Abstract
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common respiratory condition and the fourth leading cause of death in Canada. Optimal COPD management requires patients to participate in their care and physician knowledge of patients’ perceptions of their disease.
METHODS:
A prospective study in which respiratory specialist physicians completed a practice assessment questionnaire and patient assessments for 15 to 20 consecutive patients with COPD. Patients also completed a questionnaire regarding their perceptions of COPD and its management.
RESULTS:
A total of 58 respiratory specialist physicians from across Canada completed practice assessments and 931 patient assessments. A total of 640 patients with COPD (96% with moderate, severe or very severe disease) completed questionnaires. Symptom burden was high and most patients had experienced a recent exacerbation. Potential COPD care gaps were identified with respect to appropriate medication prescription, lack of an action plan, and access to COPD educators and pulmonary rehabilitation. Perceived knowledge needs and gaps differed between physicians and patients.
CONCLUSIONS:
Despite the dissemination of Canadian and international COPD clinical practice guidelines for more than a decade, potential care gaps remain among patients seen by respiratory specialist physicians. Differing perceptions regarding many aspects of COPD among physicians and patients may contribute to these care gaps.
Keywords: Care gaps, Clinical practice guidelines, COPD, Patient knowledge, Practice assessment, Survey
Abstract
INTRODUCTION :
La maladie pulmonaire obstructive chronique (MPOC), un trouble respiratoire courant, est la quatrième cause de décès en importance au Canada. Pour une prise en charge optimale de la MPOC, le patient doit participer aux soins et le médecin doit savoir comment le patient perçoit sa maladie.
MÉTHODOLOGIE :
Étude prospective au cours de laquelle des médecins spécialistes en pneumologie ont répondu à un questionnaire d’évaluation de la pratique et ont évalué de 15 à 20 patients consécutifs atteints d’une MPOC. Les patients ont également rempli un questionnaire sur leurs perceptions de la MPOC et de sa prise en charge.
RÉSULTATS :
Au total, 58 médecins spécialistes en pneumologie de partout au Canada ont rempli des évaluations de la pratique et 931 évaluations de patient. De plus, 640 patients ayant une MPOC (dont 96 % avaient une maladie modérée, grave ou très grave) ont rempli les questionnaires. Le fardeau de la maladie était élevé, et la plupart des patients avaient subi une exacerbation récente. Les chercheurs ont constaté des lacunes potentielles sur le plan des soins pour ce qui est de la prescription pertinente de médicaments, de l’absence de plan d’action et de l’accès à des éducateurs en MPOC et à une réadaptation pulmonaire. Les médecins et les patients n’avaient pas la même perception des besoins et des lacunes en matière de savoir.
CONCLUSIONS :
Malgré la diffusion de lignes de pratique clinique canadiennes et internationales sur la MPOC depuis plus d’une décennie, il reste encore d’éventuelles lacunes en matière de soins des patients traités par des médecins spécialistes en pneumologie. Des perceptions différentes des médecins et des patients à l’égard de nombreux aspects de la MPOC contribuent à ces lacunes.
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory condition caused by the inhalation of noxious substances (eg, cigarette smoke), and is associated with progressive respiratory symptoms, systemic comorbidities and exacerbations (1). Estimates of COPD prevalence indicate that approximately 4.4% of Canadian adults have been diagnosed with this condition by a physician (2). This number likely represents an underestimate of the true prevalence and diagnosis is often made late in the disease course (3).
Over the past decade, a number of national and international clinical practice guidelines that provide clinicians with evidence-based recommendations for the diagnosis and treatment of COPD (1,4,5) have been published. Despite the promulgation of these guidelines, significant care gaps have been reported in COPD management, particularly at the primary care level (6). A telephone survey of 389 individuals with self-reported COPD revealed substantial symptom and psychosocial burden related to living with this disease. In general, respondents had poor knowledge about the causes of COPD and their role in its management, especially during exacerbations (7).
Optimal COPD management requires patients to participate in their care and physician knowledge of patients’ perceptions of their disease. The purpose of the present study was to prospectively collect ‘real world’ data with respect to COPD practice patterns and perceptions among respiratory specialist physicians. A concurrent patient survey enabled comparison of perceptions of COPD between health care providers and their patients.
METHODS
Study design and participants
The present prospective study was conducted between June 15 and October 15, 2010. Physicians were required to complete a structured questionnaire (practice assessment) at study entry and a patient assessment for 15 to 20 consecutive patients with COPD seen during a regularly scheduled visit. These same patients were then invited to complete a separate study questionnaire. Patients and physicians were blinded to one anothers’ responses. Neither group received financial compensation for their involvement in the present study. The questionnaires were developed by the study authors. Because the study was conducted at individual physician clinics across the country, it received research ethics approval from a central review board (Institutional Review Board Services, Aurora, Ontario).
The target recruitment population was general respiratory physicians, regionally distributed across Canada. Physicians whose practices focused in subspeciality areas of sleep medicine, asthma, transplant, pulmonary hypertension and critical care were excluded from the survey. Patients were recruited from the practices of the respiratory physicians during a regularly scheduled visit. Inclusion criteria were either a confirmed diagnosis of COPD based on the definition in the Canadian Thoracic Society (CTS) COPD guidelines (1) or a suspected diagnosis of COPD based on referral from a family practitioner (FP) or nonrespiratory physician specialist. Patients referred by another respiratory physician were excluded.
