Abstract
The core promoter of eukaryotic genes is structurally and functionally diverse and contributes to the combinatorial control of gene-specific transcription. Recent findings identifying specific coactivators and architectural proteins as core promoter-specific basal cofactors for RNA polymerase II suggest possible mechanisms for the core promoter selectivity of certain regulators and enhancers.
Keywords: RNA polymerase II, core promoter-selective transcription, general transcription factors, TFIID, Mediator, HMG proteins, coactivators, positive and negative transcription cofactors
In eukaryotes, regulation of gene-specific transcription by RNA polymerase II (RNAP II) involves the combinatorial effects of diverse regulatory DNA sequences bound by sequence-specific DNA-binding regulators and core promoter DNA elements bound by the basal/general transcription machinery. This machinery is composed of the general transcription factors (GTFs) TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, and RNAP II, and is required for transcription of most protein-coding genes.1 Despite much progress in understanding the mechanisms of eukaryotic gene transcription, it is still unclear how differences in core promoter DNA sequence can lead to different levels of transcription and to a differential regulation by distal regulators and enhancers. Current models mostly emphasize the differential DNA-binding affinity of TFIID (and/or other GTFs) for different core promoters.1-4 However, as discussed here, several reports have now shown that the purified basal transcription machinery, including TFIID, cannot recapitulate the differential activity of core promoters observed in vivo or in crude cell-free extracts in vitro, but that additional auxiliary core promoter-selective cofactors are required. Some of these cofactors were recently purified and shown to be identical to previously characterized coregulators (e.g., coactivators) that are essential for the activities of enhancers and are thought to function as architectural and bridging factors that mediate the functions of distal sequence-specific regulators to the basal transcription apparatus.5,6 In light of these observations, this essay proposes that the long-known but still poorly understood core promoter-specific activities of enhancers and sequence-specific regulators7 could be mediated, at least in part, by coregulators with core promoter-dependent DNA/protein architectural functions. These coregulators in concert with TFIID and specific core promoter sequences are hypothesized here to modulate the differential assembly or “remodeling” of distinct stereo-specific transcription complexes on different core promoters.
The primary recognition of the core promoter is performed by the general transcription factor TFIID, which is a stable complex in metazoans composed of the TATA-binding protein (TBP) and 14 TBP-associated factors (TAFs). TFIID binds most core promoter elements identified thus far, with the exception of the BRE (BREu and BREd) sequences bound by TFIIB. Core promoter elements have been best characterized in metazoans and those recognized by TFIID include the TATA box that is bound and dramatically bent by TBP about 30 bp upstream of the transcription start site; the Initiator (INR) at the transcription start site that is bound by the TAF1 and TAF2 subunits; and several elements located at specific distances downstream of the INR, such as the downstream core element (DCE) bound by TAF1, and the motif ten element (MTE) and downstream promoter element (DPE) that are bound by TAF6 and TAF9.1,7 The INR is found in almost half of core promoters from yeast to humans, while the TATA box is present in only 10–20% of promoters for protein-coding genes. Promoters have different combinations of these elements and many lack all of the currently known core promoter motifs.1,7,8 Specific combinations of core promoter elements synergize in basal and activated transcription in vivo and in crude cell-free extracts in vitro. Accordingly, TFIID binds more efficiently to promoters containing several correctly spaced core promoter elements such as composite TATA- and INR-containing (TATA/INR) core promoters and a recently described artificial “super core promoter” composed of TATA, INR, MTE and DPE motifs.3,4 The latter super core promoter was shown to be more active in vitro and in vivo than two of the strongest natural core promoters, i.e., the cytomegalovirus (CMV) immediate early 1 and the adenovirus major late (AdML) core promoters. Consistent with the higher affinity of TFIID for promoters containing several core promoter elements, single-round transcription assays indicated that the synergistic activity of core elements is at the level of functional pre-initiation complex formation.4
