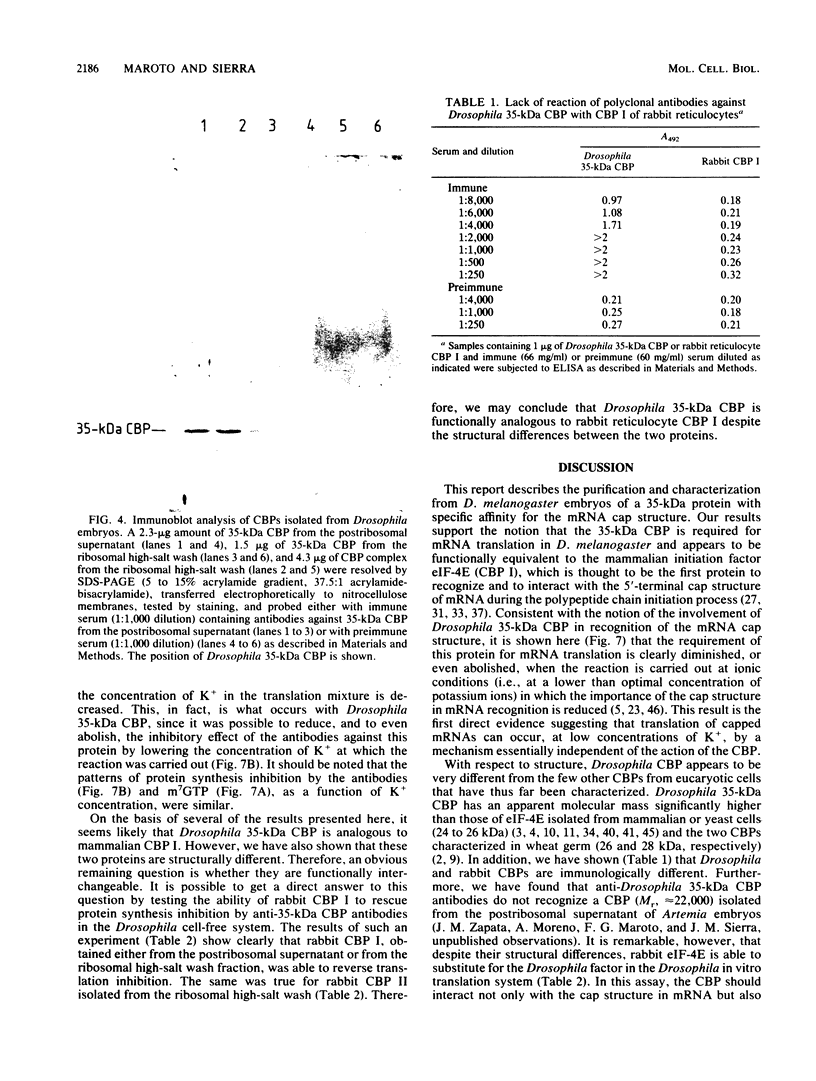
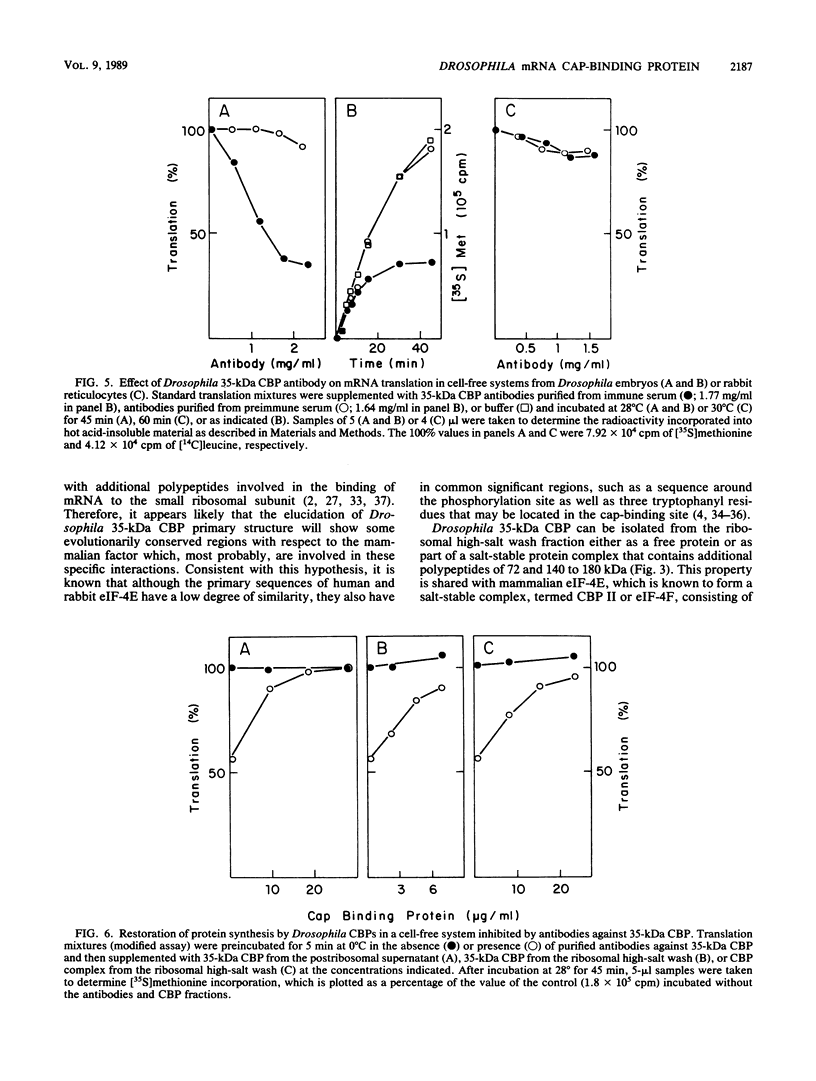
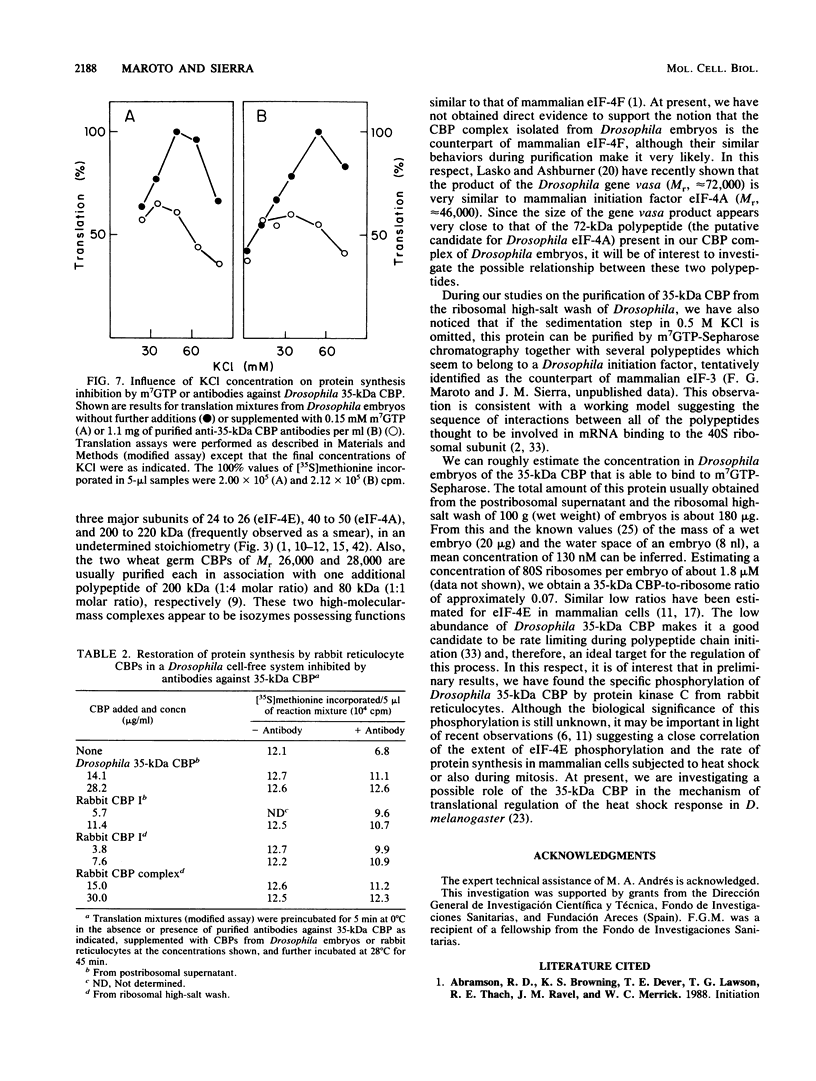
Abstract
A protein with specific affinity for the mRNA cap structure was purified both from the postribosomal supernatant and from the ribosomal high-salt wash of Drosophila melanogaster embryos by m7GTP-Sepharose chromatography. This protein had an apparent molecular mass of 35 kilodaltons (kDa) in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, a size very different from those of the cap-binding proteins that have been characterized thus far. Drosophila 35-kDa cap-binding protein (CBP) could also be isolated from the ribosomal high-salt wash as part of a salt-stable protein complex consisting of polypeptides of 35, 72, and 140 to 180 kDa. Polyclonal antibodies against Drosophila 35-kDa CBP neither reacted with eucaryotic initiation factor 4E from rabbit reticulocytes nor affected mRNA translation in a rabbit reticulocyte cell-free system. However, in a cell-free system from Drosophila embryos, mRNA translation was specifically inhibited by these antibodies. The requirement of 35-kDa CBP for mRNA translation in Drosophila was diminished under ionic conditions in which the importance of mRNA cap structure recognition was reduced. Despite the structural differences between Drosophila 35-kDa CBP and mammalian initiation factor 4E, both proteins were functionally interchangeable in the in vitro translation system from Drosophila embryos.