Questionnaires
Practice profile questionnaire:
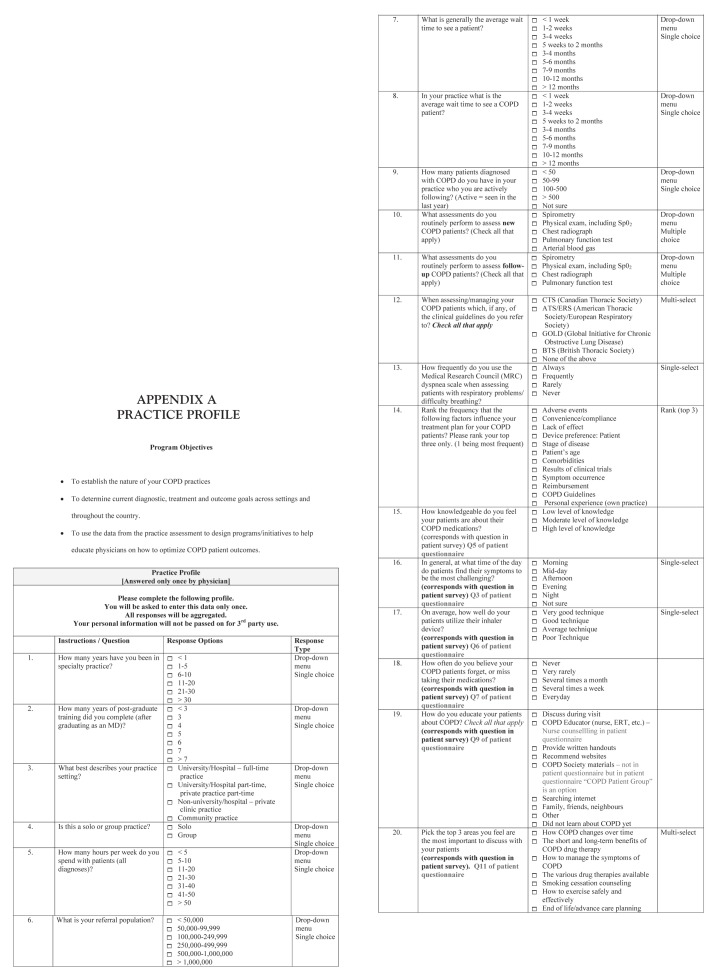
Physicians who consented to participate completed a one-time electronic questionnaire (practice profile) at the beginning of the study. The questionnaire contained 20 questions designed to collect data on the physician’s practice profile (eg, type of practice, practice setting and referral population), characteristics of their COPD patients and physician perceptions of issues relevant to COPD (eg, how knowledgeable patients are about COPD, the effect of dyspnea on patients’ daily lives, diurnal variability of patient symptoms and patient inhaler device technique). Additional details of the physician questionnaire can be found in Appendix A.
Patient questionnaire:
Consecutive patients with COPD from each respiratory physician’s practice were invited to complete an anonymous, paper-based questionnaire consisting of 18 questions. The responses were linked to a physician’s practice but not to a specific patient. The questionnaire was designed to collect data regarding management of the COPD patient population (eg, participation in pulmonary rehabilitation programs and current medication) and also to identify patient perceptions of issues relevant to COPD such as symptoms, knowledge of disease and sources of education. Additional details regarding the patient questionnaire can be found in Appendix B.
COPD patient assessment:
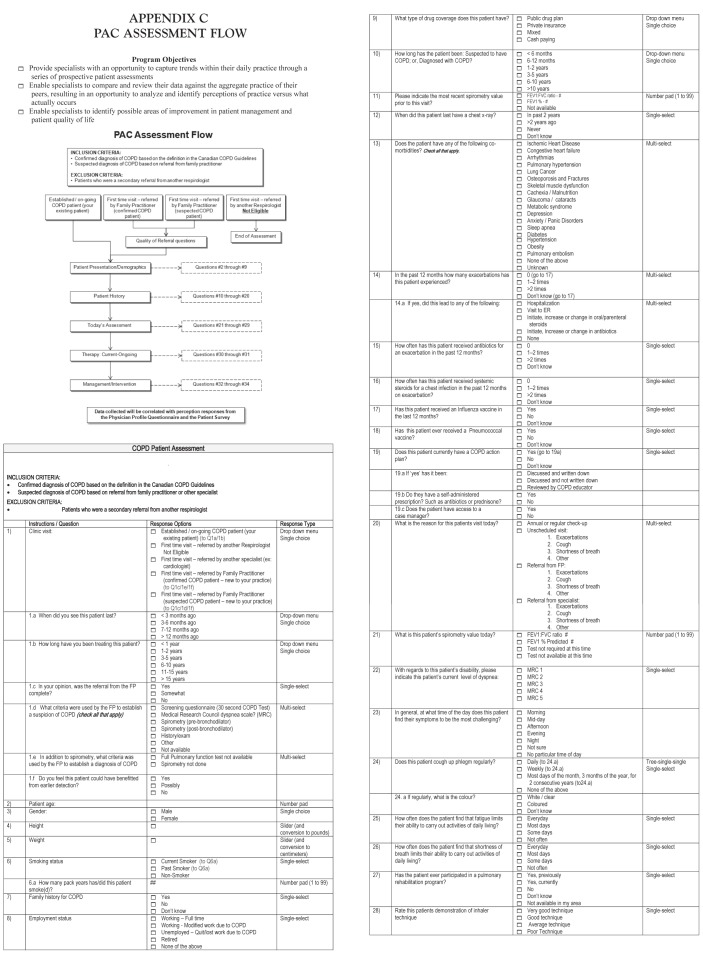
In addition to the patient and physician questionnaires, physicians recorded clinical observations and management details following the visit by their COPD patient. The patient assessment consisted of an electronic questionnaire of 33 questions, details of which can be found in Appendix C.
