Abstract
Hedgehog (Hh) signaling plays fundamental roles in morphogenesis, tissue repair, and human disease. Initiation of Hh signaling is controlled by the interaction of two multipass membrane proteins, patched (Ptc) and smoothened (Smo). Recent studies identify Smo as a G-protein coupled receptor (GPCR)-like protein that signals through large G-protein complexes which contain the Gαi subunit. We hypothesize Regulator of G-Protein Signaling (RGS) proteins, and specifically RGS5, are endogenous repressors of Hh signaling via their ability to act as GTPase activating proteins (GAPs) for GTP-bound Gαi, downstream of Smo. In support of this hypothesis, we demonstrate that RGS5 over-expression inhibits sonic hedgehog (Shh)-mediated signaling and osteogenesis in C3H10T1/2 cells. Conversely, signaling is potentiated by siRNA-mediated knock-down of RGS5 expression, but not RGS4 expression. Furthermore, using immuohistochemical analysis and co-immunoprecipitation (Co-IP), we demonstrate that RGS5 is present with Smo in primary cilia. This organelle is required for canonical Hh signaling in mammalian cells, and RGS5 is found in a physical complex with Smo in these cells. We therefore conclude that RGS5 is an endogenous regulator of Hh-mediated signaling and that RGS proteins are potential targets for novel therapeutics in Hh-mediated diseases.
Introduction
Hh signaling is an important mediator of cell proliferation, morphogenesis, and wound repair, and it plays critical roles in organogenesis, tissue fibrosis, and different forms of cancer [1]–[4]. Shh has been reported to stimulate angiogenesis [5], [6], exhibit anti-inflammatory properties [7], and maintain various stem and progenitor cell populations via its mitogenic and survival activity for these cells [8]–[10]. Despite the importance for normal development and tissue homeostatsis, a complete understanding of how Hh proteins signal in mammalian cells is still lacking. This is particularly true with regard to endogenous regulatory pathways that inhibit, rather than stimulate Hh signaling.
Genetic and biochemical evidence has shown that Smo, a seven transmembrane domain protein with structural homology to GPCRs, initiates Hh signaling in Hh responsive cell types [11]–[14]. GPCRs are among the most abundant gene families in the mammalian genome (∼1% of all coding genes) [12], and are frequent pharmaceutical targets [15], [16]. In the absence of agonist, the 3RD intercellular loop (i3) of a GPCR interacts with the large G proteins: a GDP-bound Gα protein (Gαs, Gαq, Gαi/o, and/or Gα12/13) and the Gβγ heterodimer. Upon agonist binding, GTP is exchanged with GDP on the Gα protein, which then dissociates from the Gβγ subunits and activates down-stream signaling thorough secondary messengers [17]–[19]. Regulator of G-protein Signaling (RGS) proteins, of which there are more than 20 mammalian family members [20]–[23], function as GAPs that greatly accelerate the GTP hydrolyzing activity of the Gα protein; the GDP-bound Gα subunit is inactive for signaling [24], [25]. In addition to signaling through a GPCR, Smo-mediated signaling is controlled through the coordinated localization of the signaling complex to a unique cell organelle, the primary cilia [1], [26]–[30]. Unlike most GPCRs, Smo-dependent signaling is constitutively active; however, though the localization of Ptc to primary cilia, signaling is inhibited [31], [32]. In the presence of Shh, which binds directly to Ptc, Ptc translocates out of the cilia, allowing Smo to enter the cilia and actively signal [33]–[36]. Therefore, signaling through GPCRs is the product of proper cellular localization and specific interactions between the GPCR agonist, the GPCR itself, individual large G proteins, and specific RGS proteins.
Recent studies have identified the Gα proteins which interact with Smo. In vitro, Smo is capable of signaling through Gαi1–3, Gαo, and Gαz [37]. In Drosophila, Smo signals through Gαi [38], as it does in neuronal precursor cells [39]. Given the facts that members of the RGS-R4 subfamily of RGS proteins show specificity for Gαi and Gαq, and that RGS5 interacts with Gαi1–3 [40], we hypothesize that RGS5 is a component of the Hh signaling cascade that functions to dampen Smo-dependent signaling through its interaction with Gαi.
Materials and Methods
Cell Culture
C3H10T1/2 cells (ATCC) were cultured in BME (Gibco) with 10% fetal bovine serum (PAA Laboratories) and penicillin-streptomycin. Cells were grown at 37°C and 5% CO2. For qPCR analysis, 5.0×104 cells were plated in a 6-well dish. For protein extracts, 1.0×106 cells were plated in a 15 cm plate. Where indicated, cells were treated with Shh (1 µg/mL; R&D Systems) for 2 hours, 6 hours, or 24 hours. Where indicated, cells were treated for 24 hours with SAG (100 nM; Santa Cruz) or pertussis toxin (PTX; 100 ng/mL; List Biological Laboratories Inc.).
Plasmids
The plasmids encoding the human RGS1, RGS2, RGS3, RGS4, RGS5, and RGS16 (3X HA-tagged, N-terminus) were obtained from the Guthrie cDNA Resource Center. The HA epitope tags were removed and a FLAG epitope tag was added to either the N- or C-terminus of RGS5 by standard cloning techniques. The following primers were used to add a FLAG epitope tag to the N-terminus of hRGS5 (referred to as FL-hRGS5; sequence encoding FLAG tag is underlined): 5′- AACTTTAAGCTTATGGCAGATTATAAAGATGATGATGATAAATGCAAAGGACTTGCAGCTTTGCCC CAC-3′; 5′-CAGGAGTTAATCAAGTAGCTCGAGTCTAGAGGGCCCGTTTA-3′. The following primers were used to add a FLAG epitope tag to the C-terminus of hRGS5 (referred to as hRGS5-FL; sequence encoding FLAG tag is underlined): 5′-GCTAGCGTTTAAACTTAAGCTTGGTACCACCATGTGCAAAGGACTT-3′; 5′-GAGTTTTATCAGGAGTTAATCAAGGATTATAAAGATGATGATGATAAATAACTCGAGCCCCGC-3′. The resulting plasmids were sequence verified and the size of the resultant protein was confirmed by immunonblot.
Quantitative Real-time RT-PCR (qPCR) and Data Analysis
RNA was isolated from C3H10T1/2 cells using the RNeasy RNA isolation mini kit (Qiagen) as described in Gunaje et al [41]. The mouse genes assayed via TaqMan probes were the following: RGS5 (Mm00501393_m1; Applied Biosystems), RGS2 (Mm00501385_m1; Applied Biosystems), RGS4 (Mm00501389_m1; Applied Biosystems), Smo (Mm01162710_m1; Applied Biosystems), Ptc1 (Mm00436026_m1; Applied Biosystems), Ptc2 (Mm00436047_m1; Applied Biosystems), Gli1 (Mm00494645_m1; Applied Biosystems), Gli2 (Mm01293116_m1; Applied Biosystems), and GAPDH (Mm99999915_m1; Applied Biosystems). The mouse genes assayed via SYBR-Green probes were the following: collagen, type 1, alpha 1 (Col1α1; 5′-ATGATGCTAACGTGGTTCGT-3′, 5′-TGGTTAGGGTCGATCCAGTA-3′), bone sialoprotein (Bsp; 5′-AGAACAATCCGTGCCACTCACT-3′, 5′-CCCTGGACTGGAAACCGTTT-3′), osterix (Osx; 5′-AGAGATCTGAGCTGGGTAGAGGAA-3′, 5′-AAGTTGAGGAGGTCGGAGCAT-3′), related transcription factor 2 (Runx2; 5′-ATGCCTCCGCTGTTATGAAA-3′, 5′-GAATGCGCCCTAAATCACTGA-3′), and GAPDH (5′-CTGGAGAAACCTGCCCAAGTA-3′, 5′-TGTTGCTGTAGCCGTATTCA-3′). Gene expression was calculated by the ΔΔCt method: Fold expression = 2−ΔΔCt. Specifically, gene expression was corrected for GAPDH expression within each sample and then normalized to an individual treatment condition within each dataset (as indicated in the figure legend).
siRNA Knockdown of RGS5 Expression
RGS5 was knocked-down in C3H10T1/2 cells using a specific small interfering RNA (siRNA) from Invitrogen ((1) 5′-CAGACUCUGCUGUUGACCUUGUCAU-3′, or (2) 5-GGUGAACAUUGACCACUUCACUAAA-3′ (Fig. S5)). RGS4 was knocked-down using a specific siRNA from Invitrogen (5′-AAAGCUGCCAGUCCACAUUCAUGGU-3′). In control experiments, a non-specific siRNA was utilized (Invitrogen). Cells were transfected with siRNA with Fugene 6 (Roche) following manufacturers specifications. After 24 hours, the cells were changed to serum-free media and starved for 24 hours prior to stimulation with Shh (1 µg/mL) for 2 hours, 6 hours, or 24 hours, where indicated. Alternatively, cells were stimulated with SAG (100 nM) for 24 hours, where indicated.
