Abstract
Since its discovery more than a decade ago, H3K4 methylation has become synonymous with transcription. We only now have begun to realize that the distinct states of H3K4 methylation have unique distributions and specialized roles in other chromatin-related processes. Here, I discuss recent findings addressing their regulation and functions.
Keywords: chromatin, transcription, H3K4 methylation, KMT2, MLL, Set1, Trx, Trr
Introduction
Chromatin, the nucleoprotein structure protecting eukaryotic genomes, impedes the access of protein to DNA during processes like transcription, replication, recombination and repair. The fundamental repeating unit of chromatin is the nucleosome core particle, which consists of 146 bp of DNA wrapped around an octamer of two copies of each histone, H2A, H2B, H3 and H4. Cells possess an array of conserved multiprotein complexes that posttranslationally modify histones or incorporate variant histones to regulate access to the DNA.1,2 Many chromatin changes are functionally interconnected and are believed to establish an intricately balanced ‘histone code’3. Central for the regulation of the histone code are so-called ‘effector’ domains that target associated chromatin modifiers to pre-modified nucleosomes. These effectors connect certain posttranslational modifications (PTMs) with others that are introduced by associated factors. One example is the methylation of histone H3 at lysine 4 (H3K4), which is in general implicated in transcription. The epsilon amino group of H3K4 can be mono- (H3K4me1), di- (H3K4me2) or trimethylated (H3K4me3). Although H3K4 methylation is a universal mark for nucleosomes near the transcription start sites (TSS) of expressed genes in all eukaryotes, more recent studies suggest that H3K4me1, H3K4me2 and H3K4me3 have specific roles in chromatin and might be targeted by different effectors and associated modifiers. While both methyltransferases and demethylases of H3K4 have been extensively studied, it is still unclear how they contribute to the differential methylation states of H3K4. Here, I will discuss recent findings from our and other laboratories addressing these important issues.
Distributions of H3 lysine 4 mono-, di- and trimethylation
Genome-wide distribution studies by chromatin immunoprecipitation and microarray analysis (ChIP/chip) revealed that H3K4me3 is found in nucleosomes near the transcription start sites (TSS) of expressed genes in all eukaryotes.1,4 These nucleosomes also contain, to variable extents, H3K4me2, and H3K4me1—presumably due to partial methylation of H3K4. In addition, H3K4me2 is found in nucleosomes further downstream in the body of genes, while H3K4me1 is prevalent in their 3′ regions.1 More recent ChIP/high-throughput sequencing studies (ChIP/seq) addressed whether H3K4me1 is also present in non-transcribed regions in the fly and mammalian genomes. This work revealed that developmental enhancers of these organisms are in general enriched for H3K4me1, while H3K4me2 or H3K4me3 are underrepresented or lacking.5-7
Work on H3K4me2 was less conclusive since most commercial anti-H3K4me2 antisera exhibit substantial cross-reactivity to H3K4me3 and H3K4me1. Nevertheless, some studies mapped H3K4me2 to silent hematopoietic genes in mammalian blood stem cells, kinetochores, and other specialized chromatin regions.8,9 In flies, H3K4me2 was only found in domains that were also enriched for H3K4me3,10 which is somewhat unexpected considering the otherwise conserved distributions of H3K4me1 vs. H3K4me3 in both vertebrates and invertebrates. Substantial differences between the distributions of H3K4me3 and H3K4me2 are detectable using competitive ChIP (cCHIP), which was developed by us.11 In this method, synthetic H3K4-methylated peptides are added to the primary antibody to prevent cross-reactive IgGs from binding to chromatin. Using competitive immunofluorescence microscopy (cIFM), we were able to detect regions that exclusively H3K4me1, H3K4me2 or H3K4me3 on polytene chromosomes. Due to the relatively low resolution of the microscopic pictures, differences in the chromosomal banding patterns are likely to correspond to domains of up to several hundred kilobases. This supports that all three H3K4 methylation states have unique functions in higher eukaryotes. Despite their presence at developmentally regulated genes and other specialized chromosome regions, it is still unclear how these domains are generated. As outlined in the following, preliminary evidence supports that both methyltransferases as well as demethylases might be involved in their formation.
