Abstract
Arbuscular mycorrhizal fungi (AMF) are common and important plant symbionts. They have coenocytic hyphae and form multinucleated spores. The nuclear genome of AMF is polymorphic and its organization is not well understood, which makes the development of reliable molecular markers challenging. In stark contrast, their mitochondrial genome (mtDNA) is homogeneous. To assess the intra- and inter-specific mitochondrial variability in closely related Glomus species, we performed 454 sequencing on total genomic DNA of Glomus sp. isolate DAOM-229456 and we compared its mtDNA with two G. irregulare isolates. We found that the mtDNA of Glomus sp. is homogeneous, identical in gene order and, with respect to the sequences of coding regions, almost identical to G. irregulare. However, certain genomic regions vary substantially, due to insertions/deletions of elements such as introns, mitochondrial plasmid-like DNA polymerase genes and mobile open reading frames. We found no evidence of mitochondrial or cytoplasmic plasmids in Glomus species, and mobile ORFs in Glomus are responsible for the formation of four gene hybrids in atp6, atp9, cox2, and nad3, which are most probably the result of horizontal gene transfer and are expressed at the mRNA level. We found evidence for substantial sequence variation in defined regions of mtDNA, even among closely related isolates with otherwise identical coding gene sequences. This variation makes it possible to design reliable intra- and inter-specific markers.
Introduction
Arbuscular mycorrhizal fungi (AMF) are plant root-inhabiting obligate symbionts that form symbiotic associations with approximately 80% of plant species [1], [2]. This symbiosis helps plants to acquire nutrients and protects them from soil-borne pathogens [3], [4] by inducing plant resistance [5]–[8] or inhibiting pathogen growth [9]. In return, plants provide carbohydrates, which AMF cannot acquire from extracellular sources. They are an important component of soil microbial communities, as they are able to exchange their genetic material between compatible isolates through a process called anastomosis [10]. The latter have been hypothesized to be an important factor in maintaining the genetic diversity found in Glomeromycota and to attenuate the effect of genetic drift within a population [10]–[14]. AMF are currently thought to reproduce clonally, based on the absence of a recognizable sexual stage (or apparatus). However, this hypothesis has been challenged by the identification of many orthologues of sexually-related genes [15]–[17], which suggests at least the presence of cryptic recombination. AMF spores and hyphae are multinucleated, but their true genetic organization is currently under debate [11], [18]–[21]. However, evidence strongly suggests that nuclei can be genetically divergent within an AMF individual. Thus, AMF are characterized by considerable within-isolate nuclear genetic diversity even at the expression level [22]. The presence of such diversity in AMF individuals/populations [22], [23], combined with a lack of molecular data, have hindered the use of nuclear markers to assess questions on community structure, diversity and function. In contrast, AMF mitochondrial (mt) DNA is homogeneous within single isolates [24], [25], making it a good target for marker development. Following this logic, the mitochondrial large subunit (LSU) rRNA gene has been explored for its usefulness as a marker [24], [26], [27], although determining its specificity at the isolate level is still challenging for all AMF taxa aside from the model species G. irregulare.
Comparative AMF mitochondrial genomics has been proposed as an approach to open up new possibilities for development of strain-specific molecular markers [25], [28], [29] given that the type of mitochondrial marker necessary to establish specificity at different divergence levels may vary. This approach has been shown to be a powerful tool for the study of evolutionary relationships among lower fungi [30]. Unfortunately, only three AMF mitochondrial genomes had been published until recently, including that of Glomus intraradices [25], [29] (renamed to G. irregulare [31] and changed again recently to Rhizophagus irregularis based on an exhaustive molecular phylogeny of rRNA genes [32]; in the present paper, we will use the older nomenclature) as well as those of two distant AMF species, Gigaspora rosea and Gigaspora margarita [33], [34]. Compared to G. irregulare, the Gigasporaceae genomes have an inflated mitochondrial genome size that is mainly the result of extended intergenic regions. These regions are not syntenic and both genomes harbor cox1 and rns genes with exons encoded on different strands, whose products are joined at the RNA level through either trans-splicing events of group I introns, or base-pairing. The mitochondrial protein sequences in the dataset were sufficient to confirm the phylogenetic relationship of AMF with Mortierellales as a sister group. This shows that a broader sampling of AMF mtDNA can answer questions about the evolution of these ecologically important fungi. Formey et al. (2012) have recently sequenced four isolates of G. irregulare [29] and were able to develop isolate-specific markers using variable regions That were created by the insertion of mobile elements.
Those elements, including linear or circular plasmids and mobile ORF encoding endonucleases (mORFs), are present in a broad range of fungal mitochondrial genomes (For review see [35]). Plasmids are autonomously-replicating circular or linear extrachromosomal DNA molecules. They are found in three broad types: circular plasmids encoding a DNA polymerase gene (dpo) [36], linear plasmids with terminal inverted repeats encoding either a dpo or rpo (RNA polymerase) gene or both [37], and retroplasmids, which usually encode a reverse transcriptase [38]. Free linear or circular plasmids encoding dpo can be present in the mitochondria of fungi [36] and plants [39]. Segments have been shown to integrate within the mtDNA of fungi [40]–[42], but plasmid-related dpo insertions tend to fragment, shorten (since they are not selected for) and eventually disappear from mitochondrial genomes. Plasmid-related dpo insertions have been reported in the AMF Gigaspora rosea, but are virtually absent from the closely related paraphyletic zygomycetes. The mobility of mORFs, elements that thrive in Glomus, is mediated by the site-specific DNA endonuclease they encode. This endonuclease cleaves ORF-less alleles by creating a double-strand break in DNA and initiates the insertion and fusion of the mobile element. The same process, called intron homing, has been proposed for group I introns [43]. Several lines of phylogenetic evidence support the hypothesis of the evolutionary-independent ancestral origins of mORFs [44]. These highly mobile elements have the ability to carry group I introns [45], intergenic sequences [46], and coding sequences [47]. The first reported case of mitochondrial gene transfer caused by those elements was a mORF-mediated insertion of a foreign atp6 carboxy-terminal in the blastocladiomycete Allomyces macrogynus [48].
The present study compared the mitochondrial genomes of the newly sequenced AMF species Glomus sp. DAOM226456 (a Glomus diaphanum like species based on spore morphology) with two isolates of the closely related G. irregulare. Along with a highly divergent intron insertion pattern, we found insertions of plasmid-related DNA polymerase and propagation of mobile open reading frame (mORFs) encoding endonucleases in Glomus mtDNAs. Our findings have brought to light the first evidence of AMF interspecific exchange of mitochondrial coding sequences entailing formation of gene hybrids in Glomus sp. atp6, atp9 (coding for the subunit 6 and 9 of the ATP synthetase complex), cox2 (cytochrome C oxidase subunit 2) and nad3 (NADH dehydrogenase subunit 3) genes.
Materials and Methods
Fungal material
Spores and mycelium of Glomus sp. (DAOM-229456) and G. irregulare (DAOM 197198) were cultivated in vitro on a minimal (M) medium with carrot roots transformed with Agrobacterium rhizogenes, as described in the literature [49]. The medium was liquefied using a 0.82 mM sodium citrate and 0.18 mM citric acid extraction buffer solution. The resulting fungal material was further purified by hand under a binocular microscope, to remove root fragments.
DNA extraction
Spores and mycelium were suspended in 400 µL of the DNeasy Plant Mini Kit AP1 buffer (Qiagen) and crushed with a pestle in 1.5 ml microtubes, and the DNA was purified according to the manufacturer's recommendations. Purified DNA in a final elution volume of 40 µL was stored at −20°C until use.
