Abstract
The first successfully recorded event-related potential (ERP) for taste, one of our basic senses, was published nearly half a century ago. Despite this large time span, surprisingly little is known about the early neural processing of taste perception. Here, we are providing a comprehensive and critical overview of over four decades of research, with a focus on the temporal dimension of cerebral taste processing in healthy humans. For this purpose, we review studies using techniques that permit a high temporal resolution, namely, electroencephalography and magnetoencephalography, ERP, and event-related magnetic fields (ERF). Our current knowledge of taste ERP is interpreted in the context of our understanding of other, nonchemical senses. Gaps in the existing literature are identified and discussed. Finally, we suggest directions for future investigations using gustatory ERP/ERF.
Keywords: Taste, EEG (ERP), MEG (ERF), Event-related responses
Cortical Taste Processing
Compared to the other sensory systems, relatively little is known of how the human brain processes taste. This is surprising, given the central role of gustation in food intake. The sense of taste provides a gateway for energy and nutrient balance and, as such, is instrumental in the selection of foods. Information conveyed via the gustatory system aids in identifying edible and nutritious foods sources, enables us to avoid poisonous substances, as well as drives the hedonic evaluation of potential nutrition, which can take place prior to, during, or after ingestion. Therefore, gustation serves a vital function that calls for efficient coupling of perception and behavior in order to allow a fast behavioral response, e.g., spitting out a potentially noxious food. It is for this reason, among others, that the interest in understanding gustatory processing is growing, not only for basic science, but also for clinical applications and the consumer industry.
While some of the aforementioned taste-related processes can be performed at the level of the brain stem, taste afferents project to and activate various regions of the neocortex where the intensity, quality, and reward value of taste are processed. Most of our current understanding of the cortical areas involved in human taste perception has been derived from functional neuroimaging studies using either functional magnetic resonance imaging (fMRI) or positron emission tomography (PET). These studies have shown that liquid oral stimuli activate several subdivisions of the insula, orbitofrontal cortex (OFC), and anterior cingulate cortex (Kinomura et al. 1994; Zald et al. 1998; Frey and Petrides 1999; de Araujo et al. 2003; Schoenfeld et al. 2004). According to a recent meta-analysis of all available gustatory functional imaging papers (Veldhuizen et al. 2011), a significant and widespread probability of activations were observed in the bilateral insula and overlying opercula, left lateral and right medial OFC, and the pregenual cingulate cortex, indicating that these regions are reliably and consistently activated in response to gustatory stimulation. The human insula has been associated with the sensory processing of taste, such as the intensity (Grabenhorst et al. 2008) and quality (Schoenfeld et al. 2004) of a taste. It is hence commonly referred to as the primary taste area. The OFC has been linked to the processing of hedonic aspects of taste processing (Kringelbach et al. 2003; McCabe and Rolls 2007) and is often referred to as the secondary taste area. However, cortical activation patterns change rapidly, within the millisecond time range. Thus, the slow and aggregated hemodynamic measures acquired using fMRI and PET have clear and inherent problems with providing insights into the dynamics of the early cerebral processing of gustatory stimuli. In order to study how the brain processes taste within the first second after stimulation in healthy humans, one must use either one of two techniques that provide excellent temporal resolution: electroencephalogram (EEG) or magnetoencephalogram (MEG).
This review focuses on the temporal dimension of gustatory processing. For this, we provide a critical overview of over 40 years of research on gustatory perception conducted with EEG/MEG (for an overview, see Table 1). We will discuss the particular demands on stimulus delivery that result from gustatory EEG/MEG, and we present a brief overview of the various techniques used to date. We discuss our current knowledge of gustatory event-related brain responses in the context of our understanding of other, nonchemical senses. Moreover, we highlight inconsistencies and gaps in the existing literature.
Table 1.
Overview of studies reporting gustatory ERP and ERF
| Study | Sample (f/m) | EEG/MEG (sensors) | Delivery apparatus | Flow (mL/s) | Duration (ms) | Amount (mL) | Trials | ISI (s) | Taste | ERP/EMF deflection (latency/source) |
|---|---|---|---|---|---|---|---|---|---|---|
| Funakoshi and Kawamura (1971) | 5 | EEG (1) | Open flow | – | 5,000 | 1 | 40 | Water | 150 ms | |
| 1 M sucrose | 150 ms, 500 –1,500 ms | |||||||||
| 1 M NaCl | 150 ms, 500–1,500 ms | |||||||||
| 1/20–1/160 M tartaric acid | 150 ms, 500–1,500 ms | |||||||||
| 1/200–1/400 M quinine | 150 ms, 500–1,500 ms | |||||||||
| Kobal (1985) | 5 | EEG (12) | Olfactometer | 140 | 200 | 56 | 16 | 50–60 | 42+55% acetic acid | P300, N410, P660, N860 |
| Plattig et al. (1988) | EEG (Cz) | Open flow | 0.75 | 300–1,500 | 0.22–1.12 | 8–16 | 40–50 | NaCl | N1000, P2300 | |
| Tartaric acid | P2300 | |||||||||
| Sucrose | No response | |||||||||