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson R. D., Dever T. E., Lawson T. G., Ray B. K., Thach R. E., Merrick W. C. The ATP-dependent interaction of eukaryotic initiation factors with mRNA. J Biol Chem. 1987 Mar 15;262(8):3826–3832. [PubMed] [Google Scholar]
- Altmann M., Edery I., Sonenberg N., Trachsel H. Purification and characterization of protein synthesis initiation factor eIF-4E from the yeast Saccharomyces cerevisiae. Biochemistry. 1985 Oct 22;24(22):6085–6089. doi: 10.1021/bi00343a009. [DOI] [PubMed] [Google Scholar]
- Altmann M., Handschin C., Trachsel H. mRNA cap-binding protein: cloning of the gene encoding protein synthesis initiation factor eIF-4E from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Mar;7(3):998–1003. doi: 10.1128/mcb.7.3.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Lodish H. F. Translation of capped and uncapped vesicular stomatitis virus and reovirus mRNA'S. Sensitivity to m7GpppAm and ionic conditions. J Biol Chem. 1979 Jan 25;254(2):459–468. [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J Biol Chem. 1987 Aug 15;262(23):11134–11139. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner C., Nakayama N., Goebl M., Tanaka K., Toh-e A., Matsumoto K. CDC33 encodes mRNA cap-binding protein eIF-4E of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Aug;8(8):3556–3559. doi: 10.1128/mcb.8.8.