RNA extraction
Fresh Glomus sp. fungal material was harvested from in vitro cultures. RNA extraction was performed using an E.Z.N.A. Fungal RNA Kit (Omega Biotek) according to manufacturer's recommendations. Total RNA was treated with Turbo DNase (Applied Biosystems) for 30 min at 37°C to remove residual DNA fragments that could interfere with downstream applications. In order to prevent chemical scission of the RNA during heat inactivation of the DNase at 75°C for 15 min, EDTA was added at a final concentration of 15 mM. In total, 40 µl of 100 ng/µl RNA was collected and stored at −80°C until use. The RNA concentration was determined using a Nanophotometer Pearl (Implen).
cDNA synthesis
From the total RNA previously extracted, 500 ng were used for cDNA synthesis with the SuperScript III reverse transcriptase kit (Life Technologies, Canada) according to manufacturer's recommendations, using oligo dT. The only change from these recommendations was the addition of MgCl2 to a final concentration of 15 mM to compensate for the EDTA added in the previous step. In order to remove RNA complementary to the cDNA, 1 µl of RNase ONE ribonuclease (Promega, Canada) was added to the cDNA and incubated at 37°C for 20 min. The resulting cDNA was stored at −20°C until use.
Polymerase chain reaction (PCR)
The proposed intergenic markers to discriminate between G. irregulare DAOM197198 and Glomus sp. were tested by PCR using the KAPA2G Robust Hotstart ReadyMix PCR kit (KapaBiosystems, Canada). The specific primers used were respectively rnl-cox2_197198_spec_F (5′-AAAGGAATTACATCGATTTA-3′), rnl-cox2_197198_spec_R (5′- ACAAGAAGGTTTGCATCGCTA-3′), nad6-cox3_dia_spec_F (5′- CCACTAGTTAAGCTACCCTCTA-3′) and nad6-cox3_dia_spec_R (5′- AATCATACCGTGTGAAAGCAAG -3′). The variable length primers were rnl-cox2_197198_size_F (5′- TAGGGATCAGTACTTTAGCCAT -3′), rnl-cox2_197198_size_R (5′- TCCTTACGGTATGAATGGTAAG -3′), rnl-cox2_dia_size_F (5′- AGACTTCTTCAGTTCCACAATCA -3′) and rnl-cox2_dia_size_R (5′- ATGGCTAAAGTACTGATCCCTAC -3′). For 40 µl of PCR reaction volume, 12 µl of water, 20 µl of 2× PCR buffer, 3.5 µl of (5 µM) forward and reverse primers, and 1 µl of DNA were added. Cycling parameters were 94°C/3 min, followed by 38 cycles of: 94°C/30 sec, 54°C/25 sec, 72°C/45 sec and a final elongation at 72°C. PCR products were separated by electrophoresis in a 1.5% (w/v) agarose gel and visualized with GelRed under UV light.
Reverse transcriptase – polymerase chain reaction (RT-PCR)
Our objective with regard to PCR reactions on cDNA was to assess which regions of the gene hybrid reported in atp6, atp9, cox2 and nad3 were expressed at the mRNA level. For each of the four hybrids, a forward primer designed in the conserved ‘core’ structure of the gene (atp6_core_F: 5′-AGAGCAGTTTGAGATTGTTAAG-3′, atp9_core_F: 5′-CTGGAGTAGGAGTAGGGATAGT-3′, cox2_core_F: 5′-CATGGCAATTAGGATTTCAAGA-3′ and nad3_core_F: 5′-TCGTTCCTTTGTTCGTGCTA-3′) was used in combination with three reverse primers designed respectively in the inserted C-terminal (atp6_insert_R1: 5′-AGCCTGAATAAGTGCAACAC-3′, atp9_insert_R1: 5′- GTAAGAAAGCCATCATGAGACA-3′, cox2_insert_R1: 5′-TGAGAAGAAAGCCATAACAAGT-3′ and nad3_insert_R1: 5′-AGAAGTATGAAAACCATAGCAATC-3′), the mobile ORF (atp6_mORF_R2: 5′-AGTCTTCGAATATACTGGCAG-3′, atp9_mORF_R2: 5′-TGTCGAGTCTCCAAAGTATGT-3′, cox2_mORF_R2: 5′-ACTGAATTCCTGTGTTTCGATCT-3′ and nad3_mORF_R2: 5′-TGACGAATGGTTAGACGATGT-3′) and the native C*-terminal portion of the corresponding gene (atp6_native_R3: 5′-CGTACCGTCGTAACAAGTAGA-3′, atp9_native_R3: 5′-CCATCATTAAGGCGAATAGA-3′, cox2_native_R3: 5′-CTAACAAACTCCCGACTATTACCT-3′ and nad3_native_R3: 5′-AGAATGAAGACCATTGCAAC-3′). To verify that there was no residual mitochondrial DNA in the cDNA, the primers Ctrl_positive_nad5exon4_689F (5′-ACCATTCTGTTATGTTCTAATGT-3′) and Ctrl_positive_nad5exon4_689R (5′-GTCTGACTTAGCAGGTTAGTTAAG-3′) were designed in nad5 exon 4 and used as a positive control on cDNA and negative control on RNA. The RT-PCR reactions were carried out using the KAPA2G Robust Hotstart ReadyMix PCR kit (KapaBiosystems, Canada) as described above in the PCR section.
Cloning
Cloning reactions were performed on each successful RT-PCR amplification. The ligation reactions were done using the pGEM-T Easy Vector Systems kit (Promega, Canada) according to manufacturer's recommendations. The transformation was carried out in E. coli DH5 alpha competent cells. Bacterial colonies were screened via PCR using T7 and SP6 universal primers as described in the PCR section.
Sequencing, assembly and gene annotation
Glomus sp. total DNA was sequenced using 454 Titanium Flex shotgun technology (one plate) and the respective resulting 1,078,190 reads were assembled with Newbler (Genome Quebec Innovation Center, McGill University, Montreal, Canada). Gene annotation was performed with MFannot (http://megasun.bch.umontreal.ca/cgi-bin/mfannot/mfannotInterface.pl), followed by manual inspection and introduction of missing gene features as described in Nadimi et al., (2012). G. irregulare isolates 494 and DAOM-197198 mtDNAs (accession numbers FJ648425 and HQ189519 respectively) were used for comparison. Sequencing of the cloned RT-PCR products was performed on the same sequencing platform, using Sanger technology with T7 and SP6 universal primers.
Phylogenetic analysis
For each gene of interest (atp6, atp9, cox2 and nad3) in 12 AMF species, the dataset contains the corresponding C*-terminal for: Glomus sp. DAOM-229456, G. irregulare isolate 494, G. irregulare DAOM-197198, G. irregulare DAOM-240415, G. irregulare DAOM-234179, G. irregulare DAOM-234328, G. irregulare DAOM-213198, Glomus sp. DAOM-240422, G. fasciculatum DAOM-240159, G. aggregatum DAOM-240163, G. cerebriforme DAOM-227022, Gigaspora rosea DAOM-194757 (accession number JQ693396) and 3 selected fungal representatives: Mortierella verticillata (accession number AY863211), Smittium culisetae (accession number AY863213) and Rhizopus oryzae (accession number AY863212). The sequences were deposited in databases under the accession numbers: JX074786-JX074817. The reference phylogeny was constructed using the concatenated ‘core’ sequence (without the C*-terminal portion used previously) of the same four genes. The DNA sequence alignments and the inference of maximum likelihood trees using GTR+G (with five distinct gamma categories) were performed using the integrated program MEGA version 5 [50]. Bootstrap resampling (1000 replicates) was carried out to quantify the relative support for each branch of the trees. Bayesian analysis were done using MrBayes version 3.2 using the GTR+G model (with five distinct gamma categories), four independant chains, one million cycles, tree sampling every 100 generations and a burn-in value of 25%.