RESULTS
Of the 601 respiratory physicians in Canada, 58 participated in the present study (9.7%), with good regional representation (eight practiced in British Colombia, four in Alberta, four in Saskatchewan/Manitoba, 18 in Ontario, 18 in Quebec and six in Atlantic Canada). Of the 601 respiratory physicians in Canada, those known by the authors to have a practice profile meeting the exclusion criteria of the study were not invited to participate. All physicians completed practice assessment questionnaires and also completed 931 patient assessments. From among these 931 patients, 640 subsequently completed questionnaires, yielding a response rate of 69%.
Practice profiles of participating physicians
Of the 58 participating respiratory physicians, 19 practiced in a university setting, six in a university/private practice setting and 33 in private practice (private clinic/community). The majority (64%) of respiratory physicians reported actively following (seen in the past year) between 100 and 500 patients with COPD in their practice, 21% reported actively following >500, 12% actively followed <100 and 3% were unsure. Mean wait times for COPD patients were reported to be 10.1 weeks for the four western provinces (n=16), seven weeks for Ontario (n=18), 13 weeks for Quebec (n=18) and 10.8 weeks for Atlantic Canada (n=6). The mean referral population size was estimated to be 700,000 for the four western provinces, 490,000 for Ontario, 680,000 for Quebec and 450,000 for Atlantic Canada.
The COPD clinical practice guidelines most frequently referred to by physicians were those produced by the CTS (91%), the American Thoracic Society/European Respiratory Society (43%) and the Global initiatitive for chronic Obstructive Lung Diseases (43%). The CTS clinical practice guidelines recommend using the Medical Research Council (MRC) dyspnea scale in addition to spirometry to assess COPD severity; physicians reported using the MRC dyspnea scale always (43%), frequently (31%), rarely (24%) and never (2%) during office visits (1). Physical examination, including oxygen saturation, was routinely performed to assess new (100%) and follow-up (98%) patients. In new patients, other routine assessments included chest radiograph (95%), full pulmonary function tests (84%), spirometry (81%) and arterial blood gas (26%). In follow-up patient visits, spirometry was performed in 79% of patients, and chest radiographs and full pulmonary function tests were performed in 21% of patients. Arterial blood gas measurements were not performed.
Patient demographics and clinical characteristics
Demographic data of the COPD patients (n=931) included in the present study are summarized in Table 1. There was a significant number (20% to 32%) of COPD patients who reported a positive family history of COPD, a high percentage of whom were current smokers, particularly among new referrals. The majority (82%) of patients had comorbidities (hypertension [45%], ischemic heart disease [26%], osteoporosis [19%], diabetes [17%] and depression [15%]). The mean number of comorbidities per patient was 2.7. Of the patients with comorbidities, 22% had one comorbidity, 24% had two, 16% had three and 20% had four or more. Based on CTS spirometric definitions of COPD severity, for patients with current spirometry values (n=476), the majority were moderate or severe (mild, n=20 [4%]; moderate, n=204 [43%]; severe, n=192 [40%]; very severe, n=60 [13%]) (1). The majority of patients were moderate using the MRC symptom-based disease severity classification (total n=931; no exertional dyspnea [MRC 1], n=50 [5%]; mild [MRC 2], n=220 [24%]; moderate [MRC 3 to 4], n=582 [63%]; and severe [MRC 5], n=79 [8%]).
TABLE 1.
Demographics of chronic obstructive pulmonary disease (COPD) patients
| Patient type |
Sex
|
Age, years, mean |
Smoking status
|
Family history of COPD | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Current | Former | Never | |||
| Existing (n=727) | 54 | 46 | 69 | 23 | 70 | 7 | 21 |
| Specialist referral (n=32) | 53 | 47 | 67 | 44 | 56 | 0 | 28 |
| FP referral, confirmed diagnosis (n=103) | 44 | 56 | 68 | 39 | 55 | 6 | 20 |
| FP referral, suspected diagnosis (n=69) | 52 | 48 | 67 | 42 | 52 | 6 | 32 |
Data presented as % unless otherwise indicated. FP Family practitioner
Patient visits
Patients reported that they visited their respiratory physicians an average of 2.5 times per year (12% <1 time per year, 58% one to two time[s] per year; 25% three to six times per year; 3% six to 10 times per year, 2% >10 times per year). According to physicians, the main reason for the patient visit on the day that the patient assessment questionnaire was completed was for a regular follow-up for COPD (71%), shortness of breath (17%), exacerbation (10%) and cough (8%).
Symptoms
Shortness of breath: impact on activities of daily living:
Most patients indicated that shortness of breath negatively impacted their routine activities of daily living (eg, meal preparation, bathing, dressing) – rating the degree of impact as extreme (6%), very much (29%), moderate (28%), a little (24%) and not at all (13%). There were slight differences regionally, with patients in Quebec indicating a slightly higher negative impact versus the other regions. Fifty-two per cent of patients reported that shortness of breath affected their activities of daily living every day or most days.
Sputum production:
Sixty-five per cent of patients reported having a cough productive of sputum: daily in 44%, most days in 10% and weekly in 11%. Eighty per cent reported that their sputum was usually clear or white in colour, 17% reported coloured sputum and 3% were uncertain of the colour.
Diurnal variability of symptoms:
Perception of morning as the most challenging time of day for patients due to respiratory symptoms was similarly reported by patients (n=635 [33%]) and physicians (n=931 [29%]). Patients reported that their physicians asked them about diurnal variability of respiratory symptoms very often (8%), often or sometimes (50%), and rarely or never (42%).