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning K. S., Lax S. R., Ravel J. M. Identification of two messenger RNA cap binding proteins in wheat germ. Evidence that the 28-kDa subunit of eIF-4B and the 26-kDa subunit of eIF-4F are antigenically distinct polypeptides. J Biol Chem. 1987 Aug 15;262(23):11228–11232. [PubMed] [Google Scholar]
- Buckley B., Ehrenfeld E. The cap-binding protein complex in uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1987 Oct 5;262(28):13599–13606. [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Etchison D., Milburn S. Separation of protein synthesis initiation factor eIF4A from a p220-associated cap binding complex activity. Mol Cell Biochem. 1987 Jul;76(1):15–25. doi: 10.1007/BF00219394. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Furuichi Y., Sierra J. M., Muthukrishnan S., Shatkin A. J., Ochoa S. A protein binding the methylated 5'-terminal sequence, m7GpppN, of eukaryotic messenger RNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1559–1563. doi: 10.1073/pnas.73.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hiremath L. S., Webb N. R., Rhoads R. E. Immunological detection of the messenger RNA cap-binding protein. J Biol Chem. 1985 Jul 5;260(13):7843–7849. [PubMed] [Google Scholar]
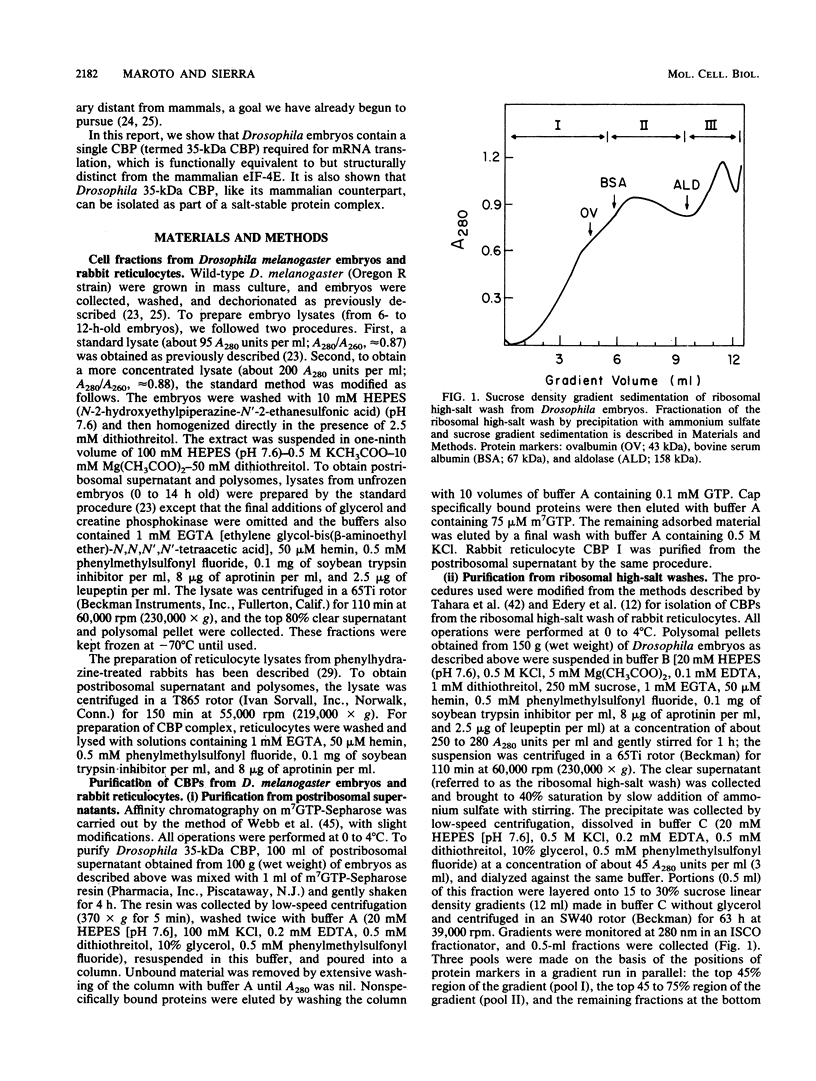
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988 Oct 13;335(6191):611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Lawson T. G., Cladaras M. H., Ray B. K., Lee K. A., Abramson R. D., Merrick W. C., Thach R. E. Discriminatory interaction of purified eukaryotic initiation factors 4F plus 4A with the 5' ends of reovirus messenger RNAs. J Biol Chem. 1988 May 25;263(15):7266–7276. [PubMed] [Google Scholar]