Histone H4K4 methyltransferases
The enzymes implicated in bulk methylation of H3K4 constitute the type 2 lysine methyltransferase (KMT2) family (reviewed in10; Figure 1A). The three prototypic members of this family are fly Trithorax (Trx), human Mixed Lineage Leukemia 1 (MLL1) and yeast Set1. All three proteins share homology in their C-terminal SET domains, which later were identified as histone methyltransferase domains. Apart from their SET domains, Trx and MLL1 are structurally rather distinct from Set1 (Fig. 1B). Despite these differences, all three proteins were considered for nearly a decade as main H3K4 trimethyltransferases with global roles in transcription.12 The major reason was that yeast does not possess any MLL/Trx-type KMT2s, while flies and mammals appeared to lack Set1-relatives. More recent studies revealed that Set1-relatives have a major role in H3K4 methylation at promoters, while MLL/Trx-type proteins appear to have acquired specialized roles in developmental gene regulation.11,13
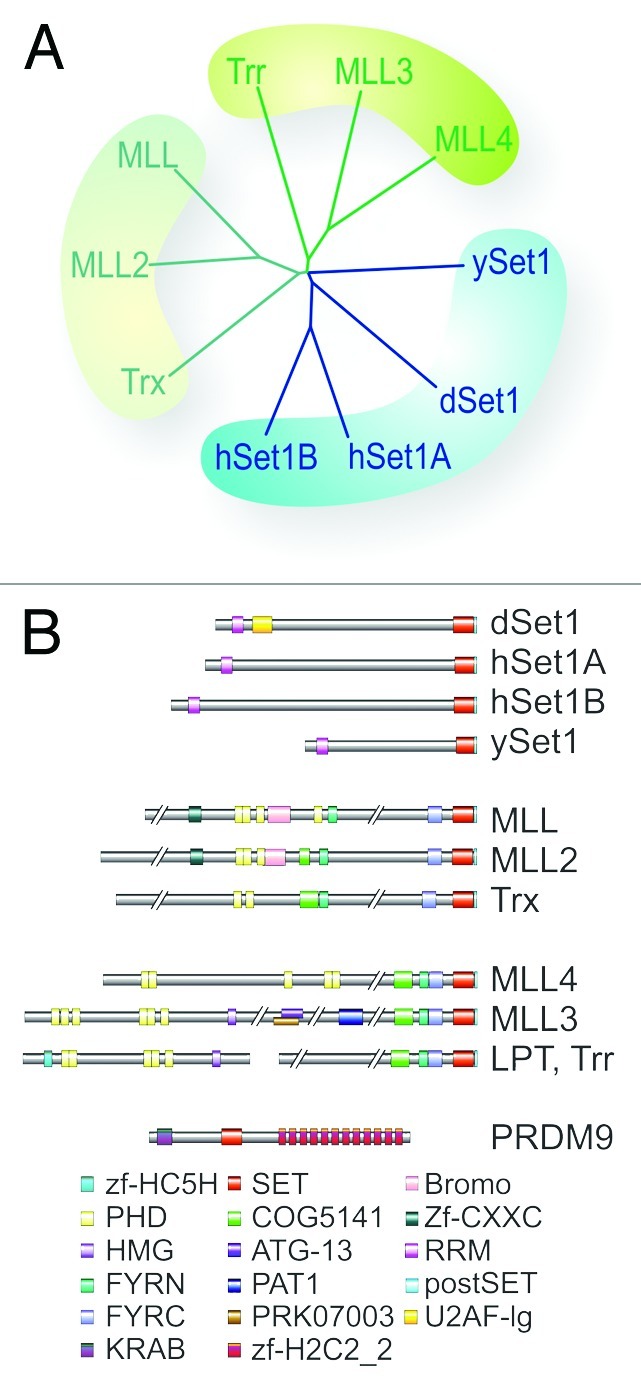
Figure 1. Comparison of H3K4 methyltransferase subfamilies. (A) Phylogenetic comparison of KMT2 superfamily from S. cerevisiae, D. melanogaster, and H. sapiens generated with Phylip (bootstrap value 1000, 15 repeats). (B) Domain structure comparison between known H3K4-specific KMT2s. LPT, a component of Trr complexes, is homologous to the N-termini of Mll3/Mll4-relatives. PRDM9 is a mammalian gonad-specific H3K4 KMT with role in meiotic recombination. Abbreviations, homology regions: zf-HC5H, Zinc finger of HC5H class; PHD, Plant Homeodomain; HMG, High Mobility Group; FYRN/C: F/Y-rich N-/C-terminal; SET, Suvar3–9, Enhancer of Zeste, Trithorax domain; COG5141, PHD zinc finger-spanning domain; ATG-13, autophagy-related 13 homolog; PAT1, PAT family N-terminal domain; PRK07003, homology to DNA polymerase II subunits gamma and tau; Bromo, bromodomain; Zf-CXXC, CXXC-type zinc finger; RRM, RNA Recognition Motif; postSET, cysteine-rich motif, typical for histone-specific KMTs; U2AF-lg, U2 snRNP auxiliary factor, large subunit; KRAB, Krüppel-associated box; zf-H2C2_2, zinc-finger double domain.
Functional diversification among H3K4 methyltransferases in higher eukaryotes