Results and Discussion
Glomus sp. genome organization and structure
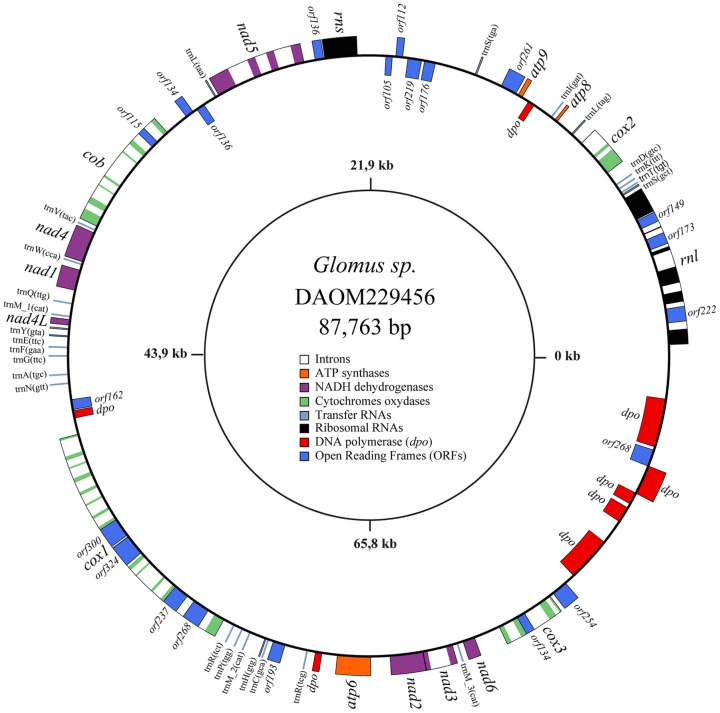
The complete sequence of the Glomus sp. 229456 mt genome was a double-stranded circular DNA molecule, exempt of polymorphism, with a size of 87,763 bp. The annotated sequence of Glomus sp. was deposited in GenBank under the accession number JX065416. Its mtDNA harbors the typical set of 41 mitochondrial genes found in other AMF (two rRNAs, 14 protein coding genes (PCGs) and 25 tRNAs). The PCGs include three ATP synthetase (atp), one cytochrome b (cob), three cytochrome C oxydase (cox) and seven NADH dehydrogenase (nad) genes. Also, 19 ORFs and 31 introns are inserted in this newly sequenced mt genome (Figure 1).
Figure 1. The Glomus sp. 229456 mitochondrial genome circular-map was opened upstream of rnl.
Genes on the outer and inner circumference are transcribed in a clockwise and counterclockwise direction, respectively. Gene and corresponding product names are atp6, 8, 9, ATP synthase subunit 6; cob, apocytochrome b; cox1–3, cytochrome c oxidase subunits; nad1–4, 4L, 5–6, NADH dehydrogenase subunits; rnl, rns, large and small subunit rRNAs; A–W, tRNAs, the letter corresponding to the amino acid specified by the particular tRNA followed by their anticodon. Open reading frames smaller than 100 amino acids are not shown.
Comparative view of three Glomus mtDNAs
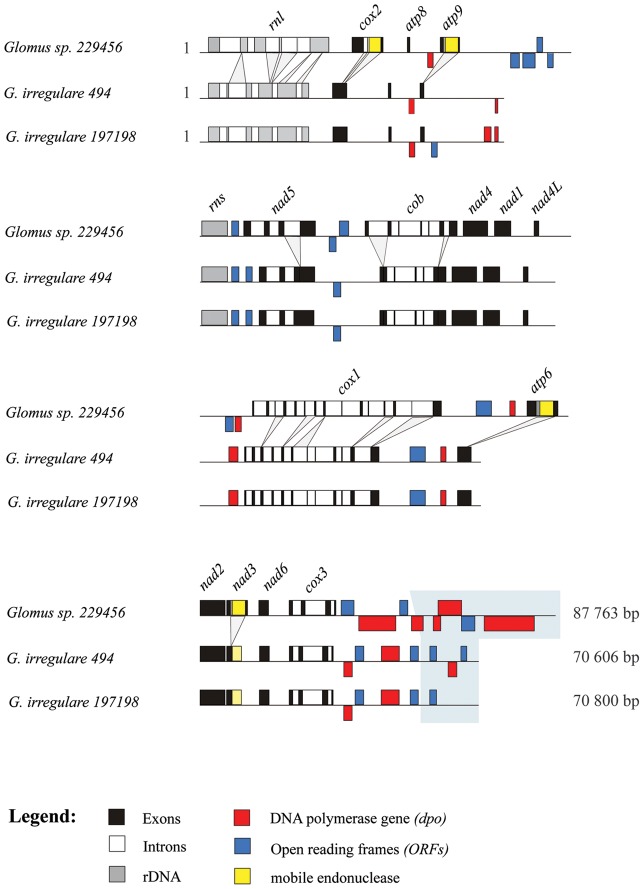
The gene content in Glomus sp. and G. irregulare mitochondrial genomes is similar to that found in zygomycetes, except for rps3 and rnpB. The mtDNAs of both AMF species have the same gene order, and all genes are transcribed from one strand with very similar coding regions except for the insertion of mobile ORF elements (mORFs) in the atp6, atp9, cox2 and nad3 genes of Glomus sp. (Figure 2). However, there are many differences in the number of introns, some of which carry more substantial sequence differences than do the coding sequences. The differences in the presence of introns and mORFs explain the inflated genome size of 87,763 bp in Glomus sp., as compared to 70,800 bp in G. irregulare 197198 (Table 1). Glomus sp. cox1 intron 8 is the homolog of an intron inserted at the same position in Rhizopus oryzae and angiosperms (with 76 and 79% of sequence identity, respectively) [28]. G. irregulare cox1 intron 7 is also inserted at the same position, but has an eroded ORF encoding the homing endonuclease gene, and thus also shares identity with the intron RNA secondary structure of R. oryzae and plants. The plant cox1 intron was thought to have been acquired from a fungal donor, due to the proximity of its clade to that of fungi rather than to the non-vascular plant Marchantia. Knowing the extent to which the intron has spread in angiosperms [51], [52], it would be interesting to see whether such an invasion has also occurred within the Glomeromycota phylum.
Figure 2. Comparative view of the three mitochondrial genomes linear map where the exons (black), introns (white), rDNA (gray), dpo plasmid insertions (red), ORFs (blue) and mobile endonuclease (yellow) are represented.
Divergence in intron insertion pattern is indicated by projections. A hyper-variable region in the cox3-rnl intergene is boxed in grayscale.