| Quinine HCl | N2300ms | |||||||||
| Water | No response | |||||||||
| Min and Sakamoto (1998) | 10 | EEG (Cz) | Sponge applicator | – | 2,000 | 1.5 | 8 | 60 | 1 M NaCl | P50, P180 |
| 0.1 M sucrose | P50, P180 | |||||||||
| 0.12 M tartaric acid | P50, P180 | |||||||||
| 0.03 M quinine HCl | P50, P180 | |||||||||
| Artificial saliva | P50 | |||||||||
| Fitzsimons et al. (1999) | 7/2 | EEG (Cz) | Electrogustometer | na | 250 | – | 64 | 8 | Tactile | N146, P223, N297 |
| Tactile+electric taste | N141, P204, P410, N485, P557 | |||||||||
| Electric taste | P447, N506, P531 | |||||||||
| Mizoguchi et al. (2002) | 5 (3/2) | EEG (5) | Flow chamber | 2.33 | 400 | 0.93 | 240 | 30 | 0.3 M NaCl | P127 |
| N263 | ||||||||||
| P432 | ||||||||||
| Wada (2005) | 11 | EEG (Cz) | Sponge applicator | – | – | 0.1 | 8 | 30 | 20% glucose | P72–197 |
| 10% NaCl | P84–188 | |||||||||
| Artificial saliva | No response | |||||||||
| Ohla et al. (2009) | 17 (8/9) | EEG (64) | Electrogustometer | na | 1,000 | – | 114 | 12–15 | 11.5 μA | P130, N220, P390 |
| Frontal, frontocentral, centroposterior | ||||||||||
| No response | ||||||||||
| Ohla et al. (2010) | 17 (8/9) | EEG (64) | Electrogustometer | – | 1,000 | – | 106 | 12–15 | 11.5 μA | P134, N219, P390 |
| 360.5 μA | P124, N186, P347 | |||||||||
| Insula+opercula+vmOFC | ||||||||||
| Franken et al. (2011) | 32 (10/22) | EEG (32) | Open flow | 2.45 | 204 | 0.5 | 41 | 8.5–10.5 | 1 M glucose | P1 100–150 ms, N1 150–230 ms, P2 250–300 ms, P3 400–1,000 ms |
| Artificial saliva | P1 100–150 ms, N1 150–230 ms, P2 250–300 ms, P3 400–1,000 ms (at F3/4, Fz, C3/4, Cz), P3 larger for sweet | |||||||||
| Hummel et al. (2010) | 17 (9/8) | EEG (3) | Olfactometer | 133 | 250 | 33.25 | 15 | 25 | 70% acetic acid | – |
| 100% acetic acid | N390, P601 | |||||||||
| Singh et al. (2011) | 17 (7/10) | EEG (5) | “Spray” gustometer | 0.2 | 250 | – | 16 | 13–18 | 200 mM NaCl | – |
| 400 mM NaCl | N506, P718 | |||||||||
| 200 mM MSG | – | |||||||||
| 400 mM MSG | – | |||||||||
| Murayama et al. (1996) | 5 (0/5) | MEG (64) | Open flow | – | 10,000 | – | 2 | – | 10% glucose | N175/bilateral opercula |
| 0.3 M NaCl | N175/bilateral opercula | |||||||||
| Water | N175/no localization | |||||||||
| Kobayakawa et al. (1996a, b) | 6 (3/3) | MEG (64) | Flow chamber | 3.33 | 400 | 1.33 | ~35 | 30 | 1 M NaCl | 130 ms/insula/operculum |
| 3 mM saccharin | 380 ms/insula/operculum | |||||||||
| Gymnemate/3 mM saccharin | No response | |||||||||
| Water | No response | |||||||||
| Saito et al. (1998) | 5 | MEG (64) | Flow chamber | – | 400 | – | 30 | 30 | 1 M NaCl | 83 ms |
| 3 | 3 mM saccharin | 173 ms | ||||||||
| 100 mM, 330 mM, 1 M NaCl | 89 ms | |||||||||
| 3 mM saccharin | 205 ms | |||||||||
| Kobayakawa et al. (1999) | 7 (3/4) | MEG (64) | Flow chamber | 3.33 | 400 | 1.33 | 30 | 1 M NaCl | 155 ms/area G/time of best fit | |
| 3 mM saccharin | 267 ms/area G | |||||||||
| Mizoguchi et al. (2002) | 5 (3/2) | MEG (64) | 2.33 | 400 | 0.93 | 240 | 30 | 0.3 M NaCl | 126 ms, 277 ms, 438 ms | |
| Area G, left central s.+right sup temp g., area G | ||||||||||
| Iwaki et al. (2004) | – | MEG (122) | Electrogustometer | na | 200 | – | >40 | – | 25–64 mA | – |
| Onoda et al. (2005) | 6 (6/0) | MEG (64) | Flow chamber | – | 400 | – | >32 | 30 | 1 M NaCl | 130 ms/bil. insula–parietal operculum |
| Yamamoto et al. (2006) | 7 (2/5) | MEG (122) | Flow chamber | 1.66 | 400 | 0.66 | 40 | 30–60 | 0.05 M citric acid | 112 ms/insula/opercula |
| 0.5 M sucrose | 396 ms/insula/opercula | |||||||||
| Miraculum/0.05 M citric acid | 373 ms/insula/opercula | |||||||||
| Kobayakawa et al. (2008) | 8 (4/4) | MEG (64) | Flow chamber | – | 400 | – | 35 | 30 | 30 mM NaCl | 160 ms/insula/opercula |
| 100 mM NaCl | 160 ms/insula/opercula | |||||||||
| 300 mM NaCl | 160 ms/insula/opercula | |||||||||
| 1 M NaCl | 160 ms/insula/opercula |
EEG, MEG, and Event-Related Responses
EEG is the measurement of minute electrical signals that originate from cortical postsynaptic potentials. EEG can be recorded noninvasively from the scalp at a relatively low cost using electrodes attached to the skin or mounted in an elastic electrode cap placed over the head. Scalp-recorded EEG reflects the summed potentials of millions of synchronously activated neurons (Nunez and Srinivasan 2006). This contrasts with invasive single-unit and multiunit recordings in patient populations, which measure action potentials.
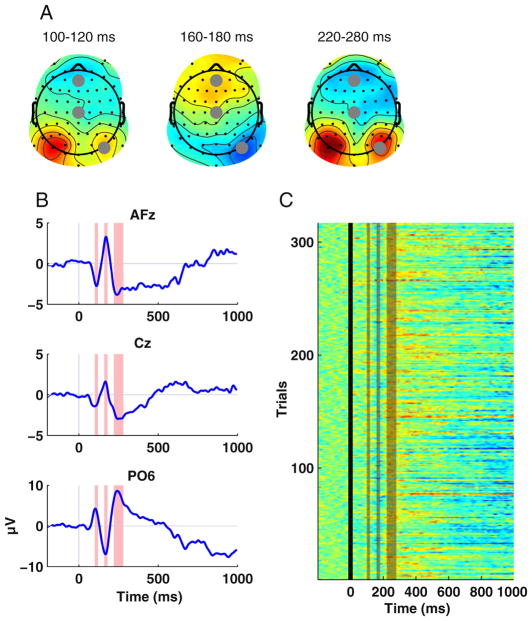
During the first decades after its discovery, analysis of the EEG signal was mostly restricted to qualitative description of the ongoing raw signal (Berger 1929; Adrian and Matthews 1934). Its use for investigating sensory or cognitive processes was limited due to the small amplitude of the evoked or event-related response relative to spontaneous EEG fluctuations and ambient noise. This situation changed, however, when methods were devised for averaging the signals of several trials, using photographic methods and analog or digital computers (for a historical account, see Collura 1995). This averaged signal that is time-locked to the onset of a stimulus is called an event-related potential (ERP)—a waveform with a characteristic sequence of positive and negative peaks. Conventional ERP analyses quantify the waveform’s amplitude, latency, or (if recorded from a sufficient number of electrodes) distribution across the scalp and compare these parameters between experimental conditions (for an excellent introduction, see Luck 2005). Figure 1 illustrates exemplary ERP, topographic maps, and single-trial activity elicited by visual objects.
Fig. 1.