- Lopo A. C., MacMillan S., Hershey J. W. Translational control in early sea urchin embryogenesis: initiation factor eIF4F stimulates protein synthesis in lysates from unfertilized eggs of Strongylocentrotus purpuratus. Biochemistry. 1988 Jan 12;27(1):351–357. doi: 10.1021/bi00401a053. [DOI] [PubMed] [Google Scholar]
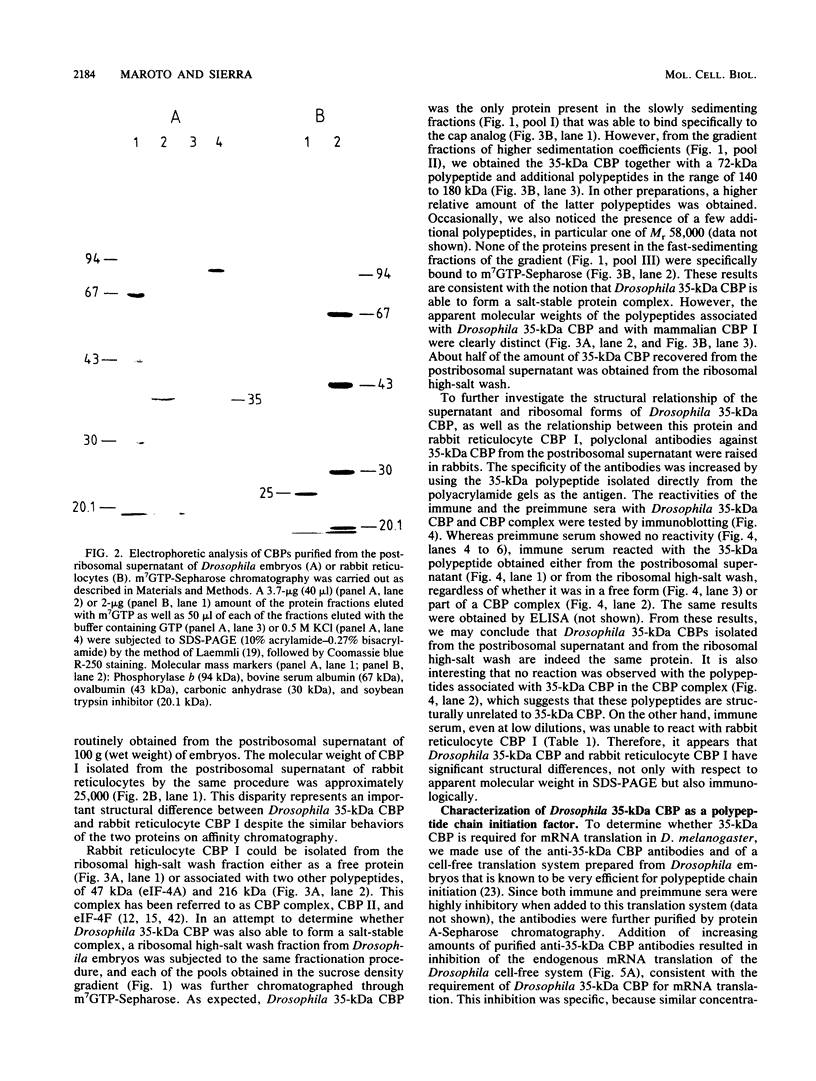
- Maroto F. G., Sierra J. M. Translational control in heat-shocked Drosophila embryos. Evidence for the inactivation of initiation factor(s) involved in the recognition of mRNA cap structure. J Biol Chem. 1988 Oct 25;263(30):15720–15725. [PubMed] [Google Scholar]
- Mateu M. G., Sierra J. M. Protein synthesis in Drosophila melanogaster embryos. Two mechanisms for guanine nucleotide exchange on eukaryotic initiation factor 2. Eur J Biochem. 1987 Jun 15;165(3):507–513. doi: 10.1111/j.1432-1033.1987.tb11468.x. [DOI] [PubMed] [Google Scholar]
- Mateu M. G., Vicente O., Sierra J. M. Protein synthesis in Drosophila melanogaster embryos. Purification and characterization of polypeptide chain-initiation factor 2. Eur J Biochem. 1987 Jan 2;162(1):221–229. doi: 10.1111/j.1432-1033.1987.tb10564.x. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Trachsel H. The mouse protein synthesis initiation factor 4A gene family includes two related functional genes which are differentially expressed. EMBO J. 1988 Jul;7(7):2097–2105. doi: 10.1002/j.1460-2075.1988.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