COPD exacerbation frequency, severity and management
Of the 931 patients assessed by physicians, 57% (n=527) had experienced an exacerbation of COPD in the previous 12 months. The probability of experiencing an exacerbation increased with increasing COPD severity, regardless of whether it was determined using spirometry or MRC dyspnea score (Table 2). However, even among patients with mild disease, a minority reported a history of recent exacerbation. Exacerbation frequency was similar between follow-up COPD patients and new referrals. Among patients who experienced an exacerbation within the past 12 months, 58% were treated with an antibiotic, 52% with an increased dose or new prescription for oral or parenteral steroids, 41% with both antibiotics and oral steroids, 30% visited an emergency department and 34% were hospitalized. The likelihood of being treated with both antibiotics and systemic steroids increased with increasing COPD severity.
TABLE 2.
Exacerbation frequency in past 12 months according to chronic obstructive pulmonary disease (COPD) severity, determined by spirometry-derived forced expiratory volume in 1 s values and Medical Research Council (MRC) dyspnea score
| Exacerbation in past 12 months |
COPD severity according to spirometry
|
|||
| Mild (n=20) | Moderate (n=204) | Severe (n=192) | Very severe (n=60) | |
|
| ||||
| Yes | 40 | 46 | 67 | 67 |
| No | 60 | 54 | 33 | 33 |
|
| ||||
|
COPD severity according to MRC dyspnea score
|
||||
| Exacerbation in past 12 months | MRC 1 (n=50) | Mild (MRC 2) (n=220) | Moderate (MRC 3–4) (n=582) | Severe (MRC 5) (n=79) |
|
| ||||
| Yes | 32 | 32 | 65 | 81 |
| No | 68 | 68 | 35 | 19 |
Data presented as %
Sixty-one per cent of COPD patients reported that they had been taught to recognize the signs and symptoms of an exacerbation of COPD, most by their physician (51%) and 20% by a COPD nurse educator (some reported being taught by both health care professionals); however, 39% claimed that they had never been taught. Of 640 patients, 248 (39%) reported having a COPD action plan; of these patients, 79% reported having been provided with a written prescription for antibiotics and/or steroids, while only 52% reported having access to review their action plan with a COPD nurse educator. Access to a COPD nurse educator to review action plans varied considerably according to region: lowest in the four western provinces and Ontario, and highest in Quebec and Atlantic Canada.
Regarding preventive measures for exacerbations, there was a higher rate of influenza vaccination in the past 12 months among existing patients who were followed in respiratory specialists’ practices (81%), compared with new patients referred by other specialists (68%), referred by FPs in cases for which COPD was confirmed (76%) and, especially, patients referred by FPs in cases for which COPD was only suspected (45%). Pneumococcal vaccination rates were similarly highest among existing patients followed in respiratory specialists’ practices (70%) versus new patients coming from the three referral sources noted above (50%, 53% and 30%, respectively).
Pharmacotherapy
Current medications prescribed to patients according to COPD disease severity determined by spirometry or MRC dyspnea score are presented in Table 3 and Table 4, respectively. The majority of patients across the disease severity spectrum were prescribed a short-acting bronchodilator, most commonly salbutamol. The number of medications prescribed increased with disease severity, although a small percentage of patients with moderate to severe disease were on no medications for COPD. According to current CTS COPD clinical practice guideline recommendations, inhaled corticosteroid/long-acting beta-agonist (ICS/LABA) combination inhalers (ie, fluticasone/salmeterol or budesonide/formoterol) were overprescribed in patients with mild disease. For patients with a history of one or more exacerbation and at least moderate severity COPD (ie, those at greatest risk for future exacerbations), only 34% were prescribed ‘triple inhaled maintenance therapy’: an ICS/LABA plus a long-acting anticholinergic, as recommended by the CTS COPD clinical practice guidelines.
TABLE 3.
Current medications prescribed to patients with chronic obstructive pulmonary disease (COPD) according to disease severity determined by spirometry
| Current medication |
COPD severity according to spirometry
|
|||
|---|---|---|---|---|
| Mild (n=20) | Moderate (n=204) | Severe (n=192) | Very severe (n=60) | |
| Salbutamol (SABA) | 70 | 74 | 79 | 80 |
| Terbutaline (SABA) | 5 | 2 | 3 | 2 |
| Ipratropium (SAAC) | 10 | 13 | 8 | 3 |
| Tiotropium (LAAC) | 70 | 67 | 78 | 88 |
| Salmeterol (LABA) | 5 | 3 | 3 | 1 |
| Budesonide/formoterol (ICS/LABA) | 25 | 22 | 29 | 18 |
| Fluticasone/salmeterol (ICS/LABA) | 35 | 49 | 54 | 73 |
| Theophylline | 0 | 2 | 11 | 18 |
| Chronic antibiotics | 0 | 1 | 2 | 0 |
| Oral steroids | 5 | 2 | 4 | 8 |
| Other | 25 | 7 | 14 | 25 |
| None | 5 | 3 | 3 | 0 |
Data presented as %. ICS Inhaled corticosteroid; LAAC Long-acting anticholinergic; LABA Long-acting beta2-agonist; SAAC Short-acting anticholinergic; SABA Short-acting beta2-agonist
TABLE 4.