Table 1. Gene and intron content in AMF and selected fungal mtDNAs.
| Genes | |||||||||||
| Species | rnl, rns | atp 6, 8, 9 | cob | cox 1, 2, 3 | nad 1–6 a | trn A–W | rnpB | rps3 | ORFs b | Intron I c | Intron II c |
| Glomus Sp. 229456 | 2 | 3 | 1 | 3 | 7 | 25 | 0 | 0 | 19 | 31 | 1 |
| Glomus irregulare 494 | 2 | 3 | 1 | 3 | 7 | 25 | 0 | 0 | 8 | 26 | 0 |
| Glomus irregulare 197198 | 2 | 3 | 1 | 3 | 7 | 25 | 0 | 0 | 8 | 26 | 0 |
| Gigaspora rosea | 2 | 3 | 1 | 3 | 7 | 25 | 0 | 0 | 4 | 13 | 1 |
| Smittium culisetae | 2 | 3 | 1 | 3 | 7 | 26 | 1 | 1 | 3 | 14 | 0 |
| Mortierella verticillata | 2 | 3 | 1 | 3 | 7 | 28 | 1 | 1 | 7 | 4 | 0 |
| Rhizopus oryzae | 2 | 3 | 1 | 3 | 7 | 23 | 1 | 0 | 4 | 9 | 0 |
| Allomyces macrogynus | 2 | 3 | 1 | 3 | 7 | 25 | 0 | 1 | 4 | 26 | 2 |
| Saccharomyces cerevisiae d | 2 | 3 | 1 | 3 | 0 | 25 | 1 | 1 | 3 | 9 | 4 |
Includes nad1, nad2, nad3, nad4, nad4L, nad5 and nad6.
Only ORFs greater than 100 amino acids in length are listed, not including intronic ORFs and dpo and rpo fragments.
Intron I and Intron II denote introns of group I and group II, respectively.
S. cerevisiae strain FY 1679 [57].
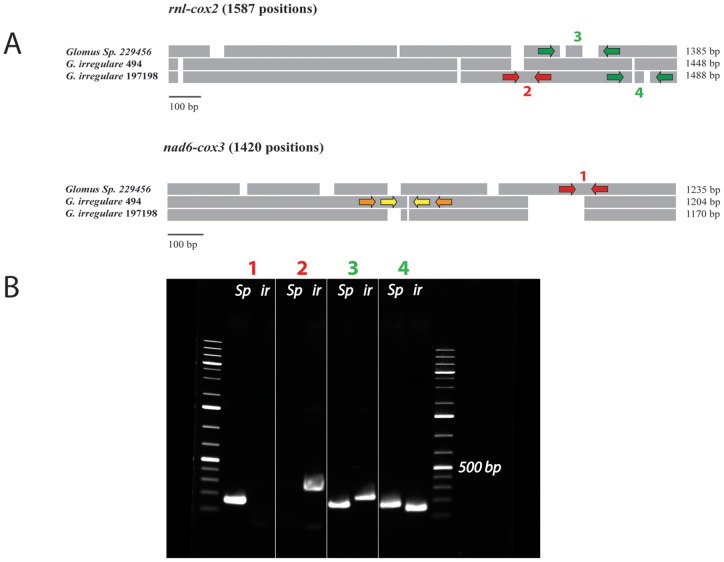
Further, intergenic regions differ substantially in sequence: some are identical while others show signs of very fast, substantial changes including point mutations, insertions, deletions and inversions (Figures 2 and 3). Most of these differences occur in the cox3-rnl intergene, a large hyper-variable region that has been invaded by dpo fragments. The variations observed in intergenic regions provide an opportunity to develop species- specific molecular markers as shown in Figure 3, and even isolate-specific markers or methods allowing reliable identification and/or quantification of these fungi. Lack of efficient and powerful molecular markers for AMF identification and quantification constitutes a major problem that limits the analysis of population genetics and field studies in AMF. Mitochondrial DNA is homogeneous within the AMF individuals studied to date, but evidence of genetic polymorphism between G. irregulare isolates has been observed in intergenic regions. They harbor highly conserved genes as well as highly variable regions, which promises to facilitate AMF barcoding at different taxonomic levels, an analysis that is currently challenging to carry out using nuclear genes. Hyper-variable intergenic regions with eroded dpo insertions and indels in intergenic regions constitute useful mitochondrial areas on which to focus attention in order to develop suitable markers for discriminating isolates of the same species. Intron insertion pattern variations, genome reorganizations (such as gene shuffling) and coding region divergences will make it possible to distinguish between different AMF species, genera and families.
Figure 3. A) Schematic alignment representation of two mitochondrial intergenic regions (rnl-cox2 and cox3-nad6) showing the presence of numerous insertions and deletions (indels).
The red arrows indicate the approximate position of the PCR primers that yield strain-specific markers, while the green arrows indicate the position of PCR primers that produce a size-specific marker. The yellow and orange arrows indicate potential regions to design, respectively, specific and size-specific markers in G. irregulare 494. B) Agarose gel electrophoresis figure showing the PCR results of the proposed markers on Glomus sp. 229456 (Gs) and G. irregulare DAOM197198 (Gi) respectively for each marker. Marker 1 shows the Glomus sp. specific amplification (156 bp), while marker 2 shows the G. irregulare 197198 specific marker (263 bp). The size-specific marker 3 yield a length of 160 bp for Glomus sp. and 226 bp for G. irregulare 197198. Finally, the size-specific marker 4 yield a length of 159 bp for Glomus sp. and 131 bp for G. irregulare 197198.
Our 454 pyrosequencing data and direct PCR sequencing showed that G. irregulare DAOM197198 and Glomus sp. mtDNAs are homogeneous, meaning that all the mitochondrial genomes in a given isolate are essentially identical, in stark contrast to the nuclear genomes. Our results confirm the previous report by Lee et al. (2009) suggesting homoplasmy in the first completed Glomeromycota mitochondrial genome of the AMF G. intraradices (G. irregulare isolate 494). A rapid and effective mitochondrial segregation mechanism was suggested to explain those findings. It was previously demonstrated that isolates of the same species can exchange nuclear material through anastomosis [10], but exchange of divergent mitochondrial haplotypes has yet to be shown. This leads us to question whether polymorphism does indeed occur through anastomosis, and for how many generations mitochondrial heteroplasmy is maintained.
Rapid expansion of plasmid-like DNA polymerase sequences in Glomus
Plasmid-related DNA polymerase genes are found in mobile mitochondrial plasmids that occur either as free linear or circular DNAs, and have been shown to also insert into mtDNA (for review see [35]). One striking feature in the comparison of the two closely related Glomus species is the presence of numerous dpo insertions in the intergenic regions of their mtDNA (Figure 2). All three Glomus mtDNAs contain a large number of dpo fragments, most of which are substantially divergent in sequence and therefore are most likely the result of independent plasmid insertion events. Even the two G. irregulare (isolates 494 and DAOM197198), otherwise almost identical in sequence, differ in dpo sequence, which supports the interpretation that dpo insertion occurs repeatedly and frequently through evolutionary time. A bona fide and complete dpo gene is present in Glomus sp., and its sequence is different from those in G. irregulare isolates. Because of its complete length, it most likely results from a recent insertion event. There is no evidence that dpo is functional when inserted in mtDNA. As in numerous other cases, dpo coding regions are fragmented in Glomus and occur on both strands, representing a good indicator of a genomic region experiencing little if any selective evolutionary constraints.
The source of the dpo insertions in Glomus mtDNA remains elusive. We did not find any free mitochondrial plasmids in our Glomus sp. and G. irregulare isolate DAOM197198 shotgun data (combining nuclear and mitochondrial DNAs), as we did for Gigaspora rosea, where a 3582 bp contig with high sequence coverage was found [34]. However, since the Glomus strains used in this study come from aseptic in vitro cultures, and even though the G. rosea fungal material was extracted from in vivo greenhouse pot cultures, we cannot rule out the possibility that an environmental vector is the source of dpo plasmids and is responsible for their propagation in G. rosea. Interestingly, dpo plasmids have been found to occur in numerous plants, notably in Daucus carota [53] which is used as a host plant for AMF cultures in vitro. The obligate biotrophic dependence of AMF on plants could be one of the reasons that dpo insertions are most abundant in Glomus mtDNAs yet virtually absent in mitochondrial sequences of the Blastocladiomycota (except for a single 100 amino acid long fragment occurrence in Smittium culisetae mtDNA), which is the closest phylogenetic group to the Glomeromycota.
