, Acharya S., Shows T. B., Rowley J. D., Sukhatme V. P. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7164–7168. doi: 10.1073/pnas.85.19.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- LaBaer J., Tsien R. Y., Fahey K. A., DeFranco A. L. Stimulation of the antigen receptor on WEHI-231 B lymphoma cells results in a voltage-independent increase in cytoplasmic calcium. J Immunol. 1986 Sep 15;137(6):1836–1844. [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L., Ledbetter J. A., Herzenberg L. A. Quantitative immunofluorescent analysis of surface phenotypes of murine B cell lymphomas and plasmacytomas with monoclonal antibodies. J Immunol. 1981 Oct;127(4):1691–1697. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- McCormack J. E., Pepe V. H., Kent R. B., Dean M., Marshak-Rothstein A., Sonenshein G. E. Specific regulation of c-myc oncogene expression in a murine B-cell lymphoma. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5546–5550. doi: 10.1073/pnas.81.17.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Tsang W., Morrison S. L., Beaven M. A., Paul W. E. Membrane IgM, IgD, and IgG act as signal transmission molecules in a series of B lymphomas. J Immunol. 1986 Oct 1;137(7):2162–2167. [PubMed] [Google Scholar]
- Mond J. J., Seghal E., Kung J., Finkelman F. D. Increased expression of I-region-associated antigen (Ia) on B cells after cross-linking of surface immunoglobulin. J Immunol. 1981 Sep;127(3):881–888. [PubMed] [Google Scholar]
- Monroe J. G., Kass M. J. Molecular events in B cell activation. I. Signals required to stimulate G0 to G1 transition of resting B lymphocytes. J Immunol. 1985 Sep;135(3):1674–1682. [PubMed] [Google Scholar]
- Monroe J. G. Up-regulation of c-fos expression is a component of the mIg signal transduction mechanism but is not indicative of competence for proliferation. J Immunol. 1988 Mar 1;140(5):1454–1460. [PubMed] [Google Scholar]
- Parker D. C. Induction and suppression of polyclonal antibody responses by anti-Ig reagents and antigen-nonspecific helper factors: a comparison of the effects of anti-Fab, anti-IgM, and anti IgD on murine B cells. Immunol Rev. 1980;52:115–139. doi: 10.1111/j.1600-065x.1980.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Paul S., Jailkhani B. L. Unmasking by neuraminidase of specific chorionic gonadotrophin binding activity of human placental syncytiotrophoblast. J Reprod Fertil. 1982 Nov;66(2):445–450. doi: 10.1530/jrf.0.0660445. [DOI] [PubMed] [Google Scholar]
- Quantin B., Breathnach R. Epidermal growth factor stimulates transcription of the c-jun proto-oncogene in rat fibroblasts. Nature. 1988 Aug 11;334(6182):538–539. doi: 10.1038/334538a0. [DOI] [PubMed] [Google Scholar]
- Ransom J. T., DiGiusto D. L., Cambier J. C. Single cell analysis of calcium mobilization in anti-immunoglobulin-stimulated B lymphocytes. J Immunol. 1986 Jan;136(1):54–57. [PubMed] [Google Scholar]
- Rhodes D., Klug A. An underlying repeat in some transcriptional control sequences corresponding to half a double helical turn of DNA. Cell. 1986 Jul 4;46(1):123–132. doi: 10.1016/0092-8674(86)90866-4. [DOI] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Scott D. W., Tuttle J., Livnat D., Haynes W., Cogswell J. P., Keng P. Lymphoma models for B-cell activation and tolerance. II. Growth inhibition by anti-mu of WEHI-231 and the selection and properties of resistant mutants. Cell Immunol. 1985 Jun;93(1):124–131. doi: 10.1016/0008-8749(85)90393-4. [DOI] [PubMed] [Google Scholar]
- Snow E. C., Fetherston J. D., Zimmer S. Induction of the c-myc protooncogene after antigen binding to hapten-specific B cells. J Exp Med. 1986 Sep 1;164(3):944–949. doi: 10.1084/jem.164.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Kartha S., Toback F. G., Taub R., Hoover R. G., Tsai-Morris C. H. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res. 1987 Sep-Oct;1(4):343–355. [PubMed] [Google Scholar]
- Teale J. M., Klinman N. R. Tolerance as an active process. Nature. 1980 Nov 27;288(5789):385–387. doi: 10.1038/288385a0. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris C. H., Cao X. M., Sukhatme V. P. 5' flanking sequence and genomic structure of Egr-1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res. 1988 Sep 26;16(18):8835–8846. doi: 10.1093/nar/16.18.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]