Current medications prescribed to patients with chronic obstructive pulmonary disease (COPD) according to disease severity determined by Medical Research Council (MRC) dyspnea score
| Current medication |
COPD severity according to MRC dyspnea score
|
|||
|---|---|---|---|---|
| MRC 1 (n=50) | Mild (MRC 2) (n=220) | Moderate (MRC 3–4) (n=582) | Severe (MRC 5) (n=79) | |
| Salbutamol (SABA) | 66 | 74 | 78 | 81 |
| Terbutaline (SABA) | 0 | 3 | 3 | 1 |
| Ipratropium (SAAC) | 12 | 11 | 12 | 8 |
| Tiotropium (LAAC) | 34 | 65 | 76 | 95 |
| Salmeterol (LABA) | 2 | 1 | 4 | 2 |
| Budesonide/formoterol (ICS/LABA) | 24 | 22 | 25 | 24 |
| Fluticasone/salmeterol (ICS/LABA) | 26 | 49 | 53 | 72 |
| Theophylline | 2 | 2 | 9 | 21 |
| Chronic antibiotics | 2 | 1 | 1 | 2 |
| Oral steroids | 0 | 4 | 4 | 16 |
| Other | 6 | 7 | 12 | 21 |
| None | 10 | 5 | 2 | 0 |
Data presented as %. ICS Inhaled corticosteroid; LAAC Long-acting anticholinergic; LABA Long-acting beta2-agonist; SAAC Short-acting anticholinergic; SABA Short-acting beta2-agonist
Pulmonary rehabilitation
Regarding pulmonary rehabilitation, 24% of patients responded that they had participated in such a program, 63% had not participated and 13% were not sure what ‘pulmonary rehabilitation’ was. Of the patients who participated (n=132), 41% had very severe COPD, 30% had severe COPD, 23% had moderate COPD and 29% had mild COPD. The majority of patients (68%) had been referred to pulmonary rehabilitation by their respiratory specialist physician, 20% by their family physician, 11% by a respiratory therapist, 5% by a nurse, 2% by another health care professional and 1% by another patient.
Patient education
In terms of how patients were educated about COPD, patients and physicians both reported that COPD education occurred most often during clinic visits (Figure 1A). There were wide gaps in perceived roles of other sources of COPD patient education (eg, COPD nurse educator, written material, handouts, websites) between physicians and patients (Figure 1B). Seventy-one per cent of physicians indicated that they also educated their patients through a COPD educator; however, only 24% of patients availed themselves of this educational resource (Figure 1A). Other educational tools were used even less by patients. The majority of respiratory physicians (70%) indicated that their patients had access to a COPD educator, with the highest percentage in Quebec and the lowest in the Atlantic provinces.
Figure 1).
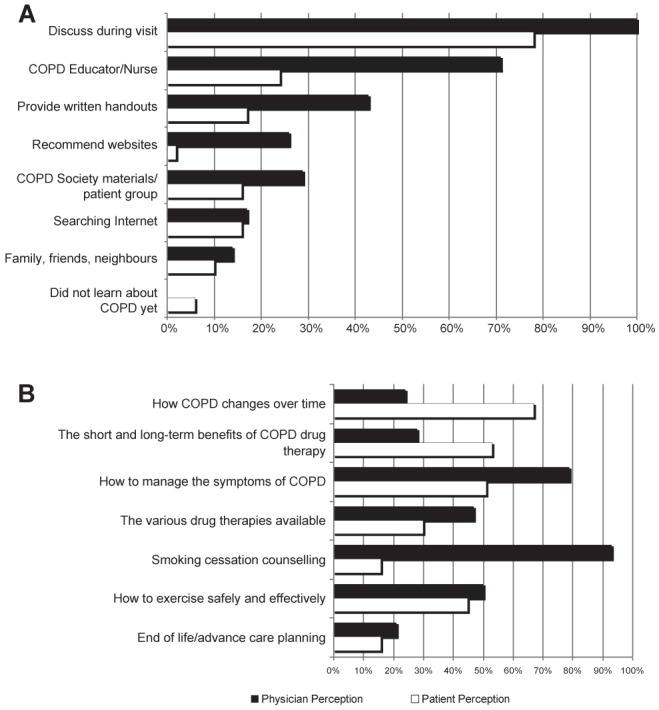
Patient and physician perception of chronic obstructive pulmonary disease (COPD) patient education sources (A) and priorities (B). A Physician question (n=58): How do you educate your patients about COPD? Check all that apply (black bars). Patient question (n=644): How do you educate yourself about COPD? Check all that apply (white bars). B Physician question (n=58): Select the top three areas where you think your patients would like to receive more information (black bars). Patient question (n=644): Select the top three areas where you would like to receive more information (white bars)
Although both groups agreed that COPD patient education was important, priorities differed between patients and physicians. The top three educational priorities for patients were how COPD changes over time, the short- and long-term benefits of COPD drug therapy and how to manage the symptoms of COPD. Among patients who selected smoking cessation counselling as an educational priority, 39% were current smokers. The top three educational priorities for patients as perceived by respiratory specialist physicians were smoking cessation and counselling, how to manage the symptoms of COPD, and how to exercise safely and effectively.
Whereas 23% of patients believed that their knowledge level about COPD was high, physicians perceived a high knowledge level among only 5% of their patients. Two specific areas in which gaps existed in perceived knowledge between patients and physicians related to adherence to taking COPD medications and inhaler technique. Eighty-four per cent of patients reported that they rarely or never forgot or missed taking their COPD medications, while physicians believed that 80% of their patients forgot or missed taking their COPD medications several times per week to several times per month. In terms of inhaler use, 78% of patients reported that they had very good or good technique, while physicians perceived very good or good technique in only 35% of their patients. Among health care professionals, patients reported that nurse educators were the most likely to directly observe their inhaler technique (Figure 2). A small percentage of patients (2%) responded that they had never been taught proper inhaler technique.
Figure 2).