Typical visualization of EEG data using data of a single participant from a visual memory study. a Topographies show the spatial distribution of the ERP across all electrodes for time ranges of interest. In this example, time windows corresponding to the first three ERP peaks were chosen. Gray markers indicate the locations of electrodes for which time courses are displayed below. b Time courses of the ERP at three electrodes. Shaded boxes indicate the time ranges used for visualizing topographies. Note that the polarity of ERP components reverses from frontal to posterior electrodes. c A visualization of single-trial EEG data. Each horizontal line represents a single trial of the experiment. Amplitudes are color-coded. Note the variability of the signal across single trials, which is lost in the averaged ERP
MEG is, in its function and methods, very similar to EEG. MEG is, as EEG, a direct measure of neuronal activity in the cortex. However, in contrast to EEG, which measures electrical activity generated by extracellular currents, MEG measures the corresponding magnetic fields (Cohen 1968). Moreover, while MEG is most sensitive to electrical dipoles with tangential orientation, EEG is equally sensitive to tangential and radial dipoles.
Cognitive neuroscience techniques are often evaluated according to their spatial and temporal resolution. For EEG and MEG, the term “spatial resolution” refers to the precision of techniques that estimate the location of the electrical sources within the brain from the electrical or magnetic signals measured on the scalp. The spatial resolution of MEG and EEG depends strongly on the number and distribution of electrodes or sensors. Furthermore, the spatial resolution of EEG is relatively poor due to the high resistance of the skull and scalp tissue, which causes the electrical field to spread out. Magnetic fields are less prone to distortion by the skull and scalp, thus resulting in a better spatial resolution for the MEG signal relative to the EEG signal
Compared to hemodynamic neuroimaging methods (i.e., PET or fMRI), both EEG and MEG have superior temporal resolution due to the fast fluctuations of the underlying postsynaptic potentials and the fast sampling rate of modern amplifiers (1,000 Hz or faster). In fact, EEG and MEG can be considered real-time measures of brain electrical activity. This represents an important advantage for studies investigating sensation, perception, or cognition since studying when these processes occur is an important tool in assessing the mechanisms of perceptual and cognitive processing. Examples include studies on the speed of visual object recognition (Thorpe et al. 1996), the timing of syntactic and semantic processing in language comprehension (Friederici et al. 1993; Hagoort 2008), and the temporal dissociation of different subprocesses involved in recognition memory (Rugg and Curran 2007) or visual change detection (Busch et al. 2010).
Although temporal resolution is an important advantage of EEG and MEG, it should be noted that the high-frequency fluctuations of electrophysiological signals also pose a limit for what types of perceptual and cognitive processes may be investigated with this method. The small evoked event-related responses in single trials will sum up neatly across trials only if these responses are precisely time-locked and phase-locked to the onset of the experimental event under investigation. This is often not a significant problem for auditory, visual, or somatosensory evoked responses or for well-defined cognitive processes immediately following stimulus onset. However, when the process under investigation occurs at variable times on different trials, this trial-to-trial jitter can severely blur EEG and MEG responses in the averaged ERP and event-related magnetic fields (ERF). Such a jitter may occur, for example, in a gustatory discrimination task or when the effective stimulus onset cannot be determined with millisecond precision, e.g., for the onset of a gustatory stimulus. In such cases, hemodynamic imaging methods indeed have a better chance to capture these neural processes due to their relative forgiveness of an imprecise stimulus onset and the method of summating responses over a time period of a few seconds.
Gustatory Event-Related Responses
Although the first successful gustatory ERP (gERP) recording was reported almost half a century ago, few gERP publications have emerged since (Funakoshi and Kawamura 1968; Plattig 1969; Funakoshi and Kawamura 1971). It is only very recently that gERP has started to gain appeal as a research and clinical tool used to study gustatory function in health (Singh et al. 2011) and disease (Hummel et al. 2010). This is surprising, given how well-established the general ERP technique is for objective and noninvasive assessments of sensory and cognitive functions. In the gustatory domain, only a marginal number of ERP studies have been conducted and the morphometry of gERP has yet to be fully characterized. Moreover, the cortical generators of gERP components remain entirely unknown. To illustrate the discrepancy between our current understanding of the gERP with that of our other, nonchemosensory modalities, a search in September 2011 of the research publications database PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) rendered a total of 40 unique hits using the keywords “gustatory,” “EEG,” and “humans.” In comparison, replacing “gustatory” with “visual” rendered 10,337 hits, for “auditory” 7,088 hits, and for “somatosensory” a total of 3,135 hits. Although one should not equate quantity with quality, there is an obvious imbalance in what we know about the sensory ERP between the various senses.
State of the Current Research
The ERP is commonly described in terms of a series of peaks, also called deflections or components, which can be linked to various perceptual or cognitive processes. Traditionally, peaks are labeled according to their polarity, i.e., P for positive-going and N for negative-going peaks, and their position in the sequence, e.g., P1 for the first observed positive peak, or their latency, e.g., P130 for a positive peak at 130 ms. The earliest ERP peaks are referred to as sensory or exogenous deflections because they are influenced mostly by physical properties of the stimulus; their time course and topographic distribution are specific to the sensory system. Later peaks, on the other hand, denoted as cognitive or endogenous components, can be elicited independent of the sensory system and exhibit a common topography, e.g., the P3, which exhibits its maximum over the vertex and which reflects stimulus evaluation and categorization, rather than sensory stimulus processing (Polich 2007).
Early sensory ERP deflections to taste have been rarely described, probably because they are transient deflections with steep flanks, thus making them vulnerable to minuscule jitters in stimulus timing. For the first positive gustatory deflection, the P1, peak latencies around 130–150 ms for salt (Mizoguchi et al. 2002; Wada 2005), glucose (Wada 2005), and electric taste (Ohla et al. 2009, 2010) have been consistently reported. In the latter study, the P1 deflection was most obvious over frontal electrodes and its neuronal origin was estimated to be in the left insula and adjacent middle temporal gyrus, the ventromedial OFC, and the anterior cingulate cortex (Ohla et al. 2010). These findings for P1 ERP latencies and localization are in agreement with observations from MEG studies showing that the bilateral insula responds to various tastes starting as early as 80–130 ms for salty and sour tastes (Kobayakawa et al. 1996a; Mizoguchi et al. 2002; Onoda et al. 2005; Yamamoto et al. 2006). The first negative gustatory deflection, the N1, has seldom been reported in the literature. To date, the most solid evidence for a clear N1 deflection has only been observed for electric taste at 200 ms, with a minimum over the vertex and neuronal generators in the bilateral insula and OFC (Ohla et al. 2009, 2010), and for salt at 265 ms at the vertex (Mizoguchi et al. 2002). Other reports of early (<250 ms) ERP deflections during tasting do indeed exist (Funakoshi and Kawamura 1968, 1971; Min and Sakamoto 1998; Franken et al. 2011). Their interpretation is, however, hampered due to concomitant gustatory and tactile stimulation.