Osteogenesis Assay
C3H10T1/2 cells were cultured in the presence or absence of SAG (100 nM) and the presence or absence of over-expressed FL-hRGS5 (375 ng/12-well dish) with Fugene 6 (Roche) following manufacturers specifications. Following 21 days of culture (media was changed every 3–4 days throughout the 3-week period), RNA was isolated and gene expression of multiple markers of bone development was determined as above.
Immunoflurescence
C3H10T1/2 cells were transfected with the FL-hRGS5 expression vector (600 ng/6-well dish) with Fugene 6 (Roche) following manufacturers specifications. Following 24 hours, cells were fixed with 4% formaldehyde (in PBS with 0.1% Triton X-100) for 5 minutes at room temperature. After washing cells 3 times in PBS with 0.1% Triton X-100, cells were blocked with 1% BSA (in PBS with 0.1% Triton X-100) for 30 minutes. Cells were incubated with primary antibody (either α-acetylated tubulin (Sigma; 2.8 µg/mL) or α-Flag (Sigma; 5 µg/mL)) for 1 hour at room temperature. Following 3 washes with PBS with 0.1% Triton X-100, cells were incubated with secondary antibody (either Alexa Fluor 594 (Invitrogen; 1/1000; for α-acetylated tubulin) or Alexa Fluor 488 (Invitrogen; 1/1000; for α-Flag)) for 1 hour at room temperature. Following 3 washes with PBS with 0.1% Triton X-100, cells were stained with DAPI (1/1000; Sigma) and visualized.
Co-Immunoprecipitation Assay and Immunoblotting
C3H10T1/2 cells were transfected with expression vectors (3 µg/100 mm plate) encoding human RGS proteins containing either a 5′- or 3′-Flag epitope tag, FL-hRGS5 and hRGS5-FL, respectively, or with a HA epitope tag (HA-RGS1, -RGS2, -RGS3, -RGS4, -RGS5, RGS8 and -RGS16) with Fugene6 (Roche) following manufacturers specifications. Whole cell extracts were prepared by resuspending the cell pellet 24 hours following transfection in cell lysis buffer (50 mM Tris/HCl, pH 8.0; 120 mM NaCl; 0.5% NP-40; 1 mM EDTA). 100 µg of protein extracts were incubated with 1.4 µg of α-acetylated tubulin (Sigma), 1 µg α-Smo (Sigma), or 1 µg β-tubulin antibody (Cell Signaling) overnight at 4°C with rocking. 20 µL of Protein A/G+-agarose (Santa Cruz; for immunoprecipitation of Smo- and β-tubulin) or Protein G+ agarose (Santa Cruz; for immunoprecipitation of acetylated tubulin-bound complexes) was added to the protein extracts and incubated for 4 hours at 4°C with rocking. The beads were washed 3X with 1 mL of lysis buffer, followed by centrifugation at 2500 rpm. The beads were resuspended in 40 µL lysis buffer and 8 µL 6X SDS/PAGE sample buffer. Extracts were boiled and proteins were separated by SDS/PAGE (10% gel) and transferred to a PVDF membrane, which was subsequently blocked with 5% nonfat dry milk (NFDM) in TBS-T (0.1% Tween). Membranes were incubated with the α-FLAG-HRP antibody (1 µg/mL; Sigma) or the α-HA antibody (1/500; Roche Applied Sciences) overnight at 4°C. After 4X washes with TBS-T, membranes were incubated with 1∶15,000 goat α-rat IgG HRP (Jackson Immuno Research). After 4X washes with TBS-T, membranes were incubated with ECL reagent (Super Signal West Pico, Pierce) and exposed to autoradiographic film.
Statistics
Data is represented as average ± standard error means (SEM) or standard deviation (SD), as indicated in the figure legend. Differences in data were considered if p<0.05, as determined by student’s t-test.
Results
To investigate the potential function of RGS5 in control of Hh signaling, we determined the effects of RGS5 over-expression on Shh reporter gene expression in C3H10T1/2 cells. C3H10T1/2 cells are a murine embryonic mesenchymal cell line [42] commonly used to study mammalian Hh signaling [43]. These cells were used to develop and evaluate antagonists of Hh-mediated signaling [44]–[46] and to establish the role of Shh and BMP-2 in chrondrogenic and osteogenic differentiation [47]–[50]. We confirmed that similar to other cells [14], [38], [51], Shh signals through a Gαi-dependent pathway in C3H10T1/2 cells. Specifically, we demonstrated that Shh reporter gene expression is inhibited in the presence of pertussis toxin (PTX; Fig. S1). Furthermore, C3H10T1/2 cells exhibit properties of a progenitor for vascular smooth muscle cells and pericytes [52] and RGS5 expression is characteristic of pericytes [53]–[55]. In pericytes, Shh signaling coordinates vascular outgrowth in the choroid plexus [56] and promotes blood brain barrier properties in perivascular astrocytes [57]. Thus, RGS5 is expressed in pericytes and mural cells and may play an important role in the regulation of Shh-mediated vascular development and angiogenesis.
RGS5 Over-expression Inhibits Shh-mediated Signaling
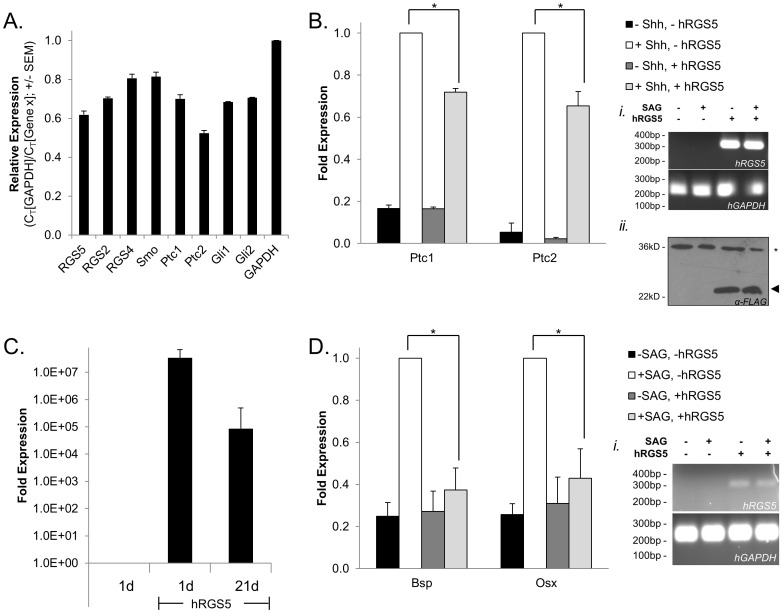
We demonstrate C3H10T1/2 cells express members of the Shh-signaling cascade (Smo, Ptc1, Ptc2, Gli1, and Gli2) and multiple members of the RGS-R4 subfamily (RGS2, RGS4, and RGS5) (Fig. 1A; Fig. S2). All of these genes are expressed at approximately equal mRNA levels (though at a lesser expression level than GAPDH), while an additional member of the RGS-R4 subfamily, RGS16, is not expressed in C3H10T1/2 cells (data not shown). Therefore, the necessary signaling components are present to assess the role of RGS5 in regulating the Shh signaling cascade. Following over-expression of RGS5 in C3H10T1/2 cells, Shh-reporter expression is inhibited. Specifically, Ptc1 and Ptc2 expression is inhibited by 28% and 35%, respectively (Fig. 1B and S3A). Furthermore, we confirm that RGS5 is over-expressed at both the mRNA and protein level (insets i and ii of Fig. 1B, respectively; see also Fig. S3C&D).
Figure 1. RGS5 inhibits Shh-mediated reporter expression.
(A) Basal expression level of various members of the RGS-R4 subfamily (RGS5, RGS2, and RGS4) and members of the Shh signaling cascade (Smo, Ptc1, Ptc2, Gli1, and Gli2) in C3H10T1/2 cells. Expression of the indicated genes was determined by qPCR and represented relative to GAPHD expression. (n = 3–7; error bars = SEM) (B) Over-expression of RGS5 inhibits Shh-mediated reporter expression. RGS5 was over-expressed in C3H10T1/2 cells by transient transfection and treated with Shh (1 µg/mL) for 24 hours. The effect of RGS5 over-expression on Ptc1 and Ptc2 was assessed by qPCR. Expression is corrected for GAPDH expression and normalized to the expression of each gene following 24hr of Shh treatment, but in the absence of RGS5 over-expression (white bar). (n = 3; error bar = SEM; *p<0.05 by t-test) (Bi&ii) Insets: traditional RT-PCR (30 cycles) demonstrating hRGS5 message (i) and protein (ii) in transfected C3H10T1/2 cells. (C&D) Over-expression of RGS5 inhibits Shh-mediated osteogenesis. RGS5 was over-expressed in C3H10T1/2 cells (C), and gene expression was monitored over 21 days by qPCR. Expression is corrected for GAPDH expression and normalized to the expression of hRGS5 in untransfected cells. Following 21 days of SAG treatment (100nM), RNA was isolated and the expression of Bsp and Osx was determined by qPCR. Expression is corrected for GAPDH expression and normalized to the expression of each gene following 21 days of SAG treatment, but in the absence of RGS5 over-expression (white bar). (n = 3; error bar = SEM; p<0.05 by t-test) (Di) Inset: traditional RT-PCR (30 cycles) demonstrating hRGS5 message expression in transfected C3H10T1/2 cells.