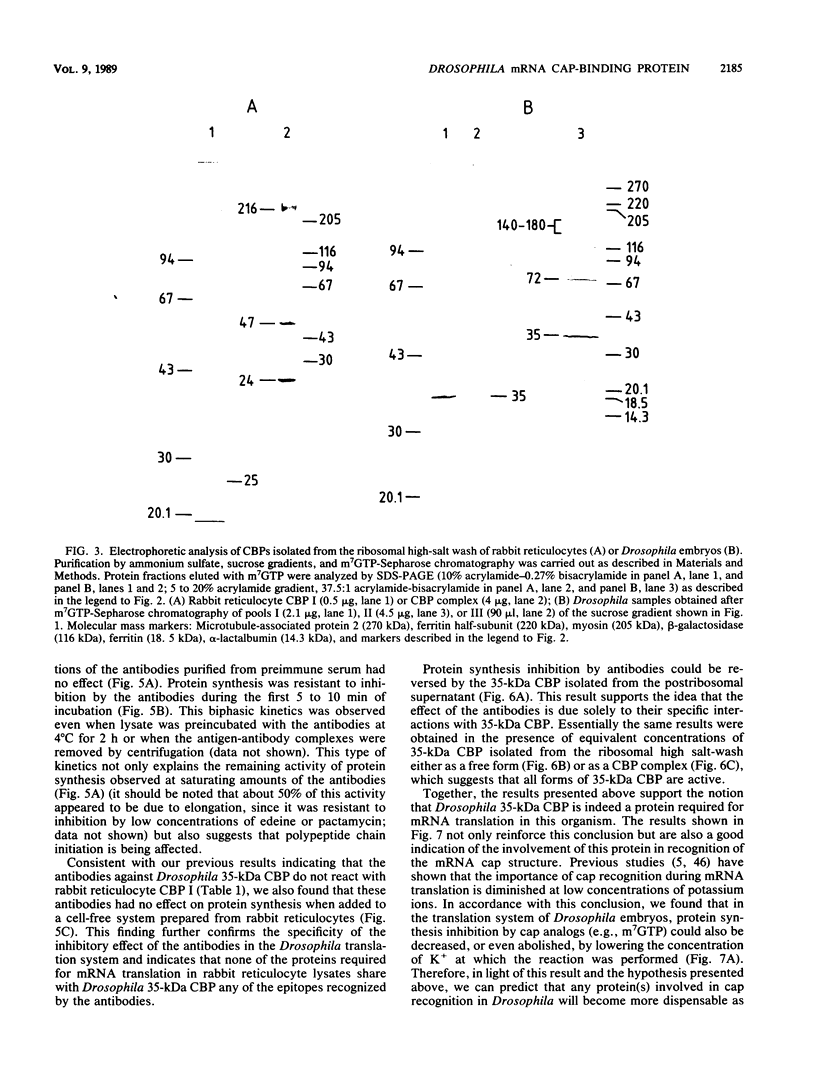
- Palomo C., Sierra J. M. Note on the activation of the heme-stabilized translational inhibitor of reticulocyte lysates by oxidized glutathione. Biochimie. 1988 Jun;70(6):827–831. doi: 10.1016/0300-9084(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Palomo C., Vicente O., Sierra J. M., Ochoa S. Studies on the activation of the heme-stabilized translational inhibitor of reticulocyte lysates by oxidized glutathione and NADPH depletion. Arch Biochem Biophys. 1985 Jun;239(2):497–507. doi: 10.1016/0003-9861(85)90718-0. [DOI] [PubMed] [Google Scholar]
- Panniers R., Stewart E. B., Merrick W. C., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich cells involves reduction of eukaryotic initiation factor 4F activity. J Biol Chem. 1985 Aug 15;260(17):9648–9653. [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. The involvement of mRNA secondary structure in protein synthesis. Biochem Cell Biol. 1987 Jun;65(6):576–581. doi: 10.1139/o87-074. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985 Jun 25;260(12):7651–7658. [PubMed] [Google Scholar]