Disregarding the latency of the deflections, an initial positive peak (P1) has been reported at latencies later than 500 ms poststimulus onset (Funakoshi and Kawamura 1971; Kobal 1985; Plattig et al. 1988; Hummel et al. 2010; Singh et al. 2011). Whether these late positive peaks reflect similar processes to the early positivity or whether these peaks are due to variation in stimulus onset between studies remains to be determined. It is conceivable that the early peaks were dismissed in these investigations because of miniscule trial-to-trial jitters of the taste stimulation or an insufficient signal-to-noise ratio (SNR). Alternatively, an undetermined delay in the taste delivery itself can account for a delayed positive peak. Additional evidence, for example, the voltage distribution of the deflections over the scalp or its cortical generators, will be needed to answer this question and to dissociate these delayed initial positivities from the P3 component, a marker of higher, cognitive stimulus processing.
Intensity-dependent shifts of the waveform toward shorter latencies and higher amplitudes have been previously found for early sensory ERP deflections, such as stimulus intensity in audition (Rapin et al. 1966) and stimulus contrast in vision (Spekreijse et al. 1973). Likewise, it has been suggested that the concentration of a tastant is signaled by the firing rate of taste-responsive cells (Ganchrow and Erickson 1970; Scott and Perrotto 1980), resulting in an increase of the amplitude of the evoked potential at the scalp. However, previous attempts to investigate the effects of taste intensity yielded inconsistent findings in that shorter latencies and higher amplitudes were observed when the concentration of acetic acid was increased (Hummel et al. 2010), while increases in salt concentration led to augmented amplitudes only (Saito et al. 1998; Kobayakawa et al. 2008).
Understanding when and where stimulus intensity is encoded in the brain has led researchers closer to identifying the primary sensory area. To date, two opposing opinions exist about the location of the primary gustatory area; evidence from functional imaging studies has led to the assumption that the transition of the insula and frontal operculum is the primary taste area (Small et al. 1999; O’Doherty et al. 2001). Evidence from MEG studies, in contrast, suggests that the transition between the parietal operculum and insula hosts the primary taste area because this area responded to taste with the shortest latencies (Kobayakawa et al. 1996a, 2005) and the observation that the frontal operculum, among other cortical areas, was activated only a few hundred milliseconds after initial activation in the parietal operculum/insula (Kobayakawa et al. 1999).
However, only the anterior and mid-insula and the overlying frontal operculum have been shown to receive taste afferents from the thalamus in the macaque, while there is no evidence for a taste projection from the thalamus to the parietal operculum (Pritchard et al. 1986). In line with this anatomical evidence, a recent EEG study found pronounced activations of the bilateral anterior insula and adjacent frontal operculum as early as 70–160 ms (Ohla et al. 2010). Similarly, it has been suggested that the posterior insula/parietal operculum are involved in oral somatosensation and attention to the mouth rather than gustation (Veldhuizen et al. 2007).
Gustatory Event-Related Responses—Why Don’t We Know More?
Progress in our knowledge of early gustatory neural processing has been hampered by several interconnected complications. First and foremost among these is the stimulus control problem. Difficulties range from the requirement of temporally precise stimulus delivery, i.e., a known time and limited variation of stimulus delivery onset to the taste bud fields, to the multimodal nature of liquid stimulation, i.e., concomitant somatosensory stimulation. Besides the technical drawbacks of taste stimulation, other problems exist which obstruct the reliability, comparability, and interpretability of the findings.
The Problem of Stimulus Control
One major constraint for most previous EEG/MEG studies derives from difficulties in controlling the delivery of the taste stimuli to the mouth. Liquid taste solutions are commonly used to elicit a taste percept. However, the exact timing of when a flowing liquid reaches taste bud fields is difficult to determine and to control. Unfortunately, temporal stimulus control is essential for measuring ERP. The timing of the stimulus onset must be temporally precise at a millisecond level in order to provide a good summation of the evoked potential over trials. Besides a very precise onset with limited variation, the stimulus must have a square-shaped stimulus characteristic as much as possible, with short or steep rise and fall times. To elicit reliable and strong taste ERP responses, the stimuli need to be of a rather strong percept. Unfortunately, to produce this strong percept, high concentrations of the tastant are needed. This leads us to the next problem; the need of a taste-free sensation between each stimulus presentation. Whereas it is comparably easy to produce a neutral sensation between stimuli for visual and auditory ERPs, the high concentrations needed to produce strong taste ERPs makes lingering taste difficult to flush away with a tasteless rinse. Similarly, as we will discuss below, these high concentrations also make sensory and neural habituation a nontrivial problem that can only be solved by a lengthy interstimulus interval (ISI) resulting in long, strenuous testing sessions.
Ideally, pure and unimodal taste stimuli should be delivered. Unfortunately, this is not a trivial task, given the inherent multisensory nature of liquid taste stimulation. Taste stimuli delivered to the surface of the tongue often produce not only a gustatory sensation, but also elicit a touch- or temperature-related percept. At the scalp, these latter sensations may result in a somatosensory EEG response superimposed on top of the gustatory response. The contributions of these modalities may be parceled out, but only if the individual unimodal responses are known. For example, the tactile response can be recorded when water or artificial saliva is presented in an identical manner as the taste stimuli and the resulting waveform can then be subtracted from the combined somatosensory–gustatory ERP, resulting in an estimate of a relatively pure gustatory response. Alternatively, somatosensory responses could be controlled for with a constant somatosensory stimulation that some methods allow. These constant stimuli desensitize/habituate the somatosensory sensory system while, in theory, leaving the gustatory system unaffected (Kobayakawa et al. 1996b; Singh et al. 2011). Various taste stimulation methods have been developed in recent decades (Table 2), each of which meets the aforementioned requirements to a greater or lesser extent.
Table 2.
Gustatory stimulation techniques
| Stimulation | Unimodal | Rise | Lateralized stimulation |
|---|---|---|---|
| Electrogustometera | Yesb/no | <20 ms | Yes |
| Hinged spoonc | No | Unknown | No |
| Flowing solutionsd | No | Unknown | No |
| Olfactometere | Yes | <20 ms | Yes |
| Sponge applicatorf | No | Unknown | Yes |
| Flow chamberg | Yes | <20 ms | Yes |
| “Spray” gustometerh | Yes | Unknown | No |
At low current densities
The first attempts to measure gERP were made more than 40 years ago using a hinged spoon which delivered a relatively large amount of taste solution upon tilting (Funakoshi and Kawamura 1968, 1971). The authors observed two major ERP components at a temporal electrode, an early one at approximately 150 ms, the tactile response to the liquid touching the tongue’s surface, and a later slow wave peaking in between 500 and 1,500 ms, interpreted as a gustatory perceptual response. However, the later potential might instead be related to higher brain functions such as gustatory recognition. At about the same time, Plattig (1969) used electrogustometry (EGM), i.e., electrical pulses applied to lingual taste bud fields to elicit a unique perception, also referred to as electric taste. This approach allows for good temporal stimulus control and results in near-rectangular stimuli with a steep rise and fall. However, electric taste, when presented at high current densities, activates not only gustatory, but also lingual somatosensory fibers. Moreover, its ecological value has been challenged because it constitutes a unique taste experience, which is difficult to compare to other tastes (Ellegard et al. 2007). More than a decade later, Kobal (1985) modified a constant flow olfactometer to generate fast-rising, nontactile gaseous taste stimuli suited to elicit an ERP with a slow, positive component between 300 and 500 ms over the vertex, in response to different concentrations of acetic acid. Despite the indisputable advantage in the control of stimulus timing, this method has not become established (for an exception, see Hummel et al. 2010). Other taste delivery setups without constant flow, such as open-flow devices (Plattig et al. 1988; Murayama et al. 1996; Franken et al. 2011) and sponge applicators (Min and Sakamoto 1998; Wada 2005), are prone to eliciting a concomitant somatosensory response.