We extended our analysis to determine whether RGS5 over-expression had a functional consequence on the Shh signaling pathway, beyond simply inhibiting expression of individual components of the Shh cascade (Ptc1 and Ptc2; Fig. 1B). A classic functional pathway used to evaluate novel agonists and antagonists of the Shh pathway is osteogenic development [43], [58], [59]. Specifically, Shh stimulates the mRNA expression osteogenesis markers in C3H10T1/2 cells [58], and we hypothesized that over-expression of RGS5 would result in the inhibition of Shh-mediated induction of individual markers of osteogenic development. As shown in Fig. 1C and 1Di, hRGS5 remains over-expressed in transfected C3H10T1/2 cells following 21 days of culture, at least as measured by mRNA expression. Furthermore, we demonstrate that SAG, a small molecule agonist of the Hh pathway through the direct binding of Smo [60], also activates bone sialoprotein (Bsp) and osterix (Osx) similar to Shh stimulation [58], whereas collagen 1, type 1, alpha 1 (Col1α1) and Runx2 are not induced by SAG (Fig. S4). Importantly, in the presence of over-expressed hRGS5, expression of both Bsp and Osx is inhibited by ∼60% (Fig. 1D). Taken together, these results suggest that RGS5 functions downstream of Smo to regulate the Shh-mediated signal cascade.
Shh Stimulation Inhibits RGS5 Message Expression
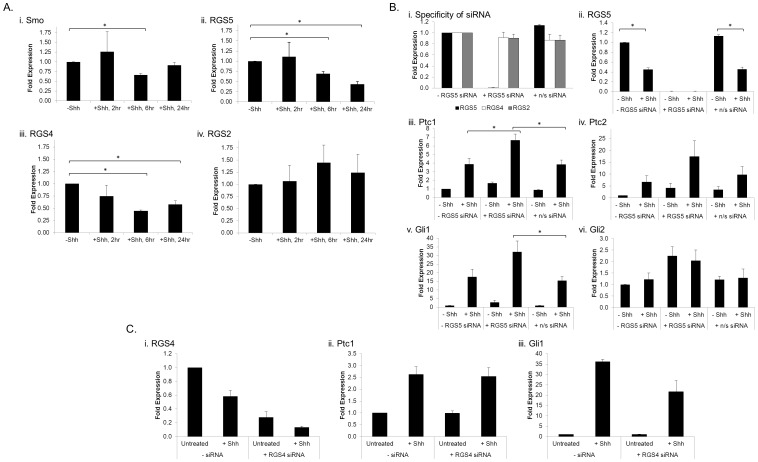
To investigate whether Shh directly regulates the expression of members of the GPCR-mediated signaling complex, the mRNA expression of Smo, RGS5, RGS4, and RGS2 was determined following Shh treatment in C3H10T1/2 cells. Smo expression is transiently, but significantly, inhibited following 6 hours of Shh stimulation, but is not significantly different from basal expression following 24 hours of stimulation (Fig. 2A, i). Conversely, Shh stimulation for both 6 hours and 24 hours did affect expression of multiple members of the RGS-R4 subfamily. Specifically, RGS5 expression was down-regulated by approximately 60% (Fig. 2A, ii), and RGS4 expression was down-regulated by approximately 40% (Fig. 2A, iii) following 24 hours of Shh treatment. Conversely, Shh stimulation had no effect upon RGS2 expression (Fig. 2A, iv) at any of the time-points assayed. The Shh-dependent down-regulation of RGS5 and RGS4 implicates a feed-forward mechanism by which Shh actively down-regulates its repressor to augment signaling. Given the fact that RGS5 has been implicated as a biomarker in multiple cancers [61]–[65] and is expressed in pericytes [53]–[55], we focused on the effect of RGS5 upon Shh-mediated signaling.
Figure 2. Direct effect of RGS5 on Shh-mediated reporter expression.
(A) RGS5 and RGS4 are inhibited by Shh stimulation. C3H10T1/2 cells were stimulated with Shh (1 µg/mL) for 2 hours, 6 hours, and 24 hours. Relative expression of (i) Smo, (ii) RGS5, (iii) RGS4, and (iv) RGS2 was determined by qPCR, and normalized to the expression of each gene in the absence of Shh (untreated cells). n = 3–7; error bar = SEM; *p<0.05 by t-test. (B) Knock-down of RGS5 activates Shh-mediated reporter expression. The expression of RGS5 was knocked-down following transfection with gene-specific siRNA in C3H10T1/2 cells (i), whereas RGS4 and RGS2 expression is not affected by the RGS5 siRNA. Cells were treated with Shh (1 µg/mL) for 24 hours, and RNA was isolated. The relative expression of (ii) RGS5, (iii) Ptc1, (iv) Ptc2, (v) Gli1, and (vi) Gli2 was assessed by qPCR. Expression is corrected for GAPDH and normalized to the expression of each gene in the absence of siRNA transfection and in the absence of Shh stimulation (-Shh, -RGS5 siRNA). n = 4–5; error bars = SEM; *p<0.05 by t-test. (C) Knock-down of RGS4 does not potentiate Shh-mediated expression of reporter genes. The expression of RGS4 was knocked-down following transfection with gene-specific siRNA in C3H10T1/2 cells. Cells were treated with Shh (1 µg/mL) for 24 hours, and RNA was isolated. The relative expression of RGS4 (i), Ptc1 (ii) and Gli1 (iii) was assessed by qPCR. Expression is corrected for GAPDH and normalized to the expression of each gene in the absence of Shh stimulation (untreated, -siRNA). n = 3–5; error bars = SD.
Knock-down of RGS5 Expression Activates Shh Reporter Expression
To determine the effect of RGS5 repression on Shh-mediated signaling, RGS5 expression was silenced by gene-specific siRNA. To confirm the specificity of the siRNA, we demonstrate that RGS5 expression is specifically knocked-down, whereas the siRNA failed to modulate expression of either RGS4 or RGS2 (Fig. 2B, i), two closely related members of the RGS-R4 subfamily of RGS proteins. Additionally, in agreement with the results in Fig. 2A, Shh treatment for 24 hours resulted in the inhibition of RGS5 expression (Fig. 2B, ii), both in the absence of gene-specific siRNA (left) and in the presence of non-specific siRNA (right).
As shown in Fig. 2B iii–vi, in the absence of gene-specific siRNA or in the presence of non-specific siRNA, Shh stimulation induced the expression of multiple Shh reporter genes: Ptc1 (iii), Ptc2 (iv), and Gli1 (v). However, Gli2 expression was not affected by Shh stimulation in C3H10T1/2 cells (Fig. 2B, vi), implying Gli2 expression might not be regulated in response to Shh in C3H10T1/2 cells. Importantly, when RGS5 expression was knocked-down by gene-specific siRNA, the expression of Ptc1, Ptc2, and Gli1 was induced approximately 2-fold (Fig. 2B), relative to expression of these Shh reporter genes in the absence of RGS5 siRNA. Similar effects were observed with an independent siRNA directed at RGS5 and in response to SAG-mediated activation of the Shh signaling cascade (Fig. S5). Therefore, in the absence of RGS5, the Shh-mediated induction of reporter gene expression is further potentiated.
In addition to RGS5, the expression of RGS4 is also inhibited by Shh stimulation (Fig. 2A, iii). To determine the effect of RGS4 expression on Shh-mediated signaling, RGS4 expression was silenced by gene specific siRNA (Fig. 2Ci). Unlike RGS5 knockdown, when RGS4 is inhibited by siRNA transfection, both Shh (Fig. 2Cii–iii) and the smoothened agonist SAG (Fig. S6) failed to further induce the expression of either Ptc1 or Gli1 (Fig. 2C; compare –siRNA, +Shh to +RGS4 siRNA, +Shh). Similar to RGS5, we over-expressed hRGS4 in C3H10T1/2 cells and demonstrated Shh-reporter gene expression is inhibited: Ptc1 by 70%, Ptc2 by 90%, and Gli1 by 95% (Fig. S7). Therefore, while expression of both RGS5 and RGS4 are inhibited by Shh stimulation and over-expression of both RGS5 and RGS4 inhibited Shh reporter expression, only the knock-down of RGS5 further potentiates the SAG- or Shh-mediated induction of Shh reporter gene expression. This data suggests that over-expression alone may result in non-specific effects, given the sensitivity of GPCR signaling to regulation by RGS proteins. However, given the correlated and antagonistic effects of both over-expression and knock-down of RGS5 expression, we are confident the inhibition of Smo-mediated signaling is unique and specific to RGS5 C3H10T1/2 cells.