While the above observations suggest that the different affinity of TFIID for structurally distinct core promoters contributes to their differential activity, there is also strong evidence indicating that additional mechanisms are involved. Indeed, there is an imperfect correlation between TFIID binding affinity and the basal activity of core promoters in vivo and in vitro. For instance, in the context of the TATA-less mouse TdT core promoter, which contains an INR and a DPE-like downstream element, the addition of a TATA box strongly increased its basal activity and its affinity for TFIID, which bound over the TATA, INR, and downstream sequences. However, mutations of the INR in this composite TATA/INR promoter did not detectably affect TFIID binding, but strongly reduced the basal activity of the promoter.9 Similarly, mutations of the INR in the TATA-containing AdML core promoter greatly reduced its activity in nuclear extracts but only reduced by 2-fold the affinity for TFIID/TFIIA complexes and did not affect TFIID interactions over the TATA, INR and downstream sequences.3 Thus, for these different TATA/INR core promoters with natural downstream TFIID recognition elements, the activity of the INR and its synergy with the TATA box do not simply correlate with TFIID binding. Moreover, in the case of the super core promoter (containing TATA, INR, MTE and DPE), mutation of TATA strongly reduced basal and activated transcription in vitro and in vivo, but had no significant effect on TFIID binding. The authors concluded that high affinity binding of TFIID might be necessary but not sufficient for high core promoter activity.4 Consistent with this, earlier reports showed that the basal transcription machinery (including TFIID) is not sufficient to support the function of the INR and its synergy with the TATA box.10 Hence, the TFIID-dependent activities of core promoter elements might not only involve effects on TFIID-promoter interactions but might also be regulated at additional levels requiring auxiliary core promoter-specific cofactors. As discussed below, core promoter-selective cofactors have now been identified and include positive cofactors for the TAF-dependent activity of the INR in synergy with the TATA box and for the function of the DPE, and negative cofactors of TBP-mediated TATA function. Notably, most of these cofactors have dual activities with either positive or negative functions depending on core promoter configuration (Table 1).
Table 1. Cofactors that regulate the core promoter-selective functions of TFIID.
| Core promoter-selective cofactors | Core promoter element regulated (promoter configuration) |
|
|---|---|---|
| Positive regulation | Negative regulation | |
| HMGA1 |
INR (TATA/INR synergy) |
TATA |
| Mediator (PC2) |
INR (TATA/INR synergy) |
? |
| Topo I (PC3) |
INR (TATA/INR synergy) |
TATA |
| PC4 |
DPE (Sp1-activated INR/DPE) |
TATA? |
| CK2 |
DPE (Sp1-activated INR/DPE) |
TATA? |
| NC2 (DR1-DRAP1) |
DPE (INR/DPE) |
TATA |
| BTAF1 (MOT1) | DPE (INR/DPE) | TATA |
? indicate unknown or possible roles postulated from published data
An activity that selectively stimulated DPE-dependent (INR/DPE type) but not TATA-dependent (TATA/INR type) core promoters in Drosophila embryo extracts was purified and identified as the “negative cofactor 2” (NC2), also known as DR1-Drap1.11 NC2 was originally identified as a repressor of TATA-dependent transcription that binds the TBP-TATA complex and blocks entry of TFIIA and TFIIB.12-14 However, the ability of NC2 to activate DPE-dependent transcription in vitro is independent of its TATA inhibitory activity.11 Loss of function experiments by RNA interference (RNAi) further demonstrated that NC2 and BTAF1/Mot1, another inhibitor of TBP that displaces TBP from TATA elements in an ATP-dependent manner,15 both inhibit TATA promoters but are needed for DPE-dependent transcription of endogenous genes in Drosophila embryonic cells.16 How NC2 (or BTAF1/Mot1) potentiates DPE-dependent transcription remains unclear. In apparent contrast, another study reported that the protein kinase CK2 and the positive cofactor/coactivator PC4, but not NC2, were required for DPE-dependent transcription.5 The latter study, however, analyzed Sp1-activated transcription of INR/DPE promoters in a system reconstituted with purified human GTFs, RNAP II, the activator Sp1, and the Mediator coactivator complex.5 From these contrasting observations it seems likely that distinct DPE cofactor requirements and mechanisms might exist, which could be context-dependent and/or influenced by specific activators. Note also that chromatin structure and the presence of competing promoters are likely to add additional levels of regulation in vivo. Hence, the above findings and earlier observations10 demonstrated the requirement of auxiliary core promoter-specific cofactors for efficient transcription of TATA-independent INR/DPE core promoters by the purified basal transcription machinery. Taken together with data indicating that TBP-DNA interactions are dispensable for the basal activity of certain TATA-less core promoters,9,17 and that NC2 facilitates maintenance of TBP on TATA-less promoter DNA,18 the above observations suggest fundamental differences in the mechanisms of transcription directed by TATA/INR and TATA-less INR/DPE promoters. Notably, however, a truly TATA-independent basal transcription initiation process in which TBP-DNA binding plays no significant role (as for the INR/DPE-like mouse TdT promoter9,17) has yet to be recapitulated in vitro with purified factors and requires several still unknown auxiliary cofactors.10 These elusive cofactors could potentially include the above DPE-specific cofactors and/or additional INR-dependent cofactors such as those described hereafter.