The identification of two human Set1 complexes with structural similarity to the yeast COMPASS (Complex Proteins Associated with Set1) complex raised questions about the contribution of Set1 vs. Trx/MLL-type KMT2s to global H3K4 methylation in metazoans.14 Moreover, the discovery of a second, Trithorax-related protein (Trr) in flies and three more MLL-like proteins from mammals suggested a functional diversification among Trx/MLL-relatives.13 This is supported by phylogenetic comparisons indicating that KMT2s fall into three distinct subfamilies (Fig. 1A).11 The first group consists of the highly conserved Set1 proteins. The MLL-relatives can be further subdivided into the Trx/MLL1/-2 subgroup and the Trr/Mll3/4 branch (Fig. 1A). Evidence for a specialization among these proteins was provided by genome-wide distribution studies on MLL1 showing that this factor only targets less than 3% of all expressed genes.13 Since a functional dissection of all six KMT2s in mammalian cells is technically extremely challenging, it is still unclear whether Set1- and Mll-relatives from mammals have unique functions or redundant roles.
Our discovery of a single-copy gene coding for fly Set1 made Drosophila ideal for such studies.11 These studies revealed that the fly and human COMPASS complexes are identically composed, indicating a high degree of conservation. Similar observations were made for the Trx and Trr complexes from flies, which—as far as characterized—also appear assemble into complexes similar to their human counterparts.15
With the identification of dSet1, we also were able to systematically assess the contribution of each of the three KMT2s to differential H3K4 methylation.11 These studies revealed that dSet1 was required for global H3K4me2, and H3K4me3. Surprisingly, Trr was mainly responsible for H3K4me1, while the loss of Trx had no major effect on H3K4me1–3. Considering that H3K4me1 is enriched at developmental enhancers, our data identified Trr-relatives as potential candidates for KMT2s with roles in these regions.
cIFM studies confirmed that dSet1 nearly fully overlapped with transcribing RNA polymerase II on chromosomes. Only few of these regions were also Trr or Trx, indicating that these might function in parallel at some expressed genes. Detailed genome-wide studies combined with functional assays should provide much needed insights into the overlapping and unique target genes of each of the three KMT2s in flies.