RGS5 is Present in the Primary Cilia
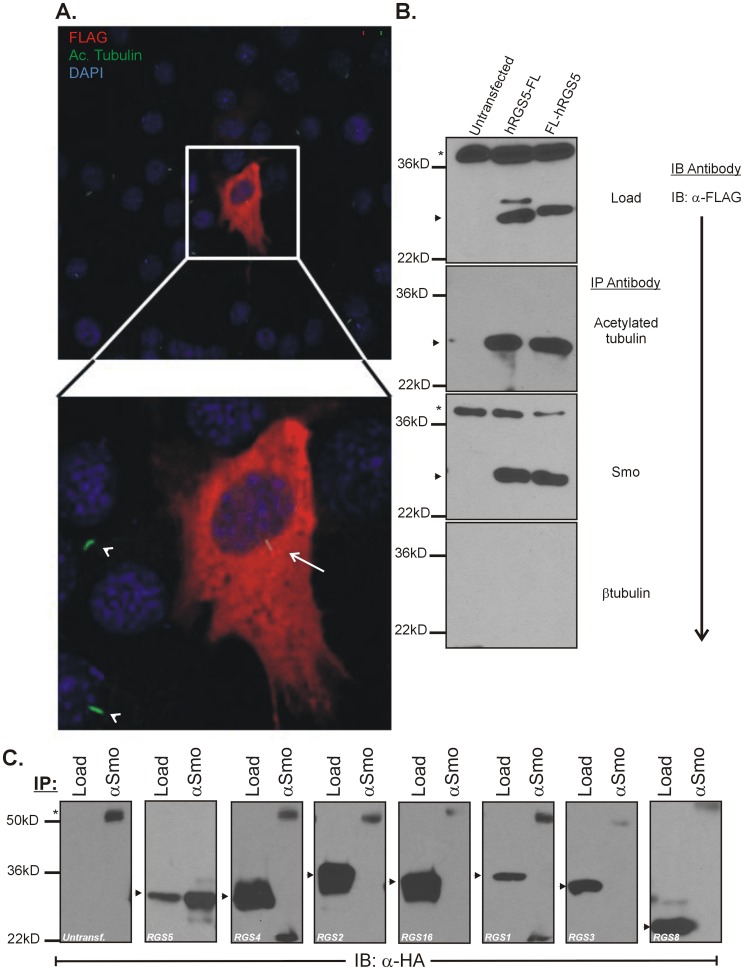
Many studies have elucidated the requirement for the localization of components of the canonical Shh signaling cascade to the primary cilia [37], [39], [66]–[68]. Therefore, C3H10T1/2 cells were transfected with FL-RGS5 and analyzed for cellular localization by immunoflurescence. As shown in Fig. 3A, cilia are observed on cells transfected by FL-RGS5, as indicated by positive staining with the α-acetylated tubulin antibody. This implies that cilia disassembly does not occur when RGS5 is over-expressed, a potential mechanism for the repressive effect of over-expressed RGS5 [69]. However, due to the high expression levels of RGS5 in transfected cells, we found it difficult to confirm RGS5 was present in the primary cilia by either immunoflurescence (Fig. 3A) or confocal microscopy (data not shown). Therefore, we attempted to confirm localization of RGS5 to the primary cilia by co-immunoprecipitation (Co-IP).
Figure 3. RGS5 is present in the primary cilia in C3H10T1/2 cells and interacts with acetylated tubulin and Smo.
(A) C3H10T1/2 cells transfected with FL-hRGS5 were visualized by immunoflurescence. Cells were fixed and stained with α-acetylated tubulin (green), α-Flag (red), and nuclei are identified by DAPI staining (blue). Shown are primary cilia located on both transfected (arrow) and untransfected (arrowhead) cells. (B) RGS5 interacts with acetylated tubulin and Smo in the primary cilia of C3H10T1/2 cells. Cells were transfected with either FL-hRGS5 or hRGS5-FL. Protein complexes involving RGS5 were isolated by immunoprecipitation and analyzed by SDS/PAGE. Shown are positive interactions between RGS5 and acetylated tubulin and RGS5 and Smo. As a negative control, the β-tubulin antibody failed to immunoprecipitate RGS5 in transfected C3H10T1/2 cells. (C) The interaction between RGS5 and Smo is specific to RGS5 within the RGS-R4 subfamily of RGS proteins. Cells were transfected with HA-hRGS1, -hRGS2, -hRGS3, -hRGS4, -hRGS5, hRGS8, and -hRGS16. Protein complexes involving RGS proteins were isolated by immunoprecipitation with the α-Smo antibody and analyzed by SDS/PAGE and immunoblotting with the α-HA antibody. A positive interaction is shown between RGS5 and Smo, while RGS1, RGS2, RGS3, RGS4, RGS8, and RGS16 failed to interact with Smo. * = non-specific protein band; arrow head = FL-hRGS5 protein band.
C3H10T1/2 cells were transfected with FLAG epitope-tagged hRGS5 (either hRGS5-FL or FL-hRGS5; FLAG epitope fused to the C- or N-terminus, respectively). Potential interactions between RGS5 and components of the primary cilia were analyzed by Co-IP and SDS-PAGE. We demonstrate in Fig. 3B that when RGS5 is over-expressed in C3H10T1/2 cells, RGS5 is capable of interacting with both acetylated tubulin and Smo, while RGS5 does not non-specifically interact with β-tubulin. Under the conditions used, acetylated tubulin (green) is observed exclusively in the primary cilia of C3H10T1/2 cells (Fig. 3A, lower panel, arrowheads). Furthermore, as a control, we demonstrated that the antibodies to acetylated tubulin and Smo are specific, as Co-IP with α-mouse IgG or α-rabbit IgG failed to immunoprecipitate FL-hRGS5 from transfected cells (Fig. S8). Therefore, not only does RGS5 regulate the Shh-mediated signaling cascade, but it is present in the primary cilia and interacts with both acetylated tubulin and the GPCR responsible for Shh-mediated signaling (Smo).
To test whether the co-localization of Smo and RGS5 to the primary cilia is specific to RGS5, we analyzed the localization of multiple additional members of the RGS-R4 subfamily by Co-IP. As shown in Fig. 3C, while RGS5 was Co-IPed with Smo, RGS1, RGS2, RGS3, RGS4, RGS8, and RGS16 failed to interact with Smo in C3H10T1/2 cells. This implies a specific interaction between Smo and RGS5 in primary cilia, at least in relation to the cell type and RGS protein assayed.
Discussion
We provide evidence that RGS proteins regulate canonical Hh signaling at the level of Smo-mediated G-protein coupling to downstream effector pathways in mammalian cells. RGS proteins accelerate GTP hydrolysis by Gα proteins and thereby inhibit GPCR-mediated signaling [25]. We found that over-expression of RGS5 inhibits gene expression downstream of Smo in C3H10T1/2 cells (Fig. 1B) and functionally inhibits Hh-dependent osteogenic development (Fig. 1D). Conversely, loss of RGS5 function led to increased levels of Shh-stimulated gene expression (Fig. 2B). Moreover, RGS5 was found to be present with acetylated tubulin in the primary cilia (Fig. 3A) and could be Co-IPed in a complex with Smo and acetylated tubulin (Fig. 3B). Taken together, these results demonstrate that RGS5 functions as an inhibitor of Hh signaling downstream of Smo. We propose the interaction between Smo, Gα subunits, and RGS proteins may provide novel targets for the control of Hh-mediated signaling in human disease.
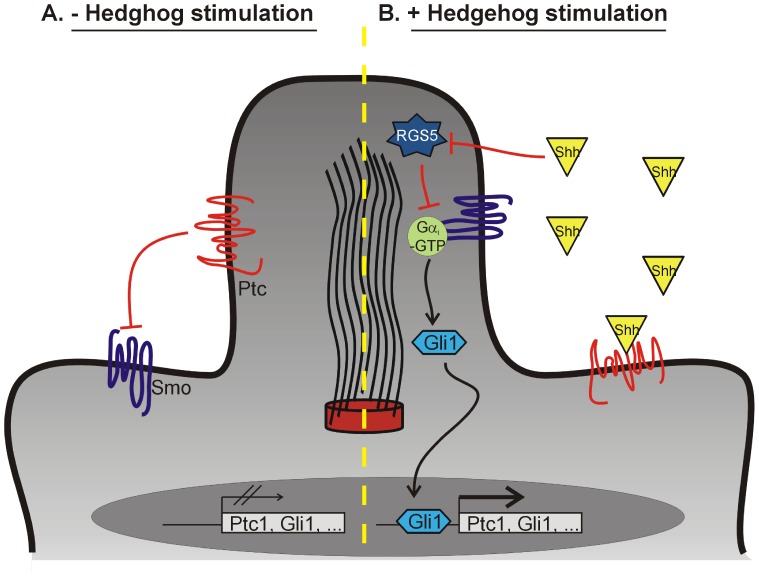
Smo is an integral membrane protein with significant structural homology to GPCRs [11]–[13]. In the unstimulated state, Ptc proteins inhibit Smo signaling, presumably by preventing Smo localization to the primary cilia ([36]; Fig. 4A). However, upon binding Hh proteins (Shh, indian hedgehog (Ihh), or desert hedgehog (Dhh)), Ptc leaves and Smo enters the primary cilia, where it resides in close proximity to other components of the Shh signaling complex: the Gli transcription factors and the large G proteins (Fig. 4B) [1]–[3], [28], [70], [71]. Multiple recent studies have characterized the interactions between Smo and members of the large G protein family. In Drosophila, Ogden et al demonstrated that Smo signals through Gαi [38]. In mammalian cells, Riobo et al demonstrated that Smo interacts with Gαi [37], and interactions between Smo and Gαi have been implicated in the control of both cell migration [68] and proliferation [39]. Interestingly, Kasai et al demonstrated that Smo may interact with Gα12/13 in neuroblastoma cells [72], however, Douglas et al recently determined that the activation of the Gli transcription factors by Gα13 does not occur in every cell type and is independent of Smo [66]. A similar argument of cell-specific activity of Gαi proteins was proposed by Hammerschmidt and McMahon, who demonstrated that blocking Gαi-mediated signaling with pertussis toxin affected some, but not all Hh-dependent developmental processes in zebrafish [51]. Finally, a recent study by Manning and colleagues demonstrated that, at least in vitro, Smo is capable of activating Gαi with an equivalent activity as the serotonin receptor [14]. We hypothesized that RGS5 functions to regulate signaling through Smo, given that fact that RGS proteins catalyze the hydrolysis of Gα-GTP to Gα-GDP, and that RGS5 specifically interacts with Gαi and Gαq [40]. Furthermore, our data suggests RGS5 regulates canonical Hh signaling [68], [73], since we observe an RGS5-dependent effect upon Hh target gene transcription and we demonstrate a physical interaction between RGS5, Smo, and acetylated tubulin in the primary cilia. Our study therefore identifies RGS5 as a novel regulator of the Shh signaling cascade (Fig. 4B).