The gustatory delivery system most often reported in the literature is a tactile-free delivery system for flowing tastes with an excellent rise time of below 20 ms, developed by Kobayakawa et al. (1996a, b, 1999a, b, 2005). In this system, the taste stimuli are embedded in a constant flow of tasteless solution where prevention of mixing between taste and rinse is accomplished by insertion of an air bubble in between the two liquids. The authors have repeatedly demonstrated that their gustometer is suitable to elicit unimodal, gustatory ERF (Kobayakawa et al. 1996a, b, 1999, 2005; Onoda et al. 2005). For the first time, their findings have provided temporally precise information regarding activation within the insula cortex for different taste sensations. Only recently, a tactile-free gustometer (GU002, Burghart, Wedel, Germany) became commercially available. This gustometer mimics constant “flow” through a continuous stream of pressure-driven spray pulses to the protruded tongue in which the taste is embedded (Singh et al. 2011). This approach aims to habituate the lingual somatosensory system in order to offer a unimodal, gustatory stimulation. Figure 2 summarizes the results from three studies (Mizoguchi et al. 2002; Ohla et al. 2009; Hummel et al. 2010) using different tactile-free stimulation approaches. It should be noted, however, that the advantages of the flow technique may at the same time reduce the method’s ecological validity. While it is desirable to investigate taste perception separate from somatosensation, these two senses are necessarily intertwined for natural situations outside the laboratory. In this regard, the flow method is unfortunately similar to most stimulation techniques in other senses, which use highly controlled but somewhat artificial stimuli.
Fig. 2.

Event-related responses to electric taste, acetic acid, and salt solution exhibit a relatively similar ERP morphology. Note that the time scales have been approximately matched. Stimulus onset is at 0 ms. a ERPs to electric taste, from 64 electrodes, exhibit a frontal P130, a frontocentral N220, and a late centroposterior positivity (P390). Reproduced from Ohla et al. (2009) with kind permission from Springer Science + Business Media. b The ERP to acetic acid, from electrode Cz, shows four distinct components: P1 at 200 ms, N1 at 350 ms, P2 at 450 ms, and a late positive component. Note that women exhibited shorter latencies and larger amplitudes. Reproduced from Hummel et al. (2010) with permission from BMJ Publishing Group Ltd. c Three major components, at 104 ms (ECD1), at 292 ms (ECD2), and at 392 ms (ECD3), were observed from simultaneously recorded EEG (upper panel) and MEG (lower panel) in a single participant. Dipole modeling revealed the temporal dynamics of the cortical activations, which started in the bilateral insula/ operculum (ECD1), spread to the central sulcus and temporal cortex (ECD2), and returned to the bilateral insula (ECD3) within 400 ms. Reproduced from Mizoguchi et al. (2002) by permission of Oxford University Press
Despite these methodological developments, most stimulation methods are not yet well-suited for clinical assessments of gustatory function using EEG, a method which constitutes an affordable and portable neuroimaging technique available in most clinical institutes. Instead, EGM has been widely used for clinical testing of taste nerve function (Stillman et al. 2003). Despite a lack of detailed understanding of the underlying mechanisms through which these sensations are mediated and the difficulty in excluding secondary trigeminal nerve innervations, EGM has been shown to be a reliable and valid tool for the assessment of taste sensitivity (Fons and Osterhammel 1966; Ajdukovic 1984; Fitzsimons et al. 1999; Stillman et al. 2003; Loucks and Doty 2004; Lawless et al. 2005). Conversely, it was recently demonstrated that electric taste activates the cortical taste pathway rather than somatosensory cortical areas using fMRI (Barry et al. 2001) and EEG (Ohla et al. 2009, 2010). As a result, EGM remains to this day the only clinical tool that is widely used to test central processing of gustatory sensations.
Limited Reliability, Comparability, and Interpretability
Repeated stimulation is needed to average event-related brain responses. However, the gustatory sensory system is much more prone to habituation than the visual and auditory senses. Hence, experiments aiming to record gERP need long ISIs, often in the time range of 20 to 60 s. This time-consuming necessity has forced most previous studies to use a relatively small number of stimulus repetitions in order to keep the duration of the experiment within a reasonable time range. Forcing participants to undergo very long recording sessions would be taxing on their attention and task performance as well as produce limited benefits with respect to experimental power due to subject fatigue. Moreover, small sample sizes (less than ten participants) are common in taste studies, probably also due to the time-consuming nature of the experiments. This inevitably results in a poor SNR, a measure of signal strength relative to the background noise. A high SNR is highly desirable, as it is a requirement for reliable and replicable findings. In order to assess whether or not an ERP is present, it should be statistically differentiated from the level of activity that is present in the baseline interval, i.e., the period prior to stimulus presentation not containing an ERP. In order to allow other researchers to make an informed assessment of the SNR of the presented findings, the ERP, averaged across participants, should be displayed at several electrodes for the time interval of the stimulation, including the prestimulus baseline period.
The use of brief transient stimuli can exacerbate analyses of the waveform due to the overlap of ON and OFF evoked responses, which affects the quantification and interpretability of ERP components. Consequently, stimuli presentation lengths in visual and auditory ERP experiments are often longer than the largest latency of any component analyzed in order to avoid superposition of components (Busch et al. 2004; Ohla et al. 2009). In comparison, olfactory ERPs have often been recorded using comparably very short stimulus durations, with the majority of publications using 250- to 350-ms-long stimuli (Morgan et al. 1999; Lundström et al. 2006). This deviance from the established norm in recordings of visual and auditory ERPs has been explained in the literature by the apparent lack of a pronounced offset ERP for olfactory stimuli, possibly a function of the hypothesized lack of a prominent thalamic neural projection. Whether gERPs also lack an offset ERP response is not known. However, the gustatory neural pathway bears more similarity to the visual and auditory pathways than the olfactory pathway in its early wiring. These anatomical similarities make offset gERPs likely and, if possible, they should be avoided by using longer stimulus duration than what is commonly used for the other chemical senses.