Differential chromatin targeting by KMT2s
Members of the Set1 subfamily do not contain any effector domains that could contribute to their chromatin targeting, but share a RNA recognition motif (RMM), which is essential for their KMT activity.13 It is still unclear how far RNA binding affects the KMT activity of these enzymes. By contrast, the members of other two subfamilies share domains in their N-termini, which are likely to contribute to their chromatin distribution and function (Fig. 1B).11 Common for these factors are tandem repeats of Plant Homeodomain (PHD) or related zinc fingers. An exception appears to be Trr, which lacks these domains; however, recent studies identified an interaction partner of Trr with high homology to the N-terminus of MLL3/-4 (Fig. 1B).15 The protein was called Lost PHDs of Trr (LPT). Although this underscores the importance of the N-terminal tandem zinc fingers in these factors, a comprehensive analysis of their effector functions has not yet been conducted.
Certain PHDs can cage methyl-groups at H3K4 via aromatic amino acids, but most PHDs in the MLL-relatives do not contain such residues in critical positions. Recent studies, however, showed that tandem PHDs can bind to acetylated histones.16 Intriguingly, MLL1 and MLL2 contain a bromodomain, which also has a well-established role in acetyl-histone binding. In fact, Trx/MLL1-relatives interact with the histone lysine acetyltransferase (KAT), CBP, but in a rather unexpected context.17 Trx/MLL1-type factors contain a protease cleavage site that is flanked by FY-rich N- and C-terminal domains. The proteolytic cleavage of these factors is believed to have activating function, and the interaction between the FY-rich domains tethers the two fragments into one complex. Unexpectedly, the N-terminal fragment of Trx was found in chromatin domains lacking H3K4 methylation or the Trx-CT. In these domains, CBP introduces H3K27 acetylation. CBP requires Trx-NT for its KAT activity, supporting that the N-termini of Trx/MLL1-relatives are utilized for the targeting of other chromatin modifiers.18
Members of the Trr/MLL3/-4 subfamily lack the protease cleavage site, suggesting that their regulation and chromatin distribution are less complex. In this context, it is tempting to speculate that the separation of Trr and LPT generates a more potent KMT and enables LPT to interact with other factors. H3K4me1 is predominant at early developmental enhancers, and our studies identified Trr as major H3K4 monomethyltransferase.11 Since early embryogenesis progresses very rapidly in flies, they might require highly active H3K4-monomethyltransferases compared with mammals. This is consistent with our findings that the SET domain of Trr has robust KMT activity in vitro.11
Despite the implications of the MLL-relatives in developmental gene regulation and leukemogenesis, their complex chromatin roles are still mostly obscure. Considering the high degree of structural conservation between mammals and flies, a combination of proteomic and genomic studies in less complex models like Drosophila should provide critical working hypotheses for studies in mammals (Fig. 1). Of particular value will be the proteomic dissection of complexes that exclusively contain the N- vs. C-terminal fragments of these proteins.
Differential H3K4 demethylation is regulated by two distinct classes of enzymes
Apart from the potential limited methylation of H3K4 by KMTs like Trr, partially methylated H3K4 could also be generated by lysine demethylases (KDMs). In fact, metazoans possess two classes of H3K4 KDMs that target distinct H3K4 methylation states (Fig. 2). These are the Lysine-specific demethylase 1 (Lsd1/KDM1)-relatives, which only can demethylate H3K4me2, and the Jumonji/AT-Rich-Interactive Domain 1 (JARID1/KDM5)-proteins that also turn over H3K4me3.19,20 Studies in Drosophila showed that JARID1 and Lsd1 interact in global H3K4 demethylation, indicating that JARID1 generates H3K4me2 or H3K4me1 that is further demethylated by Lsd1.21 The same study also showed that JARID1 prevented the silencing of expressed genes by Lsd1. This suggests that JARID1-relatives counteract Lsd1-dependent H3K4 demethylation at transcribed genes. A likely explanation is that JARID1-relatives assemble into distinct complexes with roles in gene silencing vs. transcription. Considering that H3K4me2 and H3K4me1 are found downstream of promoters of expressed genes, JARID1 could be responsible for the partial demethylation of H3K4 in these regions. The further demethylation by Lsd1 complexes is then prevented by factors that can specifically bind to H3K4me2 or H3K4me1. While repressive JARID1 complexes from flies and humans have been characterized, complexes with positive roles in transcription and the ability to generate and bind to H3K4me2 have not yet been identified.