Mobile element insertions have been shown to trigger genomic rearrangements such as gene shuffling through homologous recombination [54] and even genome linearization [35], [55], [56]. Whenever sequence repeats occur, more than one genome conformation may exist, but we have no evidence that this happens in Glomus mtDNA. It would be interesting to examine whether numerous recent dpo insertions with high sequence similarity might act as genomic repetitions and give rise to genome reorganization in closely related AMF species. Integrated plasmid segments within mitochondrial genomes, even though they are neutral or cryptic, could promote genomic rearrangements.
Mobile ORF elements (mORFs) in Glomus
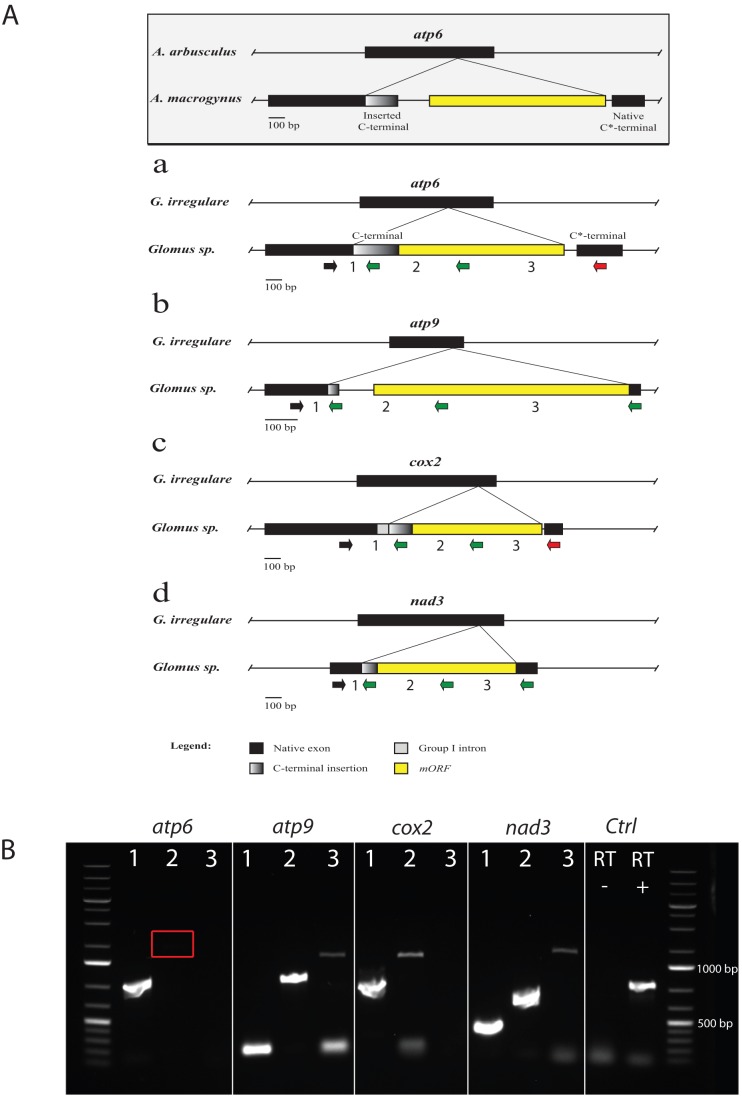
Although most ORF-encoding endonuclease genes are inserted in introns where they have been shown to play a role in propagation, they can also be present in genes in which their evolutionary impact is less obvious. We identified numerous mORFs encoding endonuclease genes unique to Glomus sp. isolate DAOM-229456 mtDNA. When we annotated the sequences of the atp6, atp9, cox2 and nad3 genes, we observed that they all have a peculiar organization. Indeed, these genes harbor a carboxy-terminal duplication (C*-terminal) that was found downstream of a mORF insertion. For example, in the atp6 gene, the duplicated portion of the C-terminal was found about 1000 bp downstream, following an inserted LAGLIDADG endonuclease ORF. When we compared the DNA sequence of the C*-terminal portion with the corresponding sequence of G. irregulare isolate 494, a close relative to Glomus sp., we found a 91.2% nucleotide identity. In contrast, the comparison between the Glomus sp. atp6 duplicated carboxy-terminals (C-terminal and C*-terminal) showed a low sequence identity of 63.5%. Interestingly, comparison of the Glomus sp. C*-terminal amino acid sequences with the corresponding portion in G. irregulare showed 100% identity, indicating that the mutations observed in DNA are all synonymous. However, the comparison of the amino acid sequences of Glomus sp. atp6 carboxy-terminals showed 91% identity.
Surprisingly, when we designed a forward primer in the upstream sequence (5′ gene portion) and two reverse primers in the C-terminal and C*-terminal respectively, we found that the C-terminal is transcribed with the upstream sequence resulting in a putative hybrid transcript while the C*-terminal was not expressed into mRNA. Thus we hypothesized that the C-terminal portion could have been acquired from a donor through horizontal gene transfer (HGT). We also observed similar organization in atp9, cox2 and nad3 genes of Glomus sp. where the carboxy-terminal portion (C*-terminal) was replaced partially or completely by one carried by a mORF (C-terminal) encoding a LAGLIDADG endonuclease (except in atp9, a GIY-YIG family endonuclease) (Figure 4A: a, b, c and d). In atp6, the insert lacks a stop codon and the ORF is in phase with the native gene. In atp9, the insert, along with the mORF, completely replaces the native 3′ end, while in cox2 and nad3 only a portion of the carboxy-terminal is replaced (Table 2). The resulting gene hybrids are expressed at the mRNA level in all four cases as shown in Figure 4B. After sequencing of the cDNA bands, we found that the mORF and the inserted C-terminal are integral parts of the transcript in all four genes. However, in atp6 and cox2, the native C*-terminal was not expressed into mRNA.
Figure 4. Comparison of gene hybrids atp6, atp9, cox2 and nad3.
A) The atp6 gene hybrid reported for Allomyces macrogynus (grayscale, boxed) is used as a reference in a comparison of the most similar atp6 (a), atp9 (b), cox2 (c) and nad3 (d) genes in Glomus sp. mtDNA. Each occurrence is put in perspective with the gene of a close relative (either Allomyces arbusculus or G. irregulare) in order to show the insertion point of the foreign element with the projections. Exons are in black, while the inserted foreign C-terminal is shaded in gray. The mobile endonuclease element is in yellow. For each gene, the black arrow indicates the position of the forward primer used in the downstream RT-PCR experiment in combination with three different reverse primers. The green arrows indicate expression at the RNA level of the corresponding portion of the gene, while the red arrows indicate a negative amplification. B) Agarose gel electrophoresis figure showing the RT-PCR results. For each gene hybrid, the expression at the RNA level was tested using a forward primer in the conserved gene core and a reverse primer respectively in the inserted C-terminal (1), the mobile endonuclease (2) and the native C*-terminal portion (3). Primers in nad5 exon 4 were used as a positive control on cDNA (RT +) and negative control on RNA (RT −). The expected size of the amplified fragments was: atp6 inserted C-terminal (684 bp), atp9 inserted C-terminal (149 bp), atp9 mORF (717 bp), atp9 native C*-terminal (1085 bp), cox2 inserted C-terminal (938 bp), cox2 mORF (1291 bp), nad3 inserted C-terminal (261 bp), nad3 mORF (597 bp), nad3 native C*-terminal (1183 bp) and the positive control in nad5 exon 4 had an expected amplicon size of 689 bp. The red box indicates a faint band that is present on the gel.