A gustometer is an apparatus that transports liquid tastes to taste bud fields, e.g., the tongue’s surface, after receiving a trigger pulse from the stimulus computer. These mechanical devices experience a time lag between the trigger signal, which, in most cases, operates a valve to release a liquid, and the actual delivery of the liquid to the tongue and a time lag that is dependent not only on numerous parameters, like the length of the tubing, but also on the flow rate and viscosity of the solutions, to mention a few variables. Since the same trigger signal also marks the stimulus onset in the EEG/MEG recordings, through averaging over the trials, the time lag artificially delays the onset of the ERP/ERF by the corresponding value, leading to a misrepresentation of the timing of the event-related waveform and its components. Consequently, the delay between the trigger and the actual stimulus delivery must be determined in order to allow for an exact interpretation of the temporal aspect of the ERP/ ERF components which provides a window into the temporal processing of taste. The delay can be determined, for example, by means of conductivity measurements, as proposed by Kelling and Halpern (1986). Given that a high temporal resolution constitutes an ostensible advantage of ERP/ERF, it is surprising that the time of stimulation has not been assessed in most previous studies (for an excellent exception, see Yamamoto et al. 2006).
Finally, the interpretability of many previous findings is compromised by the limited number of recording electrodes, which hinders conclusions about the signal distribution over the scalp and precludes estimation of the cortical origin of the waveforms. Previously, gERPs have commonly been obtained from a single electrode placed over the vertex (Plattig et al. 1988; Fitzsimons et al. 1999; Wada 2005), although other studies have used slightly more electrodes (Kobal 1985; Min and Sakamoto 1998; Mizoguchi et al. 2002; Hummel et al. 2010; Singh et al. 2011). Nevertheless, the optimal location to record a gERP in its entirety has yet to be determined. In contrast, previous MEG studies have, due to multichannel recordings, convincingly demonstrated that the first component of gustatory processing is generated in the junction of the insula/parietal operculum at roughly 70–170 ms (Kobayakawa et al. 1996a, b, 1999, 2005; Onoda et al. 2005; Yamamoto et al. 2006).
Gustatory Event-Related Responses—The Next Steps
The considerable technical difficulties associated with controlling flowing stimuli are the main reason for our limited understanding of the temporal dynamics of the cerebral processing of gustatory perception. Future studies should aim to identify the spatiotemporal cortical response pattern to taste. To gain an understanding of the morphometry of the gustatory ERP/ERF, additional observations from independent laboratories using different techniques are needed. Specifically, adherence to standards established for reporting ERP research will improve the comparability between studies (Picton et al. 2000).
The ERP—the average over trials time-locked to an experimental event—continues to be the standard method for EEG analysis in studies investigating human perception and cognition. The classical ERP method has, however, been complemented by an ever-growing number of relatively more advanced analysis techniques These techniques take advantage of the monumental increase in computer processing power currently available at affordable prices, as well as new advances in statistical computation. Several techniques are now exploiting the spatial structure of EEG signals recorded with high-density electrode montages. For example, topographic analyses use an advanced statistical framework for mining and integrating temporal as well as spatial information in complex EEG data sets (cf. Murray et al. 2008). Among others, independent component analysis aims at reconstructing physiologically and functionally distinct sources underlying the scalp-recorded EEG signal from the spatiotemporal activity patterns of the raw (unaveraged) EEG signal (Makeig et al. 2004).
Numerous algorithms for source localization, also known as electrical neuroimaging, of EEG signals have been put forward (cf. Michel et al. 2004). The spatial resolution of EEG measures will probably never compete with fMRI due to the problem of factoring out variance in tissue conductivity (the so-called inverse problem; for an in-depth explanation, see Nunez and Srinivasan 2006), and all methods for electrocortical source localization must make simplifying a priori assumptions about the nature of the EEG signal. Nevertheless, source analysis is widely regarded as an important complement to conventional, scalp-based EEG analyses. MEG, which is more sensitive to superficial cortical activity and less prone to distortion from the skull, provides an improved spatial resolution in comparison to EEG. However, MEG detects only tangential sources, i.e., in the sulci, while EEG is sensitive to both tangential and radial components of a current source, i.e., the neural signal both in the sulci and on top of the gyri. In other words, EEG is more sensitive to activity in a greater area of the brain, whereas activity that is visible in MEG can be localized with greater accuracy. In fact, source localization for gustatory ERF has provided valuable insights into the earliest gustatory activation of the cortex, which has been localized to the posterior portion of the bilateral insular cortices and the adjacent posterior opercula (Kobayakawa et al. 1996a, 2005; Onoda et al. 2005; Yamamoto et al. 2006). In fact, a first attempt of reconstructing the cortical origin of gERP over time has been made recently (Ohla et al. 2010). Applied to taste solutions of common taste qualities, the methods offer a promising tool that can be used to gain insight into the spatiotemporal dynamics of cortical taste processing.
In the temporal domain, time–frequency analysis has become an increasingly popular tool for separating the broadband EEG signal into spectral components or frequency bands (Tallon-Baudry and Bertrand 1999; Makeig et al. 2004; Herrmann et al. 2005). Among the many advantages of time–frequency analysis, when applied to the single-trial data, is an increased sensitivity for responses with temporal trial-to-trial jitter compared to the conventional ERP. Recently, studies have begun to abandon averaging across trials altogether and instead analyze the information on a single-trial level (Makeig et al. 2004; Rousselet and Pernet 2011). However, these single-trial analyses have so far only been attempted on data gathered for nonchemical stimuli and it is questionable if single-trial analyses would be feasible for gustatory recordings, given the significantly weaker signal that gustatory sensations produce. Significant advances in both recording and stimulation techniques must emerge before single-trial analyses would be feasible for gustatory EEG signals.
In conclusion, a current trend in the field of EEG/MEG research is the move towards more complex paradigms and more complex methods of data analyses (Rousselet and Pernet 2011). Fortunately, a great number of advanced analysis methods are freely available to the research community as software packages or toolboxes (cf. Baillet et al. 2011). Of course, advanced methods require advanced data sets featuring a greater number of electrodes and/or trials and superior signal quality. On the other hand, cognitive neuroscientists now have at their disposal an arsenal of techniques that provides them with an unprecedented flexibility for choosing the one analysis that suits best for answering a particular experimental question. In order for these methods to be applied to gustatory research, the first and foremost requirement remains the establishment of a precise stimulus delivery apparatus and, by doing so, solving the problem of stimulus control and timing.