Figure 4. The hedgehog-mediated signaling mechanism in the absence (A) and presence (B) of Shh.
RGS5 inhibits Shh-mediated signaling. RGS5 functions to inhibit signaling down-stream of Smo by hydrolyzing Gαi-GTP. In the absence of Shh, RGS5 inhibits Smo-dependent signaling by inactivating Gαi and blocking the expression of the Gli transcription factors and Ptc co-receptors. In the presence of Shh, RGS5 expression is repressed, leading to the potentiation of the activation of Gli and Ptc expression.
Many studies have implicated both Shh and RGS5 in the control of vascular development and remodeling in response to injury. In a model of hindlimb ischemia, the Shh signaling cascade is up-regulated in the interstitum, and blocking this pathway inhibits collateral vessel formation, whereas up-regulating the pathway enhances recovery from hindlimb ischemia [74]–[76]. Shh is also a regulator of coronary artery development [77]–[79], as well as carotid artery intimal hyperplasia [80], [81]. We are intrigued by the juxtaposition of expression domains for RGS5 and the targets of Shh signaling. For example, RGS5 is robustly expressed in pericytes [82], [83] and in arterial vascular smooth muscle cells [84]–[87]. Conversely, despite expression of Shh protein in the border zone between the media and adventitia [8], expression of Shh signaling reporters Ptc1, Ptc2, and Gli1 is restricted to the vascular adventitia [8], [88], [89]. We hypothesize that in the uninjured vessel wall, RGS5 expression in medial smooth muscle cells restricts expression of Shh and reporter genes to the adjacent adventitia. However, following vascular injury, RGS5 expression is inhibited [87], potentially as a result of local increases in PDGF-BB [41], thereby allowing the Shh signaling domain to expand to the vascular media and neointima [81].
In addition to vascular development and remodeling, Shh-mediated signaling and RGS proteins have been implicated in the development of multiple cancers [90]. Of interest to our studies, multiple members of the RGS-R4 subfamily have been associated with cancers that exhibit aberrant Shh signaling. For example, increased Shh signaling has been correlated with poor prognosis in ovarian cancer [61], breast cancer [91]–[93], medulloblastoma [90], and hepatocellular carcinoma [94]–[97]. Misexpression of RGS2 [61], RGS4 [61], and RGS5 [62]–[65] has also been reported in a number of these cancers. Recently, RGS proteins themselves have been pharmacologically targeted (reviewed in Kimple et al [98]). For example, chemical screens have identified novel inhibitors of RGS4 [99]–[101], RGS8 [102], and RGS20 [103]. Therefore, a potential treatment option for cancers in which the Shh signaling pathway is aberrantly activated would be to promote RGS5 expression or prolong its activity at the tumor site. We hypothesize this would sensitize the cancer to treatment with hedgehog antagonists, thereby requiring a lower drug treatment which might prevent undesirable off-target effects of inhibiting the hedgehog pathway [78], [104]–[106].
Our data raise several important questions going forward. For example, (i) do RGS proteins regulate Smo-mediated signaling in cell types other than C3H10T1/2; (ii) will RGS5-mediated inhibition of Shh target gene expression be followed by corresponding effects on other biological endpoints beyond simple gene expression; (iii) is trafficking of RGS proteins to the primary cilia regulated by Shh signaling and can a specific domain of the RGS protein be identified that mediates such trafficking; (iv) can RGS proteins also inhibit non-canonical forms of Shh signaling [68]; (v) does down-regulation of RGS protein expression by Shh lead to enhanced signaling by other GPCRs that couple to Gαi-dependent pathways; (vi) does RGS5 expression affect the Gli3 repressor/activator ratio in cells; and (vii) do apparently normal appearing RGS5-null mice [107]–[109] exhibit Shh signaling defects in vivo when carefully examined following exposure to injury or disease-causing stimuli?
In summary, our study presents data demonstrating RGS5 is a novel regulator of the Shh signaling cascade. In the context of the recent studies describing interactions between the heterotrimeric G proteins and Smo, it is not surprising that RGS proteins participate in the control of Shh-mediated signaling, and we propose the interaction between Shh signaling and RGS proteins may represent novel targets in the control of both cancer and vascular remodeling and disease.
Supporting Information
Effect of PTX on SAG-mediated Ptc1 and Gli1 expression. Hedgehog-mediated gene expression is sensitive to pertussis toxin (PTX) in C3H10T1/2 cells, and therefore signals through Gαi. C3H10T1/2 cells were stimulated with SAG (24 hrs; 100 nM) in the presence of PTX (24 hrs; 100 ng/mL; List Biological Laboratories Inc.) or vehicle (24 hrs; 0.1% BSA in PBS). RNA was isolated and gene expression of Ptc1 (A) and Gli1 (B) was determined as described in Methods and Materials. Gene expression was corrected for GAPDH expression and normalized to expression in the presence of SAG: Fold = 2−ΔΔCt. (n = 3; error bar = SEM; * = p<0.05 by t-test).
(TIF)
Relative Expression in C3H10T1/2 cells. Quantification of relative gene expression in C3H10T1/2 cells. The basal expression level of various members of the RGS-R4 subfamily (RGS5, RGS2, and RGS4) and members of the Shh signaling cascade (Smo, Ptc1, Ptc2, Gli1 and Gli2) in C3H10T1/2 cells. Expression of the indicated genes was determined by qPCR. Data was corrected for GAPDH expression and normalized to expression of each gene in a single sample. (n = 3–7; error bars = SEM). This data is re-plotted from frigure 1A.
(TIF)
Confirmation of hRGS5 over-expression by qPCR, RT-PCR, and immunoblot. Representative confirmation of hRGS5 expression when Shh/SAG-mediated gene expression is inhibited. C3H10T1/2 cells were cultured in the absence or presence of SAG (100 nM) and the presence or absence of transiently over-expressed FL-hRGS5 for 24 hrs. (A) RNA was isolated and the expression of Shh reporter genes (Ptc1, Ptc2, Gli1) was determined by qPCR as described. Expression values were corrected for GAPDH expression and normalized to the condition ‘+SAG, -hRGS5’ for each gene assayed (Fold Expression = 2−ΔΔCt). (B) The expression of transiently over-expressed hRGS5 was quantitated by qPCR as above. (C) The expression of transiently over-expressed RGS5 was confirmed by traditional RT-PCR. Shown is the product following 30 amplification cycles and separation on a 1% SDS-PAGE gel. (D) Protein expression for FL-hRGS5 was confirmed by immunonblot with the α-FLAG antibody. Whole cell extract from treated and/or transfected cells was separated by SDS-PAGE, probed with the α-FLAG antibody and visualized by ECL detection, as described.
(TIF)
RGS5-mediated inhibition of SAG-induced osteogenesis. RGS5 over-expression inhibits SAG-induced osteogenesis in C3H10T1/2 cells. The expression of multiple markers of osteogenesis was assayed in the presence or absence of over-expressed RGS5 and the presence or absence of SAG (SAG was used because it is more stable than the recombinant Shh protein in long-term culture). Specifically, C3H10T1/2 cells were cultured for 21 days after being transiently transfected with FL- hRGS5 and treated with SAG (100 nM). Media was changed every 3–4 days following transfection and SAG treatment. RNA was isolated and gene expression of (A) RGS5, (B) collagen, type 1, alpha 1 (Col1α1), bone sialoprotein (Bsp), osterix (Osx), and related transcription factor 2 (Runx2) was determined. Expression of both Bsp and Osx was induced by SAG treatment, and expression of both genes is inhibited by the over- expression of RGS5. Conversely, neither Col1α1 or Runx2 was significantly stimulated by 21 days of SAG treatment. Gene expression was corrected for GAPDH expression and normalized to the expression of each gene in the absence of RGS5 over-expression and in the absence of SAG treatment (white bar). (n = 3; error bar = SEM; * = p<0.05).