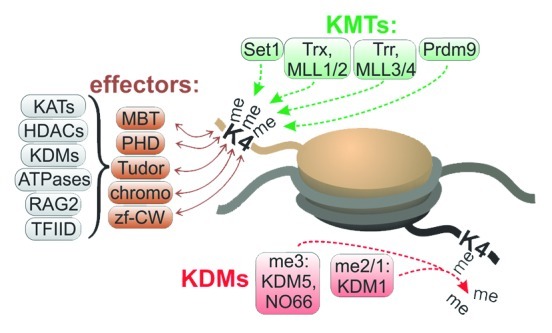
Figure 2. Regulators and effectors of H3K4 methylation. Green boxes: H3K4 methyltransferases (KMTs). Red boxes: H3K4 demethylases (KDMs) are subdivided into enzymes capable of demethylating H3K4me3 (me3) vs. H3K4me2/1 (me2/1). Left, methyl-H3K4 effectors (brown boxes) and associated factors (gray boxes). Abbreviations: KATs, Lysine Acetyltransferases; HDACs, Histone Deacetylases; ATPases, ATP-dependent chromatin modifiers; RAG2, Recombination Activating Gene 2; TfIID, Transcription Factor II D; MBT: Malignant Brain Tumor; PHD, Plant Homeodomain; Tudor, Tudordomain; Chromo, chromodomain; zf-CW, zinc finger CW.
Roles of H3K4 methylation in transcription
Obviously, the addition of methyl groups to H3K4 is not expected to have a direct impact on nucleosome stability. Therefore, methyl-H3K4 binders and their associated chromatin modifiers are likely to regulate changes in nucleosome stability. In fact, many complexes containing methyl-H3K4-specific effectors are implicated in transcription (Fig. 2). These include KATs, ATP-dependent chromatin remodelers, TFIID and others.13 Consequently, H3K4me3 has been proposed to contribute to transcription initiation or elongation. H3K4me3 was also found on mitotic chromosomes, leading to the proposal that it confers transcription ‘memory’ by re-establishing transcription programs in daughter cells after completion of mitosis.12 Recent studies showed that loss of H3K4me3 did not affect gene re-activation per se, but caused defects in transcription rates and strength.22 Therefore, H3K4me3 might function as transcription ‘booster’ by promoting RNAP II loading onto promoters or its release into elongation. We found that the loss of H3K4me3 causes the retention of RNAP II at promoters of developmental and stress genes expressed genes and stalls their upregulation.11 In fact, dSet1 is continuously exchanged at transcription puffs suggesting that K4-methylated H3 is turned over during transcription cycles in a rate-dependent manner.11 Intriguingly, K4 methylation mainly occurs at the H3 variant, H3.3, which is known to be rapidly exchanged at highly expressed genes.23 H3.3-containing nucleosomes at promoters of expressed genes are particularly unstable due to hyperacetylation and ATP-dependent H2A variant incorporation.24 Considering that KATs and chromatin remodelers with roles in nucleosome destabilization at promoters contain methyl-H3K4-binders, H3.3K4me3 is likely to have an underappreciated role in coordinating nucleosome eviction and RNAP II release into elongation (Fig. 2).4
Besides its contribution to transcription upregulation and maintenance at transcribed genes, methyl-H3K4-effectors also associate with repressive complexes including histone deacetylases (HDACs)25 or JARID1-type KDMs (Fig. 2). This suggests that H3K4 methylation functions in concert with other chromatin modifications in a context-dependent modulation of transcription responses. The underlying mechanisms are mostly obscure.