Cofactors that mediate the TAF-dependent stimulatory activities of the INR element in synergy with the TATA box were recently purified from HeLa cell nuclear extracts and identified as the chromatin architectural protein HMGA1 (formerly known as HMGI/Y) and the Mediator coregulator complex.6 By itself, HMGA1 weakly stimulated the TAF- and INR-dependent basal activity of TATA/INR promoters but repressed transcription from TATA-only core promoters in an in vitro system reconstituted with purified GTFs and RNAP II. In contrast, Mediator did not have intrinsic core promoter-selectivity. However, in the presence of HMGA1 (and TAFs), Mediator preferentially stimulated INR-dependent transcription from TATA/INR core promoters.6 Thus, HMGA1 appears to elicit a “facultative” core promoter-dependent basal stimulatory activity of Mediator. Such core promoter-selective activity of Mediator was unexpected since Mediator is typically thought of as a “general coactivator” required for activation of all protein-coding genes.19 In addition, HMGA1 and Mediator also counteracted in an INR-dependent manner the negative regulation of TATA by NC2 and DNA Topoisomerase I (Topo I). Loss of function experiments by RNAi and gene knockout further indicated an INR-dependent basal transcription function of HMGA1 from TATA/INR core promoters in mammalian cells, including a stimulatory role at endogenous TATA/INR genes in mouse embryonic stem (ES) cells.6
The mechanisms underlying the core promoter-dependent regulation of Mediator and the basal machinery by HMGA1, and the roles of other core promoter-selective cofactors, remain to be elucidated. HMGA1 does not have an intrinsic transactivation function but is thought to regulate gene-specific transcription as an architectural cofactor that coordinates the cooperative DNA binding of activators into large stereo-specific enhanceosomes. HMGA1 also influences higher order chromatin structure and induces DNA/chromatin loops that facilitate long-range enhancer communication with target promoters. HMGA1 binds and alters the conformation of DNA via its three N-terminal “AT-hook” motifs that recognize the minor groove of short A/T-rich DNA sequences (including TATA elements) and non-B-form DNA structures. HMGA1 also interacts with many nuclear proteins, including sequence-specific activators, and induces conformational changes in some of its targets (reviewed in ref. 20). Interestingly, in human cells HMGA1 interacts with TFIID and a form of Mediator that lacks the CDK8 subunit.6 Moreover, TAF3 was identified as an HMGA1-interacting protein in a phage display screening, suggesting that TAF3 could be one of the subunits contacted by HMGA1 within the TFIID complex.21 HMGA1 has an acidic C-terminal tail (C-tail) that is necessary for its interaction with TFIID and Mediator and for stimulation of TATA/INR transcription.6 While still poorly understood the molecular functions of the C-tail seem to involve attenuation of activities associated with the N-terminal domain containing the AT-hooks, including HMGA1 self-association, DNA-binding, and inhibition of TATA-directed transcription (reviewed in ref. 6). Thus, the DNA/protein architectural functions of HMGA1 probably play a role in its core promoter-dependent positive and negative activities on basal transcription and may involve concerted interactions with core promoter DNA, TFIID/TAFs, and Mediator.