(TIF)
Effect of multiple RGS5 siRNAs on SAG- mediated expression of Ptc1 and Gli1. Multiple siRNAs targeting murine RGS5 have similar effects upon Hedgehog-mediated gene expression. C3H10T1/2 cells were transiently transfected with 2 independent siRNAs targeting murine RGS5, as described in Methods and Materials. siRNA (1) is described in the manuscript, while siRNA (2) has the following sequence (5′- GGUGAACAUUGACCACUUCACUAAA-3′; Invitrogen). Cells were stimulated with SAG (24 hrs; 100 nM) either in the presence or absence of the individual siRNAs. RNA as isolated and gene expression of RGS5 (A), Ptc1 (B), and Gli1 (C) was determined. Both siRNAs inhibited RGS5 expression (A) and expression of both Ptc1 (B) and Gli1 (C) was potentiated in the absence of RGS5. Gene expression was corrected for GAPDH expression and normalized to expression in the absence of siRNA. (n = 2; error bar = SD).
(TIF)
Effect of RGS4 knockdown on SAG-mediated induction of Shh reporter gene expression. Knock-down of RGS4 does not activate SAG-mediated expression of Shh reporter genes. The expression of RGS4 was knocked-down following transfection with a gene-specific siRNA in C3H10T1/2 cells. Cells were treated with SAG (100 nM) for 24 hrs, and RNA was isolated. The relative expression of Ptc1 (i) and Gli1 (ii) was assed by qPCR. Expression is corrected for GAPDH and normalized to the expression of each gene in the absence of SAG stimulation (untreated, −siRNA). (n = 3–5; error bar = SEM; p<0.05 by t-test). Importantly, note that knock-down of RGS5 potentiated SAG-mediated up-regulation of Ptc1 and Gli1, whereas knock-down of RGS4 failed to further up-regulate the expression of these genes.
(TIF)
Effect of RGS4 over-expression on Shh- mediated gene expression. Over-expressed RGS4 inhibits Shh-mediated reporter expression. RGS4 (HA-hRGS4) was over-expressed in C3H10T1/2 cells by transient transfection and treated with SAG (100 nM) for 24 hours. The effect of RGS4 over-expression on Ptc1 and Gli1 was assessed by qPCR. Expression was corrected for GAPHD expression and normalized to the expression of each gene following 24 hr of SAG treatment, but in the absence of RGS4 over- expression (white bar). (n = 2; error bar = SD).
(TIF)
Assay for Specific Interaction between RGS5:Ac-Tubulin and RGS5:Smo. RGS5 specifically interacts with acetylated tubulin and Smoothened. C3H10T1/2 cells were transiently transfected with FL-RGS5. Protein complexes were immunoprecipitated (IPed) with antibodies to acetylated tubulin or mouse IgG control and Smo or rabbit IgG control and analyzed by SDS/PAGE. Samples were immunobloted with the α-Flag antibody, demonstrating the presence of RGS5 in the IPed protein complexes. Shown are positive and specific interactions between RGS5 and acetylated tubulin and RGS5 and Smo, but not between RGS5 and the IgG controls, respectively (arrow head = FL-hRGS5).
(TIF)
Acknowledgments
We thank members of the University of Washington Adventitial Biology Interest Group (Drs. M. Majesky, S. Schwartz, G. Daum, M. Rosenfeld, M. Bothwell, M. Reyes, W. Mahoney, and members of their labs) for useful discussions and technical advice.
Funding Statement
This work was supported by National Institutes of Health grants HL087513 and HL094374 (to WMM Jr), HL088374 (to GD) and HL019242 and HL093594 (to MWM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hooper JE, Scott MP (2005) Communicating with Hedgehogs. Nat Rev Mol Cell Biol 6: 306–317. [DOI] [PubMed] [Google Scholar]
- 2. Barakat MT, Humke EW, Scott MP (2010) Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol Med 16: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lum L, Beachy PA (2004) The Hedgehog response network: sensors, switches, and routers. Science 304: 1755–1759. [DOI] [PubMed] [Google Scholar]
- 4. Scales SJ, de Sauvage FJ (2009) Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci 30: 303–312. [DOI] [PubMed] [Google Scholar]
- 5. Nagase T, Nagase M, Yoshimura K, Fujita T, Koshima I (2005) Angiogenesis within the developing mouse neural tube is dependent on sonic hedgehog signaling: possible roles of motor neurons. Genes Cells 10: 595–604. [DOI] [PubMed] [Google Scholar]
- 6. Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, et al. (2009) Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 30: 580–588. [DOI] [PubMed] [Google Scholar]
- 7. Zacharias WJ, Li X, Madison BB, Kretovich K, Kao JY, et al. (2010) Hedgehog is an anti-inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology 138: 2368–2377 2377: e2361–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, et al. (2008) A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA 105: 9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin K, Lee J, Guo N, Kim J, Lim A, et al. (2011) Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, et al. (2008) Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells 26: 2311–2320. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz-Gomez A, Molnar C, Holguin H, Mayor F Jr, de Celis JF (2007) The cell biology of Smo signalling and its relationships with GPCRs. Biochim Biophys Acta 1768: 901–912. [DOI] [PubMed] [Google Scholar]
- 12. Bockaert J, Pin JP (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J 18: 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE (1996) The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86: 221–232. [DOI] [PubMed] [Google Scholar]
- 14. Shen F, Cheng L, Douglas AE, Riobo NA, Manning DR (2013) Smoothened is a fully competent activator of the heterotrimeric g protein gi. Mol Pharmacol 83: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lappano R, Maggiolini M (2011) G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov 10: 47–60. [DOI] [PubMed] [Google Scholar]
- 16. Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650. [DOI] [PubMed] [Google Scholar]
- 17. Osmond RI, Crouch MF, Dupriez VJ (2010) An emerging role for kinase screening in GPCR drug discovery. Curr Opin Mol Ther 12: 305–315. [PubMed] [Google Scholar]
- 18. Rozengurt E (2007) Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213: 589–602. [DOI] [PubMed] [Google Scholar]
- 19. Milligan G, Kostenis E (2006) Heterotrimeric G-proteins: a short history. Br J Pharmacol 147 Suppl 1S46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martemyanov KA, Krispel CM, Lishko PV, Burns ME, Arshavsky VY (2008) Functional comparison of RGS9 splice isoforms in a living cell. Proc Natl Acad Sci U S A 105: 20988–20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martemyanov KA, Arshavsky VY (2009) Biology and functions of the RGS9 isoforms. Prog Mol Biol Transl Sci 86: 205–227. [DOI] [PubMed] [Google Scholar]
- 22. Toro-Castillo C, Thapliyal A, Gonzalez-Ochoa H, Adams BA, Meza U (2007) Muscarinic modulation of Cav2.3 (R-type) calcium channels is antagonized by RGS3 and RGS3T. Am J Physiol Cell Physiol 292: C573–580. [DOI] [PubMed] [Google Scholar]
- 23. Liang Y, Li C, Guzman VM, Chang WW, Evinger AJ, et al. (2005) Identification of a novel alternative splicing variant of RGS5 mRNA in human ocular tissues. FEBS J 272: 791–799. [DOI] [PubMed] [Google Scholar]
- 24. Wilkie TM, Kinch L (2005) New roles for Galpha and RGS proteins: communication continues despite pulling sisters apart. Curr Biol 15: R843–854. [DOI] [PubMed] [Google Scholar]
- 25. Abramow-Newerly M, Roy AA, Nunn C, Chidiac P (2006) RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal 18: 579–591. [DOI] [PubMed] [Google Scholar]
- 26. Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19: R526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, et al. (2005) Vertebrate Smoothened functions at the primary cilium. Nature 437: 1018–1021. [DOI] [PubMed] [Google Scholar]
- 28. Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C (2010) Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev 24: 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai CK, Gupta N, Wen X, Rangell L, Chih B, et al. (2011) Functional characterization of putative cilia genes by high-content analysis. Mol Biol Cell 22: 1104–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson CW, Chuang PT (2010) Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137: 2079–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15: 3059–3087. [DOI] [PubMed] [Google Scholar]
- 32. Nybakken K, Perrimon N (2002) Hedgehog signal transduction: recent findings. Curr Opin Genet Dev 12: 503–511. [DOI] [PubMed] [Google Scholar]
- 33. Wong SY, Reiter JF (2008) The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol 85: 225–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pazour GJ, Witman GB (2003) The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15: 105–110. [DOI] [PubMed] [Google Scholar]
- 35. Goetz SC, Ocbina PJ, Anderson KV (2009) The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol 94: 199–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rohatgi R, Milenkovic L, Scott MP (2007) Patched1 regulates hedgehog signaling at the primary cilium. Science 317: 372–376. [DOI] [PubMed] [Google Scholar]
- 37. Riobo NA, Saucy B, Dilizio C, Manning DR (2006) Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A 103: 12607–12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, et al. (2008) G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature 456: 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barzi M, Kostrz D, Menendez A, Pons S (2011) Sonic Hedgehog-induced Proliferation Requires Specific Gα Inhibitory Proteins. J Biol Chem 286: 8067–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou J, Moroi K, Nishiyama M, Usui H, Seki N, et al. (2001) Characterization of RGS5 in regulation of G protein-coupled receptor signaling. Life Sci 68: 1457–1469. [DOI] [PubMed] [Google Scholar]