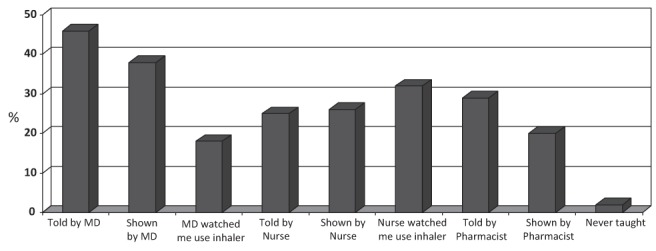
Patient education. Patient response regarding how and by whom they received instruction on proper inhaler technique. MD Physician
DISCUSSION
The present study assessed practice patterns and perceptions of COPD among respiratory specialist physicians and their COPD patients in a real-world setting. Patients reported important symptom burden and frequent exacerbations, increasing with disease severity. There was both under- and overprescription of maintenance inhaled therapies according to current clinical practice guidelines. Access to nonpharmacological therapies, such as COPD education resources and pulmonary rehabilitation in particular, was low and varied according to region. Perceived knowledge needs and preferred educational resources differed between physicians and patients.
Smoking cessation and counselling was most frequently identified by physicians as an educational priority for their patients. Despite relatively high current smoking rates among patients who participated in the present study, few patients identified this as a perceived knowledge need. Patients wanted to know more about COPD disease progression. Linking these two issues (ie, the role that smoking cessation plays in slowing disease progression) in discussion between patient and health care provider may increase the perceived importance of smoking cessation among patients.
COPD exacerbations were frequent among study patients, which places these patients at a higher risk for future exacerbations (8). The probability of experiencing an exacerbation increased with increasing COPD severity. However, consistent with other studies, even patients with ‘mild’ disease, regardless of whether assessed using symptoms or spirometry criteria, had experienced recent exacerbations (8). This apparent discrepancy validates aspects of COPD phenotyping suggested in recent COPD clinical practice guidelines – namely, that symptom burden and future risk of exacerbations should be separately assessed and managed in each patient (1,5). This fact, combined with the high prevalence of comorbidities in the study participants, emphasizes the complexity of COPD characterization, which extends beyond simply assessing degree of airflow obstruction by spirometry.
When compared with recommendations in the CTS COPD clinical practice guidelines, potential care gaps were exposed regarding interventions to prevent future COPD exacerbations. Influenza and pneumococcal vaccination rates were suboptimal. The use of a COPD action plan has previously been demonstrated to be most effective in reducing health care utilization related to exacerbations when a written prescription is provided to patients and used in combination with access to a COPD educator (9). However, fewer than 50% of patients in the present study had a written COPD action plan and only a minority of these patients had access to review this tool with a COPD educator. Furthermore, fewer than 40% of patients had ever been taught to recognize the signs and symptoms of an exacerbation. For patients at highest risk for future exacerbations, only 34% were prescribed triple-inhaled maintenance therapy (ie, ICS/LABA plus a long-acting anticholinergic). In contrast to this example of underprescription of COPD medications, there was also evidence of potential overprescription of maintenance inhaled medications in patients with mild disease.
Care gaps may result not only in worsened patient-related outcomes, but also in increased health care costs (10). Although COPD care gaps have been well described in primary care, respiratory specialist physicians are less frequently the target of care gap analysis and subsequent knowledge translation efforts (6). Barriers to adopting current evidence-based knowledge into clinical practice can exist at multiple levels, including the individual practitioner and the health care system (11). More than 90% of physicians who participated in the present study identified the CTS COPD guidelines as an information resource they referred to in the management of their COPD patients. Lack of knowledge appears unlikely to have been a major barrier to this group of respiratory specialist physicians. Other individual factors, such as lack of time, disagreement with guideline recommendations, forgetfulness or lack of awareness of local resources, may have played a role.
Alternatively, systemic barriers may have dominated as the explanation for the observed potential care gaps. Lack of access to resources was certainly an important factor contributing to under-referral of patients to COPD educators and pulmonary rehabilitation. In addition, there appears to be a need to validate the important role of COPD educators and other educational tools among patients as part of their COPD management strategy. Insurance coverage rules for medications, whether through a public or private system, may have influenced the prescribing patterns of physicians who deviated from evidence-based guidelines. Implementation efforts to narrow the care gaps in COPD will require multifaceted, evidence-based approaches targeting both individual clinicians and systemic barriers (12). The results of the present study help to identify a problem among COPD patients cared for by respiratory specialist physicians, thus warranting the attention of future knowledge translation programs by organizations such as the CTS.
Limitations of the present study include the possibility of selection bias among individuals that chose to participate, resulting in a non-representative selection of Canadian respiratory specialist physicians and their patients. Respiratory physicians with a particular interest in COPD may have been more likely to participate, thus, introducing a self ‘selection bias’. This may account for some practice behaviours reported in the present study being better than the perception of ‘actual’ practice patterns in Canada (eg, use of MRC dyspnea score to rate disease severity, referral rates to pulmonary rehabilitation). It is, therefore, conceivable that rates of potential care gaps may have been even greater without this selection bias. The study methodology did not allow for an independent audit of patient charts. Physicians may have self-reported practice behaviour consistent with guidelines that was different from their actual practice behaviour. The latter limitation would likely have served to bias the results toward an underestimation of the actual care gap. Adherence to guidelines for management decisions, such as pharmacotherapy choices, was assessed based on COPD severity. Recent spirometry results were not always available; however, MRC symptom score was provided for all patients to enable assessment of severity. Finally, the study authors did not return to the study physicians with the results of the analyses to further explore potential barriers to implementation of guideline recommendations in their individual practices.
The present study showed that despite widespread dissemination of COPD clinical practice guidelines, potential care gaps among patients managed by respiratory specialist physicians remain. Differing perceptions about many aspects of COPD among physicians and patients may contribute to these care gaps. Respiratory specialist physicians are another important target group for future knowledge translation interventions. Changes in the health care system will also likely be necessary to bridge the gap between evidence-based clinical knowledge and real-world clinical practice – only then will our COPD patients and society derive maximal benefits from growing scientific knowledge about the management of this disease.