Table 2. Description of the gene hybrids found in Glomus sp. 229456 mtDNA.
| atp6 | atp9 | cox2 | nad3 | ||
| Total length | 1569 | 1171 | 1894 | 1242 | |
| CDS length | 1569 | 225 | 837 | 1242 | |
| Features | |||||
| Group I intron | - | - | [684–922] | ||
| Inserted C-terminal | [537–774] 1 | [175–225] 1 | [923–1018] 1 | [194–313] 1 | |
| mORF | [550–1567] 1 | [334–1119] 1 | [1187–1738] 1 | [451–867] 1 | |
| Native C*-terminal | [1582–1860] | [1120–1171] 1 | [1739–1894] | [1116–1238] 1 | |
| Remarks | C-terminal and mORF in phase with native gene | C-terminal in phase with native gene. | Partial inserted C-terminal in phase with native gene. | C-terminal and mORF in phase with native gene. | |
Gene hybrid features that are expressed at the mRNA level (see Figure 4B).
These gene hybrid structures are similar to that of the atp6 gene previously described in the Allomyces macrogynus (Figure 4, grayscale box), a species that belongs to the basal fungal phylum Blastocladiomycota [48]. The same scenario has also been observed in the Rhizopus oryzae atp9 and Mortierella verticillata cox2 genes [30]. These hybrids contain a carboxy-terminal duplication as well as a mORF encoding an endonuclease, which has been biochemically demonstrated to be responsible for the element mobility. In Allomyces macrogynus, the inserted C-terminal was shown to have been recently acquired by HGT based on the divergence in sequence it had with the native C*-terminal, while the latter had a perfect sequence identity with the corresponding gene portion of the closely related species Allomyces arbusculus.
The Glomus sp. atp6, atp9, cox2 and nad3 native C*-terminals showed higher nucleotide sequence identity to those of G. irregulare 494 (91, 98, 93 and 98%, respectively) than their duplicated C-terminal counterparts (64, 71 and 81 and 73%, respectively) (Figures S1, S2, S3, and S4 and Tables S1, S2, S3, and S4). However at the protein level, the comparison of the C*-terminal amino acid sequences of the atp6, atp9, cox2 and nad3 genes with the corresponding portion in G. irregulare 494 was 100% for atp6, 94% for atp9, and 100% for cox2 and nad3. The high sequence identity of the native Glomus sp. C*-terminals with G. irregulare 494, is in stark contrast to the low similarity observed with the inserted C-terminal portions and points to a recent HGT event, as was described in Allomyces spp. [48]. However, the HGT hypothesis could likely apply to the atp6 and cox2 genes, since their native C*-terminal portion is no longer translated and could undergo rapid divergence. For the atp9 and nad3 hybrids, even though it is less parsimonious, the observed sequence divergence between the duplicated portions could have been caused by independent evolution following the mobile element insertion, since both are expressed in the mRNA transcript. It would also be interesting to see if some of the reported gene hybrids can still accomplish their functions at the protein level, given that the mORF and both C*-terminals are expressed in some cases. They are apparently expressed pseudogenes but post-translational modification mechanisms may be in place to ensure that the resulting protein is functional. We did not find a mORF-less copy of those genes that could have been transferred to the Glomus sp. nuclear genome that could explain a pseudogenization in Glomus sp. mtDNA.
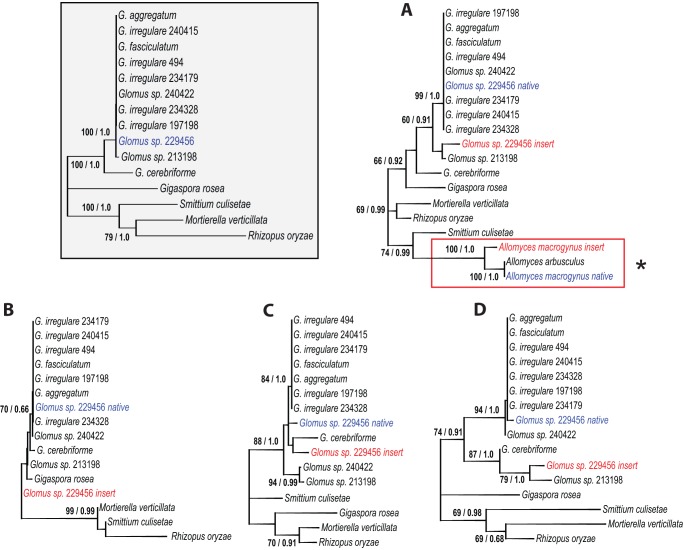
In regards to the HGT hypothesis, and in order to evaluate whether there is a plausible donor for the duplication, we compared the carboxy-terminal sequence of these genes with those in 11 Glomus spp. (to avoid redundancy we didn't add the G. margarita sequences since they are identical to G. rosea) and three phylogenetically related fungal representatives (Figure 5). In all four Glomus sp. gene hybrids (atp6, atp9, cox2 and nad3), the native C*-terminal sequences cluster within the Glomus spp. group as expected given the reference phylogeny (Figure 5, grayscale box), thereby supporting a recent insertion of the foreign element. The atp6 gene carboxy-terminal comparison (Figure 5A) shows that the mORF-derived C-terminal is related to a Glomus sp. isolate DAOM213198 with a moderate 60% bootstrap value. Surprisingly, in atp9 (Figure 5B) the inserted C-terminal is even more distantly related to Glomus spp. than to G. rosea. In cox2 (Figure 5C) the Glomus sp. inserted C-terminal and the more divergent AMF species G. cerebriforme are in the same cluster. Finally, the nad3 C-terminal clustered with Glomus sp. 213198, as it was the case for atp6, with a 79% bootstrap value (Figure 5D). Also, the nad3 gene shows high variability in length in Glomus spp., due to the insertion of those elements.
Figure 5. Unrooted maximum likelihood trees obtained with the GTR+G model (5 distinct gamma categories).
The first number at branches indicates ML bootstrap values with 1000 bootstrap replicates and the second number indicates posterior probability values of a MrBayes analysis with four independant chains. Bayesian inference predict similar trees (not shown). The concatenated tree of the atp6, atp9, cox2 and nad3 ‘core’ genes (without the duplicated C*-terminal portion) (1489 alignment positions) of selected AMF representatives (grayscale boxed) are compared with those of the atp6 (298 alignment positions), where the red box with the asterisk point out to the reference Allomyces spp. HGT event (Figure 4) (A), atp9 (51 alignment positions) (B), cox2 (106 alignment positions) (C) and nad3 (120 alignment positions) (D) C*-terminals. The Glomus sp. native C*-terminals are in blue, while the inserted C-terminals are in red.
In all four cases, the native Glomus sp. C*-terminal is nested within the Glomus spp. group and the inserted C-terminal is in a different cluster. Although it is difficult to pinpoint the donor of the sequence duplications, due to the possibly complex evolutionary history of those mobile elements with numerous insertion/loss events and 3′ end reshufflings, our data suggest HGT from a foreign AMF species, and thus the first reported occurrence in Glomeromycota. The presence of foreign DNA elements could potentially hamper mitochondrial gene phylogeny analysis unless the foreign C-terminals are carefully removed from the native portion of the gene.