- 41. Gunaje JJ, Bahrami AJ, Schwartz SM, Daum G, Mahoney WM (2011) PDGF-dependent regulation of regulator of G protein signaling-5 expression and vascular smooth muscle cell functionality. Am J Physiol Cell Physiol 301: C478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reznikoff C, Brankow D, Heidelberger C (1973) Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33: 3231–3238. [PubMed] [Google Scholar]
- 43. Pathi S, Pagan-Westphal S, Baker DP, Garber EA, Rayhorn P, et al. (2001) Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech Dev 106: 107–117. [DOI] [PubMed] [Google Scholar]
- 44. Roudaut H, Traiffort E, Gorojankina T, Vincent L, Faure H, et al. (2011) Identification and mechanism of action of the acylguanidine MRT-83, a novel potent smoothened antagonist. Mol Pharmacol 79: 453–460. [DOI] [PubMed] [Google Scholar]
- 45. Actis M, Connelly MC, Mayasundari A, Punchihewa C, Fujii N (2011) A structure-activity relationship study of small-molecule inhibitors of GLI1-mediated transcription. Biopolymers 95: 24–30. [DOI] [PubMed] [Google Scholar]
- 46. Williams KP, Rayhorn P, Chi-Rosso G, Garber EA, Strauch KL, et al. (1999) Functional antagonists of sonic hedgehog reveal the importance of the N terminus for activity. J Cell Sci 112 (Pt 23): 4405–4414. [DOI] [PubMed] [Google Scholar]
- 47. Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC (2003) BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem 90: 1112–1127. [DOI] [PubMed] [Google Scholar]
- 48. Yuasa T, Kataoka H, Kinto N, Iwamoto M, Enomoto-Iwamoto M, et al. (2002) Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol 193: 225–232. [DOI] [PubMed] [Google Scholar]
- 49. Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, et al. (2001) Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci 114: 2085–2094. [DOI] [PubMed] [Google Scholar]
- 50. Zehentner BK, Leser U, Burtscher H (2000) BMP-2 and sonic hedgehog have contrary effects on adipocyte-like differentiation of C3H10T1/2 cells. DNA Cell Biol 19: 275–281. [DOI] [PubMed] [Google Scholar]
- 51. Hammerschmidt M, McMahon AP (1998) The effect of pertussis toxin on zebrafish development: a possible role for inhibitory G-proteins in hedgehog signaling. Dev Biol 194: 166–171. [DOI] [PubMed] [Google Scholar]
- 52. Hirschi KK, Rohovsky SA, D'Amore PA (1998) PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. JCell Biol 141: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, et al. (2009) Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24: 909–969. [DOI] [PubMed] [Google Scholar]
- 54. Mitchell TS, Bradley J, Robinson GS, Shima DT, Ng YS (2008) RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 11: 141–151. [DOI] [PubMed] [Google Scholar]
- 55. Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R (2005) Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 105: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 56. Nielsen CM, Dymecki SM (2010) Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev Biol 340: 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, et al. (2011) The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 58. Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, et al. (2005) Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132: 49–60. [DOI] [PubMed] [Google Scholar]
- 59. Williams KP, Rayhorn P, Chi-Rosso G, Garber EA, Strauch KL, et al. (1999) Functional antagonists of sonic hedgehog reveal the importance of the N terminus for activity. J Cell Sci 112 (Pt 23): 4405–4414. [DOI] [PubMed] [Google Scholar]
- 60. Chen JK, Taipale J, Young KE, Maiti T, Beachy PA (2002) Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99: 14071–14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hurst JH, Mendpara N, Hooks SB (2009) Regulator of G-protein signalling expression and function in ovarian cancer cell lines. Cell Mol Biol Lett 14: 153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boss CN, Grunebach F, Brauer K, Hantschel M, Mirakaj V, et al. (2007) Identification and characterization of T-cell epitopes deduced from RGS5, a novel broadly expressed tumor antigen. Clin Cancer Res 13: 3347–3355. [DOI] [PubMed] [Google Scholar]
- 63. Bilger A, Bennett LM, Carabeo RA, Chiaverotti TA, Dvorak C, et al. (2004) A potent modifier of liver cancer risk on distal mouse chromosome 1: linkage analysis and characterization of congenic lines. Genetics 167: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen X, Cheung ST, So S, Fan ST, Barry C, et al. (2002) Gene expression patterns in human liver cancers. Mol Biol Cell 13: 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen X, Higgins J, Cheung ST, Li R, Mason V, et al. (2004) Novel endothelial cell markers in hepatocellular carcinoma. Mod Pathol 17: 1198–1210. [DOI] [PubMed] [Google Scholar]
- 66. Douglas AE, Heim JA, Shen F, Almada LL, Riobo NA, et al. (2011) The alpha subunit of the G protein G13 regulates activity of one or more Gli transcription factors independently of smoothened. J Biol Chem 286: 30714–30722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Riobo NA, Manning DR (2007) Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J 403: 369–379. [DOI] [PubMed] [Google Scholar]
- 68. Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, et al. (2011) Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286: 19589–19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Seeley ES, Nachury MV (2010) The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci 123: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15: 3059–3087. [DOI] [PubMed] [Google Scholar]
- 71. McMahon AP, Ingham PW, Tabin CJ (2003) Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53: 1–114. [DOI] [PubMed] [Google Scholar]
- 72. Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, et al. (2004) The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9: 49–58. [DOI] [PubMed] [Google Scholar]
- 73. Bijlsma MF, Damhofer H, Roelink H (2012) Hedgehog-stimulated chemotaxis is mediated by smoothened located outside the primary cilium. Sci Signal 5: ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Palladino M, Gatto I, Neri V, Straino S, Silver M, et al. (2011) Pleiotropic beneficial effects of sonic hedgehog gene therapy in an experimental model of peripheral limb ischemia. Mol Ther 19: 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, et al. (2001) The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7: 706–711. [DOI] [PubMed] [Google Scholar]
- 76. Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, et al. (2003) Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 108: 479–485. [DOI] [PubMed] [Google Scholar]
- 77. Lavine KJ, White AC, Park C, Smith CS, Choi K, et al. (2006) Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev 20: 1651–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lavine KJ, Kovacs A, Ornitz DM (2008) Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 118: 2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lavine KJ, Long F, Choi K, Smith C, Ornitz DM (2008) Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development 135: 3161–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Morrow D, Sweeney C, Birney YA, Guha S, Collins N, et al. (2007) Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am J Physiol Cell Physiol 292: C488–496. [DOI] [PubMed] [Google Scholar]
- 81. Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, et al. (2009) Sonic Hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol 29: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, et al. (2003) Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. AmJ Pathol 162: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH (2003) Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17: 440–442. [DOI] [PubMed] [Google Scholar]
- 84. Adams LD, Geary RL, McManus B, Schwartz SM (2000) A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res 87: 623–631. [DOI] [PubMed] [Google Scholar]
- 85. Adams LD, Geary RL, Li J, Rossini A, Schwartz SM (2006) Expression profiling identifies smooth muscle cell diversity within human intima and plaque fibrous cap: loss of RGS5 distinguishes the cap. Arterioscler Thromb Vasc Biol 26: 319–325. [DOI] [PubMed] [Google Scholar]
- 86. Li J, Adams LD, Wang X, Pabon L, Schwartz SM, et al. (2004) Regulator of G protein signaling 5 marks peripheral arterial smooth muscle cells and is downregulated in atherosclerotic plaque. J Vasc Surg 40: 519–528. [DOI] [PubMed] [Google Scholar]
- 87. Wang X, Adams LD, Pabon LM, Mahoney WM Jr, Beaudry D, et al. (2008) RGS5, RGS4, and RGS2 expression and aortic contractibility are dynamically co-regulated during aortic banding-induced hypertrophy. J Mol Cell Cardiol 44: 539–550. [DOI] [PubMed] [Google Scholar]
- 88. Majesky MW, Dong XR, Hoglund V, Mahoney WM, Daum G (2011) The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol 31: 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Majesky MW, Dong XR, Hoglund V, Daum G, Mahoney WM (2012) The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs 195: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Merchant AA, Matsui W (2010) Targeting Hedgehog–a cancer stem cell pathway. Clin Cancer Res 16: 3130–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cui W, Wang LH, Wen YY, Song M, Li BL, et al. (2010) Expression and regulation mechanisms of Sonic Hedgehog in breast cancer. Cancer Sci 101: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, et al. (2006) Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther 5: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, et al. (2004) Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res 64: 6071–6074. [DOI] [PubMed] [Google Scholar]