- Rhoads R. E. Cap recognition and the entry of mRNA into the protein synthesis initiation cycle. Trends Biochem Sci. 1988 Feb;13(2):52–56. doi: 10.1016/0968-0004(88)90028-x. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Domier L. L., Gardner P. R., Hellmann G. M., Rhoads R. E. Amino acid sequence of the mRNA cap-binding protein from human tissues. Proc Natl Acad Sci U S A. 1987 Feb;84(4):945–949. doi: 10.1073/pnas.84.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik W., Gardner P. R., Vanaman T. C., Rhoads R. E. Structural analysis of the messenger RNA cap-binding protein. Presence of phosphate, sulfhydryl, and disulfide groups. J Biol Chem. 1986 Jan 5;261(1):71–75. [PubMed] [Google Scholar]
- Rychlik W., Russ M. A., Rhoads R. E. Phosphorylation site of eukaryotic initiation factor 4E. J Biol Chem. 1987 Aug 5;262(22):10434–10437. [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. ATP/Mg++-dependent cross-linking of cap binding proteins to the 5' end of eukaryotic mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1643–1656. doi: 10.1093/nar/9.7.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb N. R., Chari R. V., DePillis G., Kozarich J. W., Rhoads R. E. Purification of the messenger RNA cap-binding protein using a new affinity medium. Biochemistry. 1984 Jan 17;23(2):177–181. doi: 10.1021/bi00297a001. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Baglioni C. Influence of potassium salt concentration and temperature on inhibition of mRNA translation by 7-methylguanosine5'-monophosphate. J Biol Chem. 1978 Jan 10;253(1):178–183. [PubMed] [Google Scholar]