Implications for H3K4me3 in homologous recombination
Mammals possess a un-typical H3K4 KMT, PRDM9, which only trimethylates H3K4.26 In contrast to other KMT2s, the protein has a central SET domain and 12 C-terminal zinc fingers that bind to DNA. PRDM9 is only expressed in gonads and introduces H3K4me3 at so-called meiotic ‘hot spots’, which correspond to sites that are used in higher frequency for meiotic homologous recombination (HR). In yeast, Set1 is also required for meiotic hot spots, suggesting a conserved role for H3K4me3 in meiotic DNA double-strand break (DSB) formation.27 Recent studies, however, indicated that DSB repair also requires H3K4me3.28,29 For instance, the mammalian V(D)J recombinase contains a methyl-H3K4-binding PHD, and H3K4me3 stimulates its activity.30 This suggests that methyl-H3K4-effectors might assemble with repair factors into specialized complexes regulating DSB repair by HR. Remarkably, the disruption of many transcription-linked chromatin complexes with affinity to methyl-H3K4 also affects DSB repair. It therefore is also quite likely that H3K4 methylation is targeted by the same effectors that mediate nucleosome mobilization at promoters.
Epilog
Despite its well-known role at promoters, H3K4 methylation has poorly understood and more complex roles in other chromatin regions. Considering its implications in cancer and developmental disorders, a careful functional dissection of the three metazoan KMT2 subfamilies should provide much needed insights. A good model for such studies is Drosophila, which only possesses one prototypic KMT2 for each subfamily.
Acknowledgments
I apologize to the many authors of original work that could not be cited due to space restrictions. I am indebted to Dr. David Denhardt for insightful comments on this manuscript.
Glossary
Abbreviations:
- cChIP
competitive chromatin immunoprecipitation
- cIFM
competitive immunofluorescence
- COMPASS
Complex Proteins Associated with Set1
- DSB
DNA double-strand break
- H3K4me1
-me2, -me3, H3 lysine 4 mono-, di-, trimethylation
- HR
homologous recombination
- KAT
lysine acetyltransferase
- KMT
lysine methyltransferase
- KDM
lysine demethylases
- MLL
Mixed Lineage Leukemia
- PHD
Plant Homeodomain
- PTM
posttranslational modification
- seq
massively parallel sequencing
- chip
microarray
- Trx
Trithorax
- Trr
Trithorax-related
- TSS
Transcription Start Site
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/21911
References
- 1.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–19. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Kusch T, Workman JL. Histone variants and complexes involved in their exchange. Subcell Biochem. 2007;41:91–109. doi: 10.1007/1-4020-5466-1_5. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Bonn S, Zinzen RP, Girardot C, Gustafson EH, Perez-Gonzalez A, Delhomme N, et al. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat Genet. 2012;44:148–56. doi: 10.1038/ng.1064. [DOI] [PubMed] [Google Scholar]
- 6.Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–83. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pekowska A, Benoukraf T, Zacarias-Cabeza J, Belhocine M, Koch F, Holota H, et al. H3K4 tri-methylation provides an epigenetic signature of active enhancers. EMBO J. 2011;30:4198–210. doi: 10.1038/emboj.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orford K, Kharchenko P, Lai W, Dao MC, Worhunsky DJ, Ferro A, et al. Differential H3K4 methylation identifies developmentally poised hematopoietic genes. Dev Cell. 2008;14:798–809. doi: 10.1016/j.devcel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann JH, Rodríguez MG, Martins NM, Kimura H, Kelly DA, Masumoto H, et al. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J. 2011;30:328–40. doi: 10.1038/emboj.2010.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celniker SE, Dillon LA, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, et al. modENCODE Consortium Unlocking the secrets of the genome. Nature. 