Conclusion
Taken together, the existing evidence from gustatory ERP and ERF suggests that the human cortex responds to taste much earlier than initially proposed (Funakoshi and Kawamura 1971; Kobal 1985; Plattig et al. 1988), namely, within less than 100 ms after application of a taste. Due to recent methodological advances, i.e., multichannel recordings and electrocortical source localization algorithms, we have begun to understand the spatiotemporal dynamics of brain activation to taste. Nevertheless, the underlying mechanisms and cortical origins of gERP components remain vague. Future studies need to further elucidate these activation patterns and investigate their susceptibility to taste intensity and different taste qualities. Furthermore, research is needed to assess gender-related differences and hemispheric dominance. Most importantly, we need to further our understanding of the role of cognition, in particular memory and learning, as well as the physiological status, such as hunger, satiety, or body weight, on taste perception and taste evaluation.
Acknowledgments
This work was supported by the National Institute on Deafness and other Communication Disorders—NIDCD (R03DC009869) and the Swedish Research Council (2009-2337) awarded to JNL. The authors are grateful to Andrea Lordan for the valuable comments on an earlier version of this manuscript.
Contributor Information
Kathrin Ohla, Email: kohla@monell.org, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104, USA.
Niko A. Busch, Email: niko.busch@hu-berlin.de, Institute of Medical Psychology, Charité—University Medicine, Berlin, Germany. Berlin School of Mind and Brain, Humboldt University, Luisenstrasse 56, 10099 Berlin, Germany
Johan N. Lundström, Email: jlundstrom@monell.org, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 19104, USA. Department of Psychology, University of Pennsylvania, Philadelphia, PA, USA. Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden
References
- Adrian ED, Matthews BHC. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57(4):355. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Ajdukovic D. The relationship between electrode area and sensory qualities in electrical human tongue stimulation. Acta Otolaryngol. 1984;98(1–2):152–157. doi: 10.3109/00016488409107548. [DOI] [PubMed] [Google Scholar]
- Baillet S, Friston K, Oostenveld R. Academic software applications for electromagnetic brain mapping using MEG and EEG. Comput Intell Neurosci. 2011;2011:972050. doi: 10.1155/2011/972050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MA, Gatenby JC, Zeiger JD, Gore JC. Hemispheric dominance of cortical activity evoked by focal electrogustatory stimuli. Chem Senses. 2001;26(5):471–482. doi: 10.1093/chemse/26.5.471. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektroencephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- Busch NA, Debener S, Kranczioch C, Engel AK, Herrmann CS. Size matters: effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin Neurophysiol. 2004;115 (8):1810–1820. doi: 10.1016/j.clinph.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Busch NA, Fründ I, Herrmann CS. Electrophysiological evidence for different types of change detection and change blindness. J Cogn Neurosci. 2010;22(8):1852–1869. doi: 10.1162/jocn.2009.21294. [DOI] [PubMed] [Google Scholar]
- Cohen D. Magnetoencephalography: evidence of magnetic fields produced by alpha-rhythm currents. Science. 1968;161 (843):784–786. doi: 10.1126/science.161.3843.784. [DOI] [PubMed] [Google Scholar]
- Collura TF. History and evolution of computerized electroencephalography. J Clin Neurophysiol. 1995;12(3):214–229. doi: 10.1097/00004691-199505010-00001. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18(7):2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Ellegard EK, Goldsmith D, Hay KD, Stillman JA, Morton RP. Studies on the relationship between electrogustometry and sour taste perception. Auris Nasus Larynx. 2007;34(4):477–480. doi: 10.1016/j.anl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Fitzsimons M, Sheahan NF, van der Putten W, Malone JF. The application of d.c. electrical stimulation in evoking and recording gustatory brain potentials. Physiol Meas. 1999;20(4):385–400. doi: 10.1088/0967-3334/20/4/306. [DOI] [PubMed] [Google Scholar]
- Fons M, Osterhammel PA. Electrogustometry. Arch Otolaryngol. 1966;83(6):538–542. doi: 10.1001/archotol.1966.00760020540008. [DOI] [PubMed] [Google Scholar]
- Franken IH, Huijding J, Nijs IM, van Strien JW. Electrophysiology of appetitive taste and appetitive taste conditioning in humans. Biol Psychol. 2011;86(3):273–278. doi: 10.1016/j.biopsycho.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Re-examination of the human taste region: a positron emission tomography study. Eur J Neurosci. 1999;11(8):2985–2988. doi: 10.1046/j.1460-9568.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Pfeifer E, Hahne A. Event-related brain potentials during natural speech processing: effects of semantic, morphological and syntactic violations. Brain Res Cogn Brain Res. 1993;1 (3):183–192. doi: 10.1016/0926-6410(93)90026-2. [DOI] [PubMed] [Google Scholar]
- Funakoshi M, Kawamura Y. Summated cortical response to taste stimulation in man. Nihon Seirigaku Zasshi. 1968;30(4):282–283. [PubMed] [Google Scholar]
- Funakoshi M, Kawamura Y. Summated cerebral evoked responses to taste stimuli in man. Electroencephalogr Clin Neurophysiol. 1971;30(3):205–209. doi: 10.1016/0013-4694(71)90055-1. [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Erickson RP. Neural correlates of gustatory intensity and quality. J Neurophysiol. 1970;33(6):768–783. doi: 10.1152/jn.1970.33.6.768. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18(7):1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- Hagoort P. Should psychology ignore the language of the brain? Curr Dir Psychol Sci. 2008;17(2):96–101. [Google Scholar]
- Herrmann CS, Grigutsch M, Busch NA. EEG oscillations and wavelet analysis. In: Handy TC, editor. Event-related potentials—a methods handbook. MIT; Cambridge: 2005. pp. 229–259. [Google Scholar]
- Hummel T, Genow A, Landis BN. Clinical assessment of human gustatory function using event related potentials. J Neurol Neurosurg Psychiatry. 2010;81(4):459–464. doi: 10.1136/jnnp.2009.183699. [DOI] [PubMed] [Google Scholar]
- Iwaki S, Yamamoto C, Tonoike M, Yamamoto T. Rejection of stimulus-related MEG artifacts using independent component analysis. Neurol Clin Neurophysiol. 2004;2004:17. [PubMed] [Google Scholar]
- Kelling ST, Halpern BP. The physical characteristics of open flow and closed flow taste delivery apparatus. Chemical Senses. 1986;11:89–104. [Google Scholar]
- Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, Yamaguchi T, Matsui H, Miyazawa H, Itoh H, et al. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 1994;659(1–2):263–266. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- Kobal G. Gustatory evoked potentials in man. Electroencephalogr Clin Neurophysiol. 1985;62(6):449–454. doi: 10.1016/0168-5597(85)90055-3. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, Takeda T, Saito S, Ogawa H. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett. 1996a;212(3):155–158. doi: 10.1016/0304-3940(96)12798-1. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Saito S, Ayabe-Kanamura S, Kikuchi Y, Yamaguchi Y, Ogawa H, Takeda T. Trial measurements of gustatory-evoked magnetic fields. Electroencephalogr Clin Neurophysiol Suppl. 1996b;47:133–141. [PubMed] [Google Scholar]