Conclusion
The inclusion of mitochondrial sequences from phylogenetically distant AMF species in the database is essential for developing a better understanding and classification of AMF within fungi. The mitochondrial genome comparison presented here for two closely related AMF species reveals substantial changes in mitochondrial gene sequences, resulting from dpo plasmid insertions and mobile ORFs invasions, along with intergenic sequence variation. This illustrates the importance of adding closely related species to the numerous isolates of the same species in the AMF mitochondrial genome collection. Comparative mitochondrial genomics, together with a broader sequencing effort in AMF, opens new avenues for the development of molecular markers at different evolutionary distances. It would be interesting to identify the source of plasmid-related DNA polymerase in AMF mtDNA, which should provide an estimate of the extent to which it is present within the Glomeromycota phylum and an assessment of the consequences on mitochondrial genome organization. Also, the mORF-carried foreign C-terminal described here represents the first reported evidence of HGT in AMF. The intimate relationship between AMF, the roots of their plant symbiont and soil microorganisms might be a perfect biological context to facilitate such transfers. To what extent the mobilome and HGT may have contributed to AMF evolution is a topic that merits exploration in future studies.
Supporting Information
Multiple DNA sequence alignment of numerous AMF representatives of the atp6 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Multiple DNA sequence alignment of numerous AMF representatives of the atp9 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Multiple DNA sequence alignment of numerous AMF representatives of the cox2 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Multiple DNA sequence alignment of numerous AMF representatives of the nad3 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Sequence identity matrix of the atp6 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Sequence identity matrix of the atp9 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Sequence identity matrix of the cox2 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Sequence identity matrix of the nad3 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Acknowledgments
We thank Dr. B.F. Lang for bioinformatics assistance and access to an automated organelle genome annotation software and to Dr. David Morse and Dr. Terrence Bell for comments on the manuscript. We also like to thank Biopterre centre du développement des bioproduits and CRBM for their help.
Funding Statement
This work is a part of a research project organized and coordinated by Premier Tech. The authors are grateful for financial support from NSERC Cooperative Research and Development (CRD) grant number RDPJ 395241-09, Premier Tech and CRIBIQ. The authors would also like to thank Biopterre centre développement des bioproduits and CRBM for their support. This does not alter the authors′ adherence to all the PLOS ONE policies on sharing data and materials. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16: 299–363. [DOI] [PubMed] [Google Scholar]
- 2.Smith S, Read D (2008) Mycorrhizal Symbiosis. Cambridge: UK: Academic Press. [Google Scholar]
- 3. Azcón-Aguilar C, Barea JM (1997) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens – an overview of the mechanisms involved. Mycorrhiza 6: 457–464. [Google Scholar]
- 4.St-Arnaud M, Vujanovik V (2007) Effects of Arbuscular Mycorrhizal Fungi on Plant Diseases and Pests Mycorrhizae in Crop Production: Applying knowledge. Binghamton, NY: Haworth Press. [Google Scholar]
- 5. Datnoff LE, Nemec S, Pernezny K (1995) Biological Control of Fusarium Crown and Root Rot of Tomato in Florida Using Trichoderma harzianum and Glomus intraradices. Biological Control 5: 427–431. [Google Scholar]
- 6. Cordier C, Pozo MJ, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (1998) Cell Defense Responses Associated with Localized and Systemic Resistance to Phytophthora parasitica Induced in Tomato by an Arbuscular Mycorrhizal Fungus. Molecular Plant-Microbe Interactions 11: 1017–1028. [Google Scholar]
- 7. Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, et al. (2002) Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. Journal of Experimental Botany 53: 525–534. [DOI] [PubMed] [Google Scholar]
- 8. Ismail Y, Hijri M (2012) Arbuscular mycorrhisation with Glomus irregulare induces expression of potato PR homologues genes in response to infection by Fusarium sambucinum. Functional Plant Biology 39: 236–245. [DOI] [PubMed] [Google Scholar]
- 9. Ismail Y, McCormick S, Hijri M (2011) A Fungal Symbiont of Plant-Roots Modulates Mycotoxin Gene Expression in the Pathogen Fusarium sambucinum. PLoS ONE 6: e17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Croll D, Giovannetti M, Koch AM, Sbrana C, Ehinger M, et al. (2009) Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist 181: 924–937. [DOI] [PubMed] [Google Scholar]
- 11. Bever JD, Wang M (2005) Arbuscular mycorrhizal fungi: Hyphal fusion and multigenomic structure. Nature 433: E3–E4. [DOI] [PubMed] [Google Scholar]
- 12. Angelard C, Sanders IR (2011) Effect of segregation and genetic exchange on arbuscular mycorrhizal fungi in colonization of roots. New Phytologist 189: 652–657. [DOI] [PubMed] [Google Scholar]
- 13. Colard A, Angelard C, Sanders IR (2011) Genetic Exchange in an Arbuscular Mycorrhizal Fungus Results in Increased Rice Growth and Altered Mycorrhiza-Specific Gene Transcription. Applied and Environmental Microbiology 77: 6510–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corradi N, Croll D, Colard A, Kuhn G, Ehinger M, et al. (2007) Gene Copy Number Polymorphisms in an Arbuscular Mycorrhizal Fungal Population. Applied and Environmental Microbiology 73: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halary S, Malik S-B, Lildhar L, Slamovits CH, Hijri M, et al. (2011) Conserved Meiotic Machinery in Glomus spp., a Putatively Ancient Asexual Fungal Lineage. Genome Biology and Evolution 3: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, et al. (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytologist 193: 755–769. [DOI] [PubMed] [Google Scholar]
- 17. Sanders Ian R (2011) Fungal Sex: Meiosis Machinery in Ancient Symbiotic Fungi. Current biology: CB 21: R896–R897. [DOI] [PubMed] [Google Scholar]
- 18. Kuhn G, Hijri M, Sanders IR (2001) Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature 414: 745–748. [DOI] [PubMed] [Google Scholar]
- 19. Hijri M, Sanders IR (2005) Low gene copy number shows that arbuscular mycorrhizal fungi inherit genetically different nuclei. Nature 433: 160–163. [DOI] [PubMed] [Google Scholar]
- 20. Pawlowska TE, Taylor JW (2004) Organization of genetic variation in individuals of arbuscular mycorrhizal fungi. Nature 427: 733–737. [DOI] [PubMed] [Google Scholar]
- 21. Pawlowska TE, Taylor JW (2005) Arbuscular mycorrhizal fungi: Hyphal fusion and multigenomic structure (reply). Nature 433: E4–E4. [DOI] [PubMed] [Google Scholar]
- 22. Boon E, Zimmerman E, Lang BF, Hijri M (2010) Intra-isolate genome variation in arbuscular mycorrhizal fungi persists in the transcriptome. Journal of Evolutionary Biology 23: 1519–1527. [DOI] [PubMed] [Google Scholar]
- 23. VanKuren NW, den Bakker HC, Morton JB, Pawlowska TE (2013) Ribosomal RNA gene diversity, effective population size, and evolutionary longevity in asexual Glomeromycota. Evolution 67: 207–224. [DOI] [PubMed] [Google Scholar]
- 24. Raab PA, Brennwald A, Redecker D (2005) Mitochondrial large ribosomal subunit sequences are homogeneous within isolates of Glomus (arbuscular mycorrhizal fungi, Glomeromycota) Mycological Research. 109: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Young JP (2009) The mitochondrial genome sequence of the arbuscular mycorrhizal fungus Glomus intraradices isolate 494 and implications for the phylogenetic placement of Glomus. New Phytol 183: 200–211. [DOI] [PubMed] [Google Scholar]