ACKNOWLEDGEMENTS/DISCLOSURES:
The authors thank the physicians and patients who participated in this COPD practice assessment. This study was supported by AstraZeneca Canada Inc. Statisticians working at AstraZeneca Canada Inc. undertook the initial data analysis; however, authors had complete access to the data and independently conducted data interpretation. Miriam Banner of inScience Communications, Springer Healthcare provided editing assistance to PH with the initial draft, funded by AstraZeneca; however, the authors otherwise had complete control and independence over the content and subsequent drafts of the manuscript. All of the listed authors substantially contributed to study design, analysis and interpretation of data, revising the manuscript critically, and providing approval of the submitted version of the manuscript.
APPENDIX A. PRACTICE PROFILE
APPENDIX B. PRACTICE ASSESSMENT IN COPD
Thank you for volunteering to answer some questions concerning your COPD. We will be presenting the combined responses to these questions to a group of physicians who are developing an educational program to help physicians better treat and manage patients with COPD. Please note that your responses are completely confidential and at no time will you be identified in this program. Your responses will NOT be seen by your physician at any time as you will place this form into the envelope provided. Your responses will be grouped with those of other patients from across Canada for the purpose of Continuing Education.
Please be sure to fill the bubbles in completely for the options you select:
Below are some questions related to how you feel at this moment:
-
1. How does shortness of breath impact your ability to complete daily living activities e.g. meal preparation, bathing, dressing etc.? (Select one)
○ Not at all ○ A little ○ Moderately ○ Very much ○ Extremely
-
2. Regarding daily living activities, how often does your physician ask about what time of day your COPD is most challenging for you? (Select one)
○ Never ○ Rarely ○ Sometimes ○ Often ○ Very Often
-
3. In general, at what time of day do you find your symptoms to be the most troublesome? (Select one)
○ Morning ○ Mid-day ○ Afternoon ○ Evening ○ Night ○ Not sure
Below are some questions related to your knowledge of COPD and the medication you are taking:
-
4. How long have you been taking a prescription medication to control your COPD? (Select one)
○ Less than 6 months ○ 6–12 months ○ 1 year to 2 year 11 months ○ 3–5 years ○ More than 5 years
- 5. How knowledgeable do you feel about your COPD medications? (Select one)
- ○ Low level of knowledge
- ○ Moderate level of knowledge
- ○ High level of knowledge
- 6. On average, how well do you think you use your inhaler device? (Select one)
- ○ Very good technique
- ○ Good technique
- ○ Average technique
- ○ Poor technique
- 7. How often do you forget, or miss taking your COPD medications? (Select one)
- ○ Never
- ○ Very rarely
- ○ Several times a month
- ○ Several times a week
- ○ Everyday
- 8. On average, how often do you see your physician/respirologist for your COPD condition? (Select one)
- ○ Less than once per year
- ○ 1–2 times per year
- ○ 3–6 times per year
- ○ 6–10 times per year
- ○ More than 10 times per year
- 9. How do you learn more about COPD? (Select all that apply)
- ○ Physician discusses
- ○ Nurse counseling
- ○ Physician/team provides
- ○ Physician/team recommends during visit
- ○ Written handouts
- ○ Websites
- ○ COPD Patient Group
- ○ Searching the internet
- ○ Family, friend, or neighbour
- ○ Other
- ○ Did not learn about COPD yet
- 10. How were you taught to use your COPD medications? (Select all that apply)
- ○ Told by my Doctor
- ○ Shown by my Doctor
- ○ Told by a Nurse Educator
- ○ Shown by a Nurse Educator
- ○ The Doctor watched me use it correctly
- ○ A Nurse educator watched me use it correctly
- ○ Told by my pharmacist
- ○ Shown by my pharmacist
- ○ Other
- ○ Never taught
- 11. Select the top 3 areas where you would like to receive more information.
- ○ How COPD changes over time
- ○ How to manage COPD symptoms
- ○ The short and long-term benefits of COPD drug therapy
- ○ Various drug therapies
- ○ Smoking cessation counseling
- ○ How to exercise safely and effectively
- ○ End-of-life/advanced care planning
- 12. Have you been taught to recognize the signs of an exacerbation (i.e. symptom worsening, increase of medication, hospitalization) of your COPD?
- ○ Yes, by my doctor
- ○ Yes, by a nurse educator
- ○ No
- 13. Do you currently have a plan of action to self-manage exacerbations?
- ○ Yes
- ○ No
If you do not have an action plan, please skip questions 14–16
- 14. Regarding your action plan, please select one of the two options below:
- ○ Discussed with healthcare provider and written down
- ○ Discussed with healthcare provider and not written down
- 15. Do you also have a prescription for antibiotics or prednisone that you can fill when needed?
- ○ Yes
- ○ No
- 16. Does your action plan include access to a case manager (i.e. respiratory therapist, nurse)?
- ○ Yes
- ○ No
- 17. Have you participated in a pulmonary rehab program?
- ○ Yes
- ○ No
- ○ Not sure what this is
- 18. Who referred you to pulmonary rehab?
- ○ Respirologist
- ○ Family Physician
- ○ Respiratory Therapist
- ○ Nurse
- ○ Another patient
- ○ Website
- ○ Other healthcare professional
- ○ Other
Please, return this form to Isis Healthcare (3365 Harvester Road, 2nd Floor, Burlington, ON L7N 3N2) in the prepaid envelope provided.