2009;459:927–30. doi: 10.1038/459927a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardehali MB, Mei A, Zobeck KL, Caron M, Lis JT, Kusch T. Drosophila Set1 is the major histone H3 lysine 4 trimethyltransferase with role in transcription. EMBO J. 2011;30:2817–28. doi: 10.1038/emboj.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eissenberg JC, Shilatifard A. Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev Biol. 2010;339:240–9. doi: 10.1016/j.ydbio.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith E, Lin C, Shilatifard A. The super elongation complex (SEC) and MLL in development and disease. Genes Dev. 2011;25:661–72. doi: 10.1101/gad.2015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Skalnik DG. CpG-binding protein (CXXC finger protein 1) is a component of the mammalian Set1 histone H3-Lys4 methyltransferase complex, the analogue of the yeast Set1/COMPASS complex. J Biol Chem. 2005;280:41725–31. doi: 10.1074/jbc.M508312200. [DOI] [PubMed] [Google Scholar]
- 15.Mohan M, Herz HM, Smith ER, Zhang Y, Jackson J, Washburn MP, et al. The COMPASS family of H3K4 methylases in Drosophila. Mol Cell Biol. 2011;31:4310–8. doi: 10.1128/MCB.06092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange M, Kaynak B, Forster UB, Tönjes M, Fischer JJ, Grimm C, et al. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 2008;22:2370–84. doi: 10.1101/gad.471408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz YB, Kahn TG, Stenberg P, Ohno K, Bourgon R, Pirrotta V. Alternative epigenetic chromatin states of polycomb target genes. PLoS Genet. 2010;6:e1000805. doi: 10.1371/journal.pgen.1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131–41. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19:857–64. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Benevolenskaya EV. Histone H3K4 demethylases are essential in development and differentiation. Biochem Cell Biol. 2007;85:435–43. doi: 10.1139/O07-057. [DOI] [PubMed] [Google Scholar]
- 21.Di Stefano L, Walker JA, Burgio G, Corona DF, Mulligan P, Näär AM, et al. Functional antagonism between histone H3K4 demethylases in vivo. Genes Dev. 2011;25:17–28. doi: 10.1101/gad.1983711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muramoto T, Müller I, Thomas G, Melvin A, Chubb JR. Methylation of H3K4 Is required for inheritance of active transcriptional states. Curr Biol. 2010;20:397–406. doi: 10.1016/j.cub.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Hödl M, Basler K. Transcription in the absence of histone H3.3. Curr Biol. 2009;19:1221–6. doi: 10.1016/j.cub.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, et al. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet. 2009;41:941–5. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim T, Buratowski S. Dimethylation of H3K4 by Set1 recruits the Set3 histone deacetylase complex to 5′ transcribed regions. Cell. 2009;137:259–72. doi: 10.1016/j.cell.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ségurel L, Leffler EM, Przeworski M. The case of the fickle fingers: how the PRDM9 zinc finger protein specifies meiotic recombination hotspots in humans. PLoS Biol. 2011;9:e1001211. doi: 10.1371/journal.pbio.1001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borde V, Robine N, Lin W, Bonfils S, Géli V, Nicolas A. Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J. 2009;28:99–111. doi: 10.1038/emboj.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faucher D, Wellinger RJ. Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi K, Matsui Y. Meisetz, a novel histone tri-methyltransferase, regulates meiosis-specific epigenesis. Cell Cycle. 2006;5:615–20. doi: 10.4161/cc.5.6.2572. [DOI] [PubMed] [Google Scholar]
- 30.Shimazaki N, Tsai AG, Lieber MR. H3K4me3 stimulates the V(D)J RAG complex for both nicking and hairpinning in trans in addition to tethering in cis: implications for translocations. Mol Cell. 2009;34:535–44. doi: 10.1016/j.molcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