Hypothetical mechanisms for the core promoter-specific regulation of TFIID-dependent basal transcription by HMGA1 and other core promoter-selective cofactors are proposed in Figure 1. This model postulates not only differential core promoter-specific effects of these cofactors on recruitment of TFIID and assembly of preinitiation complexes, but also architectural effects either in the formation of distinct stereo-specific intermediary complexes and/or in the remodeling of these structural intermediates into functional RNAP II initiation complexes. For instance, the TFIIA-dependent interactions of TFIID with the INR and specific downstream sequences observed on certain promoters, which involve several TAFs and probably the HMG-box or AT-hook motifs in human and Drosophila TAF1, respectively, have been proposed to bend/wrap promoter DNA3,22 and could be facilitated by HMGA1 (or other cofactors). These stereo-specific interactions could either enhance subsequent transcription steps or require further remodeling to allow recruitment of other GTFs and/or the unwinding of the DNA and its entry into the RNAP II active site. Note that while not discussed here, the core promoter structure can also affect transcription elongation,23 and both physical and functional links between HMGA1 and the positive transcription elongation factor b (P-TEFb) have been described.24 In support of the view that structural remodeling and allosteric processes could underlie the mechanisms of core promoter-specific transcription, most (if not all) core promoter-selective cofactors identified (Table 1), in addition to the GTFs that directly bind core promoter elements (i.e., TFIID, TFIIA, and TFIIB), recognize or induce specific conformations in DNA or in other DNA-binding proteins. For Mediator, such architectural roles have only recently emerged. The binding of the Mediator head module to RNAP II was shown to trigger the opening of the RNAP II DNA cleft, which presumably could facilitate the entry of DNA into the RNAP II active site.25 Moreover, structural remodeling of Mediator by its interacting regulators has also been reported (reviewed in ref. 25). In this respect, it would be interesting to determine whether HMGA1 reconfigures the composition/architecture of the Mediator complex or simply binds selectively to a Mediator species that lacks the CDK8 subunit/module.6 In contrast, CK2 might have structural functions indirectly via its phosphorylation and possible regulation of TFIID/TAFs, PC4, and/or the acidic C-tail of HMGA1.5,20 Other enzymes and posttranslational modifications (PTMs) could also impact on the core promoter-specific functions of these cofactors. For instance, HMGA1 is a substrate for extensive modification by various PTMs, including acetylation by coactivators with histone acetyltransferase (HAT) activity.20,26
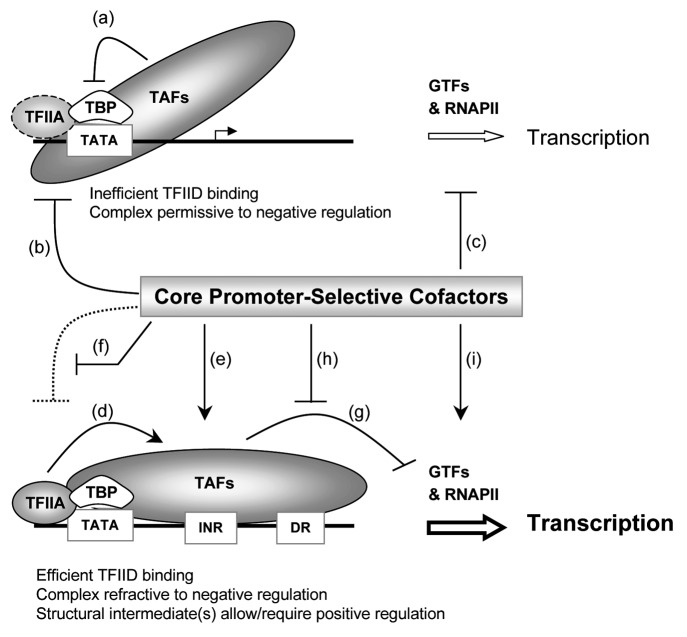
Figure 1. Hypothetical model depicting possible effects of core promoter-selective cofactors on TFIID-dependent transcription from distinct core promoters. (Top) TATA-only core promoters bind TFIID inefficiently perhaps due in part to reported negative effects of TAFs (e.g., TAF1 N-terminal domain) on TBP binding to TATA (a) and to the lack of TFIID/TAFs interactions with the downstream promoter region. The transcription start site (bent arrow) and downstream sequences are not bound by TAFs and available for interactions with other GTFs and RNAP II. However, the TFIID-DNA complex structure is proposed to be poorly responsive to the stabilizing effects of TFIIA and permissive to negative regulation by core promoter-selective cofactors such as NC2 that competes with TFIIA and TFIIB (b), and HMGA1 that could potentially inhibit TBP binding to TATA (b) and/or hamper efficient progression to a complete/functional preinitiation complex (c). (Bottom) Composite TATA/INR core