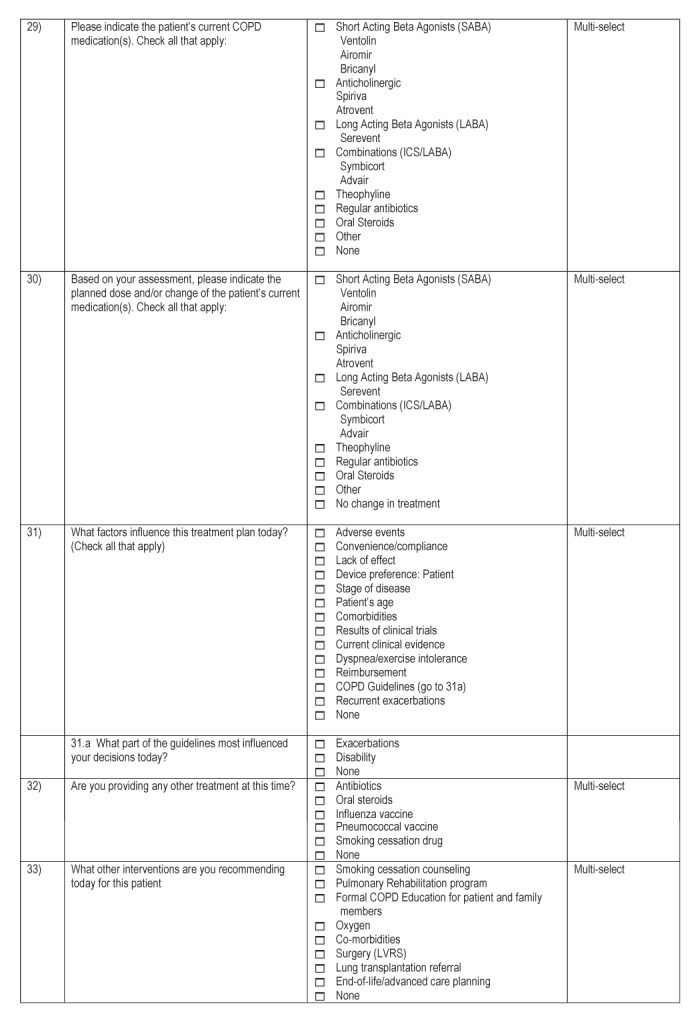
APPENDIX C. PAC ASSESSMENT FLOW
Footnotes
CONFLICT OF INTEREST STATEMENTS: Paul Hernandez has participated on medical advisory boards for pharmaceutical companies, as a researcher on industry funded clinical trials, and/or in the development of material and as a speaker for industry funded continuing medical education for the following companies: Actelion, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Nycomed and Pfizer. Meyer Balter has served on medical advisory boards and has received honoraria for speaking and/or contributing to the development of materials to be used for CME programs for the following pharmaceutical companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Nycomed and Pfizer. Jean Bourbeau has participated on medical advisory boards, conducted continuing health education activities and/or industry-sponsored clinical research trials for the following companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer and Talecris. Charles Chan has served on advisory boards or speaker bureau for the following companies: Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, InterMune, Merck and Pfizer. Darcy Marciniuk has undertaken Consulting or participated on Advisory Boards for the Canadian Lung Association, Health Canada, Health Quality Council, Public Health Agency of Canada, Saskatchewan Medical Association and Saskatoon Health Region. He has received research support (directed to the University of Saskatchewan) from AstraZeneca, Boehringer Ingelheim, Canadian Agency for Drugs and Technology in Health, Canadian Institute of Health Research, GlaxoSmithKline, Forest, Lung Association of Saskatchewan, Novartis, Nycomed, Pfizer, Saskatchewan Health Research Foundation, Saskatchewan Ministry of Health and Schering-Plough. He holds Fiduciary Positions with the Canadian COPD Alliance, American College of Chest Physicians, Chest Foundation, and Lung Association of Saskatchewan; and is an employee of the University of Saskatchewan. Shannon Walker has participated on medical advisory boards for pharmaceutical companies, and/or in the development of material and as a speaker for industry funded continuing medical education for the following companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Nycomed and Pfizer.
REFERENCES
- 1.O’Donnell DE, Hernandez P, Kaplan A, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2008 update – highlights for primary care. Can Respir J. 2008;(15 Suppl A):1A–8A. doi: 10.1155/2008/641965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Respiratory Disease in Canada. <www.phac-aspc.gc.ca/publicat/rdc-mrc01/pdf/rdc0901e.pdf> (Accessed December 4, 2007).
- 3.Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182:673–8. doi: 10.1503/cmaj.091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli B, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 5.Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease (revised 2011). Global initiative for chronic Obstructive Lung Disease (GOLD). <www.goldcopd.com> (Accessed February 29, 2012).
- 6.Bourbeau J, Sebalt RJ, Day A, et al. Practice patterns in the management of COPD in primary practice: The CAGE study. Can Respir J. 2008;15:13–19. doi: 10.1155/2008/173904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez P, Balter M, Bourbeau J, Hodder R. Living with COPD: A survey of patients’ knowledge and attitudes. Respir Med. 2009;103:1004–12. doi: 10.1016/j.rmed.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 9.Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: A disease-specific self-management intervention. Arch Intern Med. 2003;163:585–91. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 10.Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J COPD. 2012;7:201–9. doi: 10.2147/COPD.S25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimshaw J, Eccles M, Tetro J. Implementing clinical guidelines: Current evidence and future implications. J Contin Educ Health Prof. 2004;(24 Suppl 1):S31–7. doi: 10.1002/chp.1340240506. [DOI] [PubMed] [Google Scholar]
- 12.Graham ID, Logan J, Harrison MB, et al. Lost in translation: Time for a map? J Contin Educ Health Prof. 2006;26:13–24. doi: 10.1002/chp.47. [DOI] [PubMed] [Google Scholar]