- 26. Börstler B, Raab PA, Thiéry O, Morton JB, Redecker D (2008) Genetic diversity of the arbuscular mycorrhizal fungus Glomus intraradices as determined by mitochondrial large subunit rRNA gene sequences is considerably higher than previously expected. New Phytologist 180: 452–465. [DOI] [PubMed] [Google Scholar]
- 27. Thiéry O, Börstler B, Ineichen K, Redecker D (2010) Evolutionary dynamics of introns and homing endonuclease ORFs in a region of the large subunit of the mitochondrial rRNA in Glomus species (arbuscular mycorrhizal fungi, Glomeromycota). Molecular Phylogenetics and Evolution 55: 599–610. [DOI] [PubMed] [Google Scholar]
- 28. Lang B, Franz, Hijri M (2009) The complete Glomus intraradices mitochondrial genome sequence – a milestone in mycorrhizal research. New Phytologist 183: 3–6. [DOI] [PubMed] [Google Scholar]
- 29. Formey D, Moles M, Haouy A, Savelli B, Bouchez O, et al. (2012) Comparative analysis of mitochondrial genomes of Rhizophagus irregularis – syn. Glomus irregulare – reveals a polymorphism induced by variability generating elements. New Phytol 196: 1217–1227. [DOI] [PubMed] [Google Scholar]
- 30. Seif E, Leigh J, Liu Y, Roewer I, Forget L, et al. (2005) Comparative mitochondrial genomics in zygomycetes: bacteria-like RNase P RNAs, mobile elements and a close source of the group I intron invasion in angiosperms. Nucleic Acids Research 33: 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stockinger H, Walker C, Schüßler A (2009) ‘Glomus intraradices DAOM197198’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytologist 183: 1176–1187. [DOI] [PubMed] [Google Scholar]
- 32. Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytologist 193: 970–984. [DOI] [PubMed] [Google Scholar]
- 33. Pelin A, Pombert J-F, Salvioli A, Bonen L, Bonfante P, et al. (2012) The mitochondrial genome of the arbuscular mycorrhizal fungus Gigaspora margarita reveals two unsuspected trans-splicing events of group I introns. New Phytologist 194(3): 836–845. [DOI] [PubMed] [Google Scholar]
- 34.Nadimi M, Beaudet D, Forget L, Hijri M, Lang BF (2012) Group I intron-mediated trans-splicing in mitochondria of Gigaspora rosea, and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with Mortierellales. Molecular Biology and Evolution. doi: 10.1093/molbev/mss088. [DOI] [PubMed]
- 35.Hausner G (2012) Introns, Mobile Elements, and Plasmids. In: Bullerwell CE, editor. Organelle Genetics. Berlin: Springer. 329–357. [Google Scholar]
- 36. Griffiths AJF, Yang X (1995) Recombination between heterologous linear and circular mitochondrial plasmids in the fungus Neurospora . Molecular and General Genetics 249: 25–36. [DOI] [PubMed] [Google Scholar]
- 37.Klassen R, Meinhardt F (2007) Linear protein primed replicating plasmids in eukaryotic microbes. Microbial linear plasmids. Berlin: Springer. 188–226. [Google Scholar]
- 38.Kennel JC, Cohen SM (2004) Fungal mitochondria: genomes, genetic elements and gene expression. In: Arora DK, editor. The handbook of fungal biotechnology 2nd ed. New York: Marcel Dekker Inc. 131–143. [Google Scholar]
- 39.Brown GG, Zhang M (1995) Mitochondrial plasmids: DNA and RNA. In: Levings CS III, Vasil IK, editors. The molecular biology of plant mitochondria. Dordrecht: Kluwer. 61–91. [Google Scholar]
- 40. Bertrand H, Griffiths AJF (1989) Linear plasmids that integrate into mitochondrial DNA in Neurospora. Genome 31: 155–159. [Google Scholar]
- 41. Cahan P, Kennell J (2005) Identification and distribution of sequences having similarity to mitochondrial plasmids in mitochondrial genomes of filamentous fungi. Molecular Genetics and Genomics 273: 462–473. [DOI] [PubMed] [Google Scholar]
- 42. Ferandon C, Chatel SEK, Castandet B, Castroviejo M, Barroso G (2008) The Agrocybe aegerita mitochondrial genome contains two inverted repeats of the nad4 gene arisen by duplication on both sides of a linear plasmid integration site. Fungal Genetics and Biology 45: 292–301. [DOI] [PubMed] [Google Scholar]
- 43. Dujon B (1980) Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the co and rib-1 loci. Cell 20: 185–197. [DOI] [PubMed] [Google Scholar]
- 44. Bell-Pedersen D, Quirk S, Clyman J, Belfort M (1990) Intron mobility in phage T4 is dependent upon a distinctive class of endonucleases and independent of DNA sequences encoding the intron core: mechanistic and evolutionary implications. Nucleic Acids Research 18: 3763–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dalgaard JZ, Garrett RA, Belfort M (1993) A site-specific endonuclease encoded by a typical archaeal intron. Proceedings of the National Academy of Sciences 90: 5414–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma M, Ellis RL, Hinton DM (1992) Identification of a family of bacteriophage T4 genes encoding proteins similar to those present in group I introns of fungi and phage. Proceedings of the National Academy of Sciences 89: 6658–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eddy S (1992) Introns in the T-seven bacteriophages [Dissertation]. Boulder: University of Colorado. [Google Scholar]
- 48. Paquin B, Laforest MJ, Lang BF (1994) Interspecific transfer of mitochondrial genes in fungi and creation of a homologous hybrid gene. Proceedings of the National Academy of Sciences 91: 11807–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bécard G, Fortin JA (1988) Early events of vesicular–arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytologist 108: 211–218. [DOI] [PubMed] [Google Scholar]
- 50.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al.. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed]
- 51. Cho Y, Qiu Y-L, Kuhlman P, Palmer JD (1998) Explosive invasion of plant mitochondria by a group I intron. Proceedings of the National Academy of Sciences 95: 14244–14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanchez-Puerta MV, Cho Y, Mower JP, Alverson AJ, Palmer JD (2008) Frequent, Phylogenetically Local Horizontal Transfer of the cox1 Group I Intron in Flowering Plant Mitochondria. Molecular Biology and Evolution 25: 1762–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robison MM, Wolyn DJ (2005) A mitochondrial plasmid and plasmid-like RNA and DNA polymerases encoded within the mitochondrial genome of carrot Daucus carota . Current Genetics 47: 57–66. [DOI] [PubMed] [Google Scholar]
- 54. Brügger K, Torarinsson E, Redder P, Chen L, Garrett R (2004) Shuffling of Sulfolobus genomes by autonomous and non-autonomous mobile elements. Biochemical Society Transactions 32: 179–183. [DOI] [PubMed] [Google Scholar]
- 55. Biessmann H, Valgeirsdottir K, Lofsky A, Chin C, Ginther B, et al. (1992) HeT-A, a transposable element specifically involved in “healing” broken chromosome ends in Drosophila melanogaster. Molecular and Cellular Biology 12: 3910–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fricova D, Valach M, Farkas Z, Pfeiffer I, Kucsera J, et al. (2010) The mitochondrial genome of the pathogenic yeast Candida subhashii: GC-rich linear DNA with a protein covalently attached to the 5′ termini. Microbiology 156: 2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foury F, Roganti T, Lecrenier N, Purnelle B (1998) The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae . FEBS Lett 440: 325–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple DNA sequence alignment of numerous AMF representatives of the atp6 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Multiple DNA sequence alignment of numerous AMF representatives of the atp9 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Multiple DNA sequence alignment of numerous AMF representatives of the cox2 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Multiple DNA sequence alignment of numerous AMF representatives of the nad3 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(TIF)
Sequence identity matrix of the atp6 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Sequence identity matrix of the atp9 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Sequence identity matrix of the cox2 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)
Sequence identity matrix of the nad3 native C-terminals along with the Glomus sp. 229456 putative foreign inserted C*-terminal.
(DOC)