promoters bind TFIID efficiently via cooperative effects of TBP binding to TATA and TAFs interactions with the INR and other sequences in the downstream region (DR), which may include MTE, DPE, or DCE elements. The interactions of TAFs with the INR and DR regions are facilitated by TFIIA (d) and perhaps by HMGA1 and Mediator (e), and may prevent negative regulation of the TBP-TATA complex by TAF1, NC2, HMGA1, and/or Topo I (f). The resulting nucleoprotein complex containing TFIID bound to the transcription initiation site and downstream region is likely to be refractory to subsequent entry of other GTFs and RNAP II, and/or may interfere with the drastic remodeling of DNA and GTFs/RNAP II that occurs during open complex formation and the transition to transcription initiation and elongation (g). Core promoter selective cofactors (e.g., HMGA1 and Mediator) could also regulate these subsequent steps by, for instance, remodeling the TAF-DNA complex over the INR/DR regions to facilitate the recruitment of GTFs/RNAP II (h), or by facilitating structural transitions in RNAP II (and associated GTFs) that are needed to generate productive initiation and early elongation complexes (i). Core promoter-selective architectural cofactors such as HMGA1 could potentially act transiently by a “hit-and-run” mechanism on structural intermediates. Additional or alternative core promoter-selective cofactors may be required depending on the core promoter configuration (e.g., CK2 and PC4 for DPE function). TFIIA, TFIID/TAFs, and core promoter-selective cofactors (gray shaded) are coregulators - e.g., coactivators - and their functions above may be regulated by sequence-specific DNA-binding regulators and perhaps by signaling pathways involving posttranslational modifications. See text for references and further details.
It is interesting to note that most of the core promoter-selective cofactors identified have well established coactivator functions in vitro and in vivo. Moreover, both HMGA1 and Mediator have also been implicated in long-range DNA looping and enhancer function (reviewed in refs. Six and 20). Hence, it is tempting to speculate that via their ability to form multiple interactions within nucleoprotein enhancer and core promoter complexes these (and perhaps other) core promoter-selective coregulators could directly mediate the still poorly understood core promoter-dependent regulatory activities of certain enhancers and sequence-specific DNA-binding activators.7 Alternatively, as eluded above, activators or enhancers could act indirectly via their recruitment of coactivators with catalytic activities (e.g., HATs), which in turn could modify TFIID/TAFs, Mediator, HMGA1 and other core promoter-selective cofactors.
To conclude, we have only begun to understand the regulatory potential of the core promoter and the diversity of core promoter structure-dependent pathways that control, in combination with distal regulators, the final assembly of a productive RNAP II initiation complex. Cooperative low affinity protein-DNA interactions and dynamic stereo-specific or allosteric mechanisms will probably play important roles at most eukaryotic core promoters. Given the diversity of core promoter structures it is likely that additional core promoter-specific architectural cofactors will be identified. Of particular interest are other members of the HMG superfamily of architectural proteins, including HMGB proteins, which have long been known as coactivators/coregulators and in a few cases have also been reported to regulate specific core promoters from yeast to mammals (e.g., refs. 27–29). Important future challenges will be to reconstitute core promoter-dependent regulatory pathways in vitro for the diverse types of natural core promoters and to characterize the mechanisms, including the molecular dynamics at play in vitro and in vivo.