- Kobayakawa T, Ogawa H, Kaneda H, Ayabe-Kanamura S, Endo H, Saito S. Spatio-temporal analysis of cortical activity evoked by gustatory stimulation in humans. Chem Senses. 1999;24 (2):201–209. doi: 10.1093/chemse/24.2.201. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Wakita M, Saito S, Gotow N, Sakai N, Ogawa H. Location of the primary gustatory area in humans and its properties, studied by magnetoencephalography. Chem Senses. 2005;30(Suppl):1i226–1i227. doi: 10.1093/chemse/bjh196. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Saito S, Goto N, Ogawa H. Representation of salty taste stimulus concentrations in the primary gustatory area in humans. Chemosensory Perception. 2008;1:227–234. [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13 (10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Stevens DA, Chapman KW, Kurtz A. Metallic taste from electrical and chemical stimulation. Chem Senses. 2005;30 (3):185–194. doi: 10.1093/chemse/bji014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks CA, Doty RL. Effects of stimulation duration on electrogustometric thresholds. Physiol Behav. 2004;81(1):1–4. doi: 10.1016/j.physbeh.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. MIT; Cambridge: 2005. [Google Scholar]
- Lundström JN, Seven S, Olsson MJ, Schaal B, Hummel T. Olfactory event-related potentials reflect individual differences in odor valence perception. Chem Senses. 2006;31(8):705–711. doi: 10.1093/chemse/bjl012. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- McCabe C, Rolls ET. Umami: a delicious flavor formed by convergence of taste and olfactory pathways in the human brain. Eur J Neurosci. 2007;25(6):1855–1864. doi: 10.1111/j.1460-9568.2007.05445.x. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115 (10):2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Min BC, Sakamoto K. Influence of sweet suppressing agent on gustatory brain evoked potentials generated by taste stimuli. Appl Human Sci. 1998;17(1):9–17. doi: 10.2114/jpa.17.9. [DOI] [PubMed] [Google Scholar]
- Mizoguchi C, Kobayakawa T, Saito S, Ogawa H. Gustatory evoked cortical activity in humans studied by simultaneous EEG and MEG recording. Chem Senses. 2002;27(7):629–634. doi: 10.1093/chemse/27.7.629. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Geisler MW, Covington JW, Polich J, Murphy C. Olfactory P3 in young and older adults. Psychophysiology. 1999;36 (3):281–287. doi: 10.1017/s0048577299980265. [DOI] [PubMed] [Google Scholar]
- Murayama N, Nakasato N, Hatanaka K, Fujita S, Igasaki T, Kanno A, Yoshimoto T. Gustatory evoked magnetic fields in humans. Neurosci Lett. 1996;210(2):121–123. doi: 10.1016/0304-3940(96)12680-x. [DOI] [PubMed] [Google Scholar]
- Murray MM, Brunet D, Michel CM. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008;20 (4):249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford University Press; New York: 2006. [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Ohla K, Hudry J, le Coutre J. The cortical chronometry of electrogustatory event-related potentials. Brain Topogr. 2009;22 (2):73–82. doi: 10.1007/s10548-009-0076-7. [DOI] [PubMed] [Google Scholar]
- Ohla K, Toepel U, le Coutre J, Hudry J. Electrical neuroimaging reveals intensity-dependent activation of human cortical gustatory and somatosensory areas by electric taste. Biol Psychol. 2010;85 (3):446–455. doi: 10.1016/j.biopsycho.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Onoda K, Kobayakawa T, Ikeda M, Saito S, Kida A. Laterality of human primary gustatory cortex studied by MEG. Chem Senses. 2005;30(8):657–666. doi: 10.1093/chemse/bji059. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37(2):127–152. [PubMed] [Google Scholar]
- Plattig KH. Electric taste. Stimulus intensity dependent evoked brain potentials following electric stimulation of the tongue in humans. Z Biol. 1969;116(3):161–211. [PubMed] [Google Scholar]
- Plattig KH, Dazert S, Maeyama T. A new gustometer for computer evaluation of taste responses in men and animals. Acta Otolaryngol Suppl. 1988;458:123–128. doi: 10.3109/00016488809125115. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244(2):213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Rapin I, Schimmel H, Tourk LM, Krasnegor NA, Pollak C. Evoked responses to clicks and tones of varying intensity in waking adults. Electroencephalogr Clin Neurophysiol. 1966;21 (4):335–344. doi: 10.1016/0013-4694(66)90039-3. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Pernet CR. Quantifying the time course of visual object processing using ERPs: it’s time to up the game. Front Psychol. 2011;2:107. doi: 10.3389/fpsyg.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Saito S, Endo H, Kobayakawa T, Ayabe-Kanamura S, Kikuchi Y, Takeda T, Ogawa H. Temporal process from receptors to higher brain in taste detection studied by gustatory-evoked magnetic fields and reaction times. Ann N Y Acad Sci. 1998;855:493–497. doi: 10.1111/j.1749-6632.1998.tb10612.x. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf JM, Heinze HJ. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127(2):347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Scott TR, Perrotto RS. Intensity coding in pontine taste area: gustatory information is processed similarly throughout rat’s brain stem. J Neurophysiol. 1980;44(4):739–750. doi: 10.1152/jn.1980.44.4.739. [DOI] [PubMed] [Google Scholar]
- Singh PB, Iannilli E, Hummel T. Segregation of gustatory cortex in response to salt and umami taste studied through event-related potentials. Neuroreport. 2011;22(6):299–303. doi: 10.1097/WNR.0b013e32834601e8. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10(1):7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Spekreijse H, van der Twell LH, Zuidema T. Contrast evoked responses in man. Vision Res. 1973;13(8):1577–1601. doi: 10.1016/0042-6989(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Stillman JA, Morton RP, Hay KD, Ahmad Z, Goldsmith D. Electrogustometry: strengths, weaknesses, and clinical evidence of stimulus boundaries. Clin Otolaryngol Allied Sci. 2003;28(5):406–410. doi: 10.1046/j.1365-2273.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry Bertrand. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3(4):151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381(6582):520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32(6):569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN. Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp. 2011;32:2256–2266. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M. Evoked responses to taste stimulation. Int Tinnitus J. 2005;11 (1):43–47. [PubMed] [Google Scholar]
- Yamamoto C, Nagai H, Takahashi K, Nakagawa S, Yamaguchi M, Tonoike M, Yamamoto T. Cortical representation of taste-modifying action of miracle fruit in humans. NeuroImage. 2006;33 (4):1145–1151. doi: 10.1016/j.neuroimage.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121:1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]