- 94. Chen XL, Cheng QY, She MR, Wang Q, Huang XH, et al. (2010) Expression of sonic hedgehog signaling components in hepatocellular carcinoma and cyclopamine-induced apoptosis through Bcl-2 downregulation in vitro. Arch Med Res 41: 315–323. [DOI] [PubMed] [Google Scholar]
- 95. Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, et al. (2007) Selective down-regulation of glioma-associated oncogene 2 inhibits the proliferation of hepatocellular carcinoma cells. Cancer Res 67: 3583–3593. [DOI] [PubMed] [Google Scholar]
- 96. Omenetti A, Diehl AM (2008) The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol 294: G595–598. [DOI] [PubMed] [Google Scholar]
- 97. Yang L, Wang Y, Mao H, Fleig S, Omenetti A, et al. (2008) Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol 48: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kimple AJ, Bosch DE, Giguère PM, Siderovski DP (2011) Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev 63: 728–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Blazer LL, Roman DL, Chung A, Larsen MJ, Greedy BM, et al. (2010) Reversible, allosteric small-molecule inhibitors of regulator of G protein signaling proteins. Mol Pharmacol 78: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kimple AJ, Willard FS, Giguère PM, Johnston CA, Mocanu V, et al. (2007) The RGS protein inhibitor CCG-4986 is a covalent modifier of the RGS4 Galpha-interaction face. Biochim Biophys Acta 1774: 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Roman DL, Talbot JN, Roof RA, Sunahara RK, Traynor JR, et al. (2007) Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol Pharmacol 71: 169–175. [DOI] [PubMed] [Google Scholar]
- 102. Blazer LL, Zhang H, Casey EM, Husbands SM, Neubig RR (2011) A nanomolar-potency small molecule inhibitor of regulator of G-protein signaling proteins. Biochemistry 50: 3181–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang Y, Young KH (2004) Analysis of RGSZ1 protein interaction with Galphai subunits. Methods Enzymol 390: 31–52. [DOI] [PubMed] [Google Scholar]
- 104. Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, et al. (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455: 406–410. [DOI] [PubMed] [Google Scholar]
- 105. Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, et al. (2004) Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell 6: 229–240. [DOI] [PubMed] [Google Scholar]
- 106. Zhang X, Harrington N, Moraes RC, Wu MF, Hilsenbeck SG, et al. (2009) Cyclopamine inhibition of human breast cancer cell growth independent of Smoothened (Smo). Breast Cancer Res Treat 115: 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nisancioglu MH, Mahoney WM, Kimmel DD, Schwartz SM, Betsholtz C, et al. (2008) Generation and characterization of rgs5 mutant mice. Mol Cell Biol 28: 2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho H, Park C, Hwang IY, Han SB, Schimel D, et al.. (2008) Rgs5 Targeting Leads to Chronic Low Blood Pressure and a Lean Body Habitus. Mol Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang H, Gu S, Al-Sabeq B, Wang S, He J, et al. (2012) Origin-specific epigenetic program correlates with vascular bed-specific differences in Rgs5 expression. FASEB J 26: 181–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of PTX on SAG-mediated Ptc1 and Gli1 expression. Hedgehog-mediated gene expression is sensitive to pertussis toxin (PTX) in C3H10T1/2 cells, and therefore signals through Gαi. C3H10T1/2 cells were stimulated with SAG (24 hrs; 100 nM) in the presence of PTX (24 hrs; 100 ng/mL; List Biological Laboratories Inc.) or vehicle (24 hrs; 0.1% BSA in PBS). RNA was isolated and gene expression of Ptc1 (A) and Gli1 (B) was determined as described in Methods and Materials. Gene expression was corrected for GAPDH expression and normalized to expression in the presence of SAG: Fold = 2−ΔΔCt. (n = 3; error bar = SEM; * = p<0.05 by t-test).
(TIF)
Relative Expression in C3H10T1/2 cells. Quantification of relative gene expression in C3H10T1/2 cells. The basal expression level of various members of the RGS-R4 subfamily (RGS5, RGS2, and RGS4) and members of the Shh signaling cascade (Smo, Ptc1, Ptc2, Gli1 and Gli2) in C3H10T1/2 cells. Expression of the indicated genes was determined by qPCR. Data was corrected for GAPDH expression and normalized to expression of each gene in a single sample. (n = 3–7; error bars = SEM). This data is re-plotted from frigure 1A.
(TIF)
Confirmation of hRGS5 over-expression by qPCR, RT-PCR, and immunoblot. Representative confirmation of hRGS5 expression when Shh/SAG-mediated gene expression is inhibited. C3H10T1/2 cells were cultured in the absence or presence of SAG (100 nM) and the presence or absence of transiently over-expressed FL-hRGS5 for 24 hrs. (A) RNA was isolated and the expression of Shh reporter genes (Ptc1, Ptc2, Gli1) was determined by qPCR as described. Expression values were corrected for GAPDH expression and normalized to the condition ‘+SAG, -hRGS5’ for each gene assayed (Fold Expression = 2−ΔΔCt). (B) The expression of transiently over-expressed hRGS5 was quantitated by qPCR as above. (C) The expression of transiently over-expressed RGS5 was confirmed by traditional RT-PCR. Shown is the product following 30 amplification cycles and separation on a 1% SDS-PAGE gel. (D) Protein expression for FL-hRGS5 was confirmed by immunonblot with the α-FLAG antibody. Whole cell extract from treated and/or transfected cells was separated by SDS-PAGE, probed with the α-FLAG antibody and visualized by ECL detection, as described.
(TIF)
RGS5-mediated inhibition of SAG-induced osteogenesis. RGS5 over-expression inhibits SAG-induced osteogenesis in C3H10T1/2 cells. The expression of multiple markers of osteogenesis was assayed in the presence or absence of over-expressed RGS5 and the presence or absence of SAG (SAG was used because it is more stable than the recombinant Shh protein in long-term culture). Specifically, C3H10T1/2 cells were cultured for 21 days after being transiently transfected with FL- hRGS5 and treated with SAG (100 nM). Media was changed every 3–4 days following transfection and SAG treatment. RNA was isolated and gene expression of (A) RGS5, (B) collagen, type 1, alpha 1 (Col1α1), bone sialoprotein (Bsp), osterix (Osx), and related transcription factor 2 (Runx2) was determined. Expression of both Bsp and Osx was induced by SAG treatment, and expression of both genes is inhibited by the over- expression of RGS5. Conversely, neither Col1α1 or Runx2 was significantly stimulated by 21 days of SAG treatment. Gene expression was corrected for GAPDH expression and normalized to the expression of each gene in the absence of RGS5 over-expression and in the absence of SAG treatment (white bar). (n = 3; error bar = SEM; * = p<0.05).
(TIF)
Effect of multiple RGS5 siRNAs on SAG- mediated expression of Ptc1 and Gli1. Multiple siRNAs targeting murine RGS5 have similar effects upon Hedgehog-mediated gene expression. C3H10T1/2 cells were transiently transfected with 2 independent siRNAs targeting murine RGS5, as described in Methods and Materials. siRNA (1) is described in the manuscript, while siRNA (2) has the following sequence (5′- GGUGAACAUUGACCACUUCACUAAA-3′; Invitrogen). Cells were stimulated with SAG (24 hrs; 100 nM) either in the presence or absence of the individual siRNAs. RNA as isolated and gene expression of RGS5 (A), Ptc1 (B), and Gli1 (C) was determined. Both siRNAs inhibited RGS5 expression (A) and expression of both Ptc1 (B) and Gli1 (C) was potentiated in the absence of RGS5. Gene expression was corrected for GAPDH expression and normalized to expression in the absence of siRNA. (n = 2; error bar = SD).
(TIF)
Effect of RGS4 knockdown on SAG-mediated induction of Shh reporter gene expression. Knock-down of RGS4 does not activate SAG-mediated expression of Shh reporter genes. The expression of RGS4 was knocked-down following transfection with a gene-specific siRNA in C3H10T1/2 cells. Cells were treated with SAG (100 nM) for 24 hrs, and RNA was isolated. The relative expression of Ptc1 (i) and Gli1 (ii) was assed by qPCR. Expression is corrected for GAPDH and normalized to the expression of each gene in the absence of SAG stimulation (untreated, −siRNA). (n = 3–5; error bar = SEM; p<0.05 by t-test). Importantly, note that knock-down of RGS5 potentiated SAG-mediated up-regulation of Ptc1 and Gli1, whereas knock-down of RGS4 failed to further up-regulate the expression of these genes.
(TIF)
Effect of RGS4 over-expression on Shh- mediated gene expression. Over-expressed RGS4 inhibits Shh-mediated reporter expression. RGS4 (HA-hRGS4) was over-expressed in C3H10T1/2 cells by transient transfection and treated with SAG (100 nM) for 24 hours. The effect of RGS4 over-expression on Ptc1 and Gli1 was assessed by qPCR. Expression was corrected for GAPHD expression and normalized to the expression of each gene following 24 hr of SAG treatment, but in the absence of RGS4 over- expression (white bar). (n = 2; error bar = SD).
(TIF)
Assay for Specific Interaction between RGS5:Ac-Tubulin and RGS5:Smo. RGS5 specifically interacts with acetylated tubulin and Smoothened. C3H10T1/2 cells were transiently transfected with FL-RGS5. Protein complexes were immunoprecipitated (IPed) with antibodies to acetylated tubulin or mouse IgG control and Smo or rabbit IgG control and analyzed by SDS/PAGE. Samples were immunobloted with the α-Flag antibody, demonstrating the presence of RGS5 in the IPed protein complexes. Shown are positive and specific interactions between RGS5 and acetylated tubulin and RGS5 and Smo, but not between RGS5 and the IgG controls, respectively (arrow head = FL-hRGS5).
(TIF)