Acknowledgments
I would like to thank collaborators and members of my laboratory for their contributions to the studies cited in this article and especially Muyu Xu for stimulating discussions and insightful comments. This work was supported by a grant from NSF (MCB-1021696).
Glossary
Abbreviations:
- RNA polymerase II
RNAP II
- general transcription factors
GTFs
- TATA-binding protein
TBP
- TBP-associated factors
TAFs
- Initiator
INR
- downstream core element
DCE
- downstream promoter element
DPE
- motif ten element
MTE
- cytomegalovirus
CMV
- adenovirus major late
AdML
- high mobility group
HMG
- embryonic stem cell
ES cell
- terminal deoxynucleotidyltransferase
TdT
- RNA interference
RNAi
- posttranslational modification
PTM
- histone acetyltransferase
HAT
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/21846
References
- 1.Hahn S. Structure and mechanism of the RNA polymerase II transcription machinery. Nat Struct Mol Biol. 2004;11:394–403. doi: 10.1038/nsmb763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verrijzer CP, Chen JL, Yokomori K, Tjian R. Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell. 1995;81:1115–25. doi: 10.1016/S0092-8674(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 3.Emami KH, Jain A, Smale ST. Mechanism of synergy between TATA and initiator: synergistic binding of TFIID following a putative TFIIA-induced isomerization. Genes Dev. 1997;11:3007–19. doi: 10.1101/gad.11.22.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juven-Gershon T, Cheng S, Kadonaga JT. Rational design of a super core promoter that enhances gene expression. Nat Methods. 2006;3:917–22. doi: 10.1038/nmeth937. [DOI] [PubMed] [Google Scholar]
- 5.Lewis BA, Sims RJ, 3rd, Lane WS, Reinberg D. Functional characterization of core promoter elements: DPE-specific transcription requires the protein kinase CK2 and the PC4 coactivator. Mol Cell. 2005;18:471–81. doi: 10.1016/j.molcel.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Xu M, Sharma P, Pan S, Malik S, Roeder RG, Martinez E. Core promoter-selective function of HMGA1 and Mediator in Initiator-dependent transcription. Genes Dev. 2011;25:2513–24. doi: 10.1101/gad.177360.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juven-Gershon T, Kadonaga JT. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev Biol. 2010;339:225–9. doi: 10.1016/j.ydbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Bolotin E, Jiang T, Sladek FM, Martinez E. Prevalence of the initiator over the TATA box in human and yeast genes and identification of DNA motifs enriched in human TATA-less core promoters. Gene. 2007;389:52–65. doi: 10.1016/j.gene.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez E, Zhou Q, L’Etoile ND, Oelgeschläger T, Berk AJ, Roeder RG. Core promoter-specific function of a mutant transcription factor TFIID defective in TATA-box binding. Proc Natl Acad Sci U S A. 1995;92:11864–8. doi: 10.1073/pnas.92.25.11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez E, Ge H, Tao Y, Yuan CX, Palhan V, Roeder RG. Novel cofactors and TFIIA mediate functional core promoter selectivity by the human TAFII150-containing TFIID complex. Mol Cell Biol. 1998;18:6571–83. doi: 10.1128/mcb.18.11.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willy PJ, Kobayashi R, Kadonaga JT. A basal transcription factor that activates or represses transcription. Science. 2000;290:982–5. doi: 10.1126/science.290.5493.982. [DOI] [PubMed] [Google Scholar]
- 12.Meisterernst M, Roeder RG. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991;67:557–67. doi: 10.1016/0092-8674(91)90530-C. [DOI] [PubMed] [Google Scholar]
- 13.Inostroza JA, Mermelstein FH, Ha I, Lane WS, Reinberg D. Dr1, a TATA-binding protein-associated phosphoprotein and inhibitor of class II gene transcription. Cell. 1992;70:477–89. doi: 10.1016/0092-8674(92)90172-9. [DOI] [PubMed] [Google Scholar]
- 14.Goppelt A, Stelzer G, Lottspeich F, Meisterernst M. A mechanism for repression of class II gene transcription through specific binding of NC2 to TBP-promoter complexes via heterodimeric histone fold domains. EMBO J. 1996;15:3105–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Auble DT, Hansen KE, Mueller CG, Lane WS, Thorner J, Hahn S. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8:1920–34. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JY, Juven-Gershon T, Marr MT, 2nd, Wright KJ, Tjian R, Kadonaga JT. TBP, Mot1, and NC2 establish a regulatory circuit that controls DPE-dependent versus TATA-dependent transcription. Genes Dev. 2008;22:2353–8. doi: 10.1101/gad.1681808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez E, Chiang CM, Ge H, Roeder RG. TATA-binding protein-associated factor(s) in TFIID function through the initiator to direct basal transcription from a TATA-less class II promoter. EMBO J. 1994;13:3115–26. doi: 10.1002/j.1460-2075.1994.tb06610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilfillan S, Stelzer G, Piaia E, Hofmann MG, Meisterernst M. Efficient binding of NC2.TATA-binding protein to DNA in the absence of TATA. J Biol Chem. 2005;280:6222–30. doi: 10.1074/jbc.M406343200. [DOI] [PubMed] [Google Scholar]
- 19.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–9. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Reeves R. HMGA proteins: flexibility finds a nuclear niche? Biochem Cell Biol. 2003;81:185–95. doi: 10.1139/o03-044. [DOI] [PubMed] [Google Scholar]
- 21.Malini E, Maurizio E, Bembich S, Sgarra R, Edomi P, Manfioletti G. HMGA Interactome: new insights from phage display technology. Biochemistry. 2011;50:3462–8. doi: 10.1021/bi200101f. [DOI] [PubMed] [Google Scholar]
- 22.Oelgeschläger T, Chiang CM, Roeder RG. Topology and reorganization of a human TFIID-promoter complex. Nature. 1996;382:735–8. doi: 10.1038/382735a0. [DOI] [PubMed] [Google Scholar]
- 23.Dikstein R. The unexpected traits associated with core promoter elements. Transcription. 2011;2:201–6. doi: 10.4161/trns.2.5.17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eilebrecht S, Benecke BJ, Benecke A. 7SK snRNA-mediated, gene-specific cooperativity of HMGA1 and P-TEFb. RNA Biol. 2011;8:1084–93. doi: 10.4161/rna.8.6.17015. [DOI] [PubMed] [Google Scholar]
- 25.Cai G, Chaban YL, Imasaki T, Kovacs JA, Calero G, Penczek PA, et al. Interaction of the mediator head module with RNA polymerase II. Structure. 2012;20:899–910. doi: 10.1016/j.str.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Wang Y. High mobility group proteins and their post-translational modifications. Biochim Biophys Acta. 2008;1784:1159–66. doi: 10.1016/j.bbapap.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shykind BM, Kim J, Sharp PA. Activation of the TFIID-TFIIA complex with HMG-2. Genes Dev. 1995;9:1354–65. doi: 10.1101/gad.9.11.1354. [DOI] [PubMed] [Google Scholar]
- 28.Paull TT, Carey M, Johnson RC. Yeast HMG proteins NHP6A/B potentiate promoter-specific transcriptional activation in vivo and assembly of preinitiation complexes in vitro. Genes Dev. 1996;10:2769–81. doi: 10.1101/gad.10.21.2769. [DOI] [PubMed] [Google Scholar]
- 29.Reeves R. Nuclear functions of the HMG proteins. Biochim Biophys Acta. 2010;1799:3–14. doi: 10.1016/j.bbagrm.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]