Abstract
An estimated 650,000 Americans will have ESRD by 2010. Young adults with kidney failure often develop progressive chronic kidney disease (CKD) in childhood and adolescence. The Chronic Kidney Disease in Children (CKiD) prospective cohort study of 540 children aged 1 to 16 yr and have estimated GFR between 30 and 75 ml/min per 1.73 m2 was established to identify novel risk factors for CKD progression; the impact of kidney function decline on growth, cognition, and behavior; and the evolution of cardiovascular disease risk factors. Annually, a physical examination documenting height, weight, Tanner stage, and standardized BP is conducted, and cognitive function, quality of life, nutritional, and behavioral questionnaires are completed by the parent or the child. Samples of serum, plasma, urine, hair, and fingernail clippings are stored in biosamples and genetics repositories. GFR is measured annually for 2 yr, then every other year using iohexol, HPLC creatinine, and cystatin C. Using age, gender, and serial measurements of Tanner stage, height, and creatinine, compared with iohexol GFR, a formula to estimate GFR that will improve on traditional pediatric GFR estimating equations when applied longitudinally is expected to be developed. Every other year, echocardiography and ambulatory BP monitoring will assess risk for cardiovascular disease. The primary outcome is the rate of decline of GFR. The CKiD study will be the largest North American multicenter study of pediatric CKD.
It has been estimated that 650,000 Americans will have ESRD by 2010 with an annual cost of $28 billion per year (1,2). Although alarming increases in ESRD rates have occurred in older Americans, young adults with ESRD bear a sizable burden of the disease. For example, in 2001, 37% of prevalent US transplant patients were between 20 and 44 yr of age (1). In addition, many of the 12,753 young adults who presented with kidney failure (incident patients with ESRD) in 2001 likely developed early stages of chronic kidney disease (CKD) in childhood (1). Identification of modifiable risk factors to slow progression of CKD in children will aid in decreasing the burden of CKD and may yield further insights into risk factors for progression in adults before the onset of multiple comorbidities (3).
CKD in children carries a significant impact. The mortality that is associated with ESRD in children who receive dialysis is estimated to be at least 30 times higher than that in the general pediatric population (4). Unlike adults, who have completed their physiologic and intellectual maturation, children are in formative stages of development and therefore are particularly vulnerable to the adverse effects of CKD. Early detection and aggressive management have the potential to improve outcomes in young patients with CKD. Few sizable prospective studies of CKD in children have been performed (5,6), and relatively little is known about the natural history of early stages of CKD in this population.
With funding from the National Institute of Diabetes and Digestive and Kidney Diseases, in collaboration with the National Institute of Neurologic Disorders and Stroke, the National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute, we have established a prospective cohort study of children with CKD, called the Chronic Kidney Disease in Children (CKiD) study. The specific aims of the CKiD study are to (1) identify novel and traditional risk factors for the progression of CKD; (2) characterize the impact of a decline in kidney function on neurodevelopment, cognitive abilities, and behavior; (3) identify the prevalence and the evolution of cardiovascular (CV) disease risk factors in children with CKD; and (4) examine the effects of declining GFR on growth and assess the consequences of growth failure on morbidity in children with CKD.
Materials and Methods
Study Organization
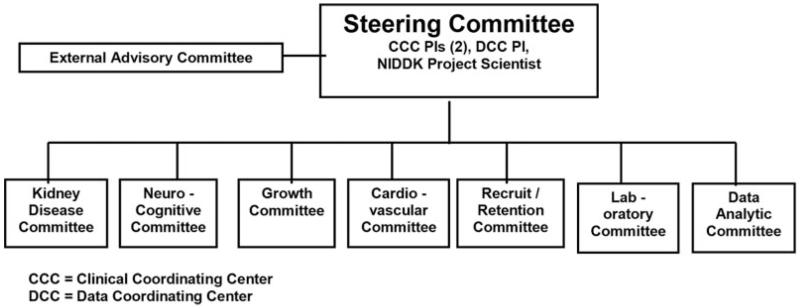
The CKiD study consists of two clinical coordinating centers in Baltimore, MD, and Kansas City, MO, which include 57 clinical sites (locations and principal investigators are listed in the Appendix); a data coordinating center in Baltimore, MD; a central laboratory in Rochester, NY; a laboratory for cystatin C (Kansas City, MO); central reading centers for echocardiograms and carotid intima-media thickness (Cincinnati, OH), magnetic resonance imaging (MRI; Bronx, NY), ambulatory BP monitoring (ABPM; Houston, TX), and neurocognitive testing (Chapel Hill, NC); and central repositories for biologic (Rockville, MD) and genetic material (New Brunswick, NJ). A study web site for CKiD is located at http://www.statepi.jhsph.edu/ckid. An External Advisory Committee meets annually to evaluate study progress, and the Steering Committee has semimonthly conference calls, including review of any adverse events related to study measurements (see Figure 1 for study organization chart).
Figure 1.
Chronic Kidney Disease in Children (CKiD) study organization.
Study Population
The CKiD study is a prospective, observational cohort of 540 children who have CKD and are enrolled during a 24-mo period. Inclusion and exclusion criteria are presented in Table 1. For eligibility, GFR is estimated by the Schwartz formula (7) on the basis of height and two measurements of serum creatinine obtained within 18 mo before enrollment.
Table 1.
Inclusion and exclusion criteriaa
| Inclusion |
| age 1 to 16 yr |
| eGFR 30 to 75 ml/min per 1.73 m2 |
| informed consent |
| Exclusion |
| renal, other solid-organ, bone marrow, or stem cell transplantation |
| dialysis treatment within the past 3 mo |
| cancer/leukemia diagnosis or HIV diagnosis/ treatment within past 12 mo |
| current pregnancy or pregnancy within the past 12 mo |
| inability to complete major data collection procedures (e.g., allergic to iohexol) |
| current enrollment in a randomized clinical trial in which specific treatment is unknown |
| plans to relocate away from any participating CKiD site |
| history of structural heart disease |
| genetic syndromes involving the central nervous system (e.g., Down syndrome) |
| history of severe to profound mental retardation (i.e., IQ < 40, significant impairment in adaptive |
| function, and/or inability to independently execute self-care skills) |
CKiD, Chronic Kidney Disease in Children; eGFR, estimated GFR (via the Schwartz formula).
Table 2 describes the expected number of children in each stratum of age and estimated GFR (eGFR). The expected age distribution is based on data from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) (8) registry and surveys of participating sites. The lower age limit of 1 yr was chosen to facilitate testing for CKD progression, as well as CV and neurocognitive measurements. The upper age limit of 16 yr was chosen to optimize follow-up, because 16-yr-old participants likely would remain under the care of the enrolling pediatric nephrology center for 2 to 5 yr.
Table 2.
Expected number of children by age and eGFR categories
| GFR (ml/min per 1.73 m2) |
Age (yr) |
Total | ||
|---|---|---|---|---|
| 1 to 4 | 5 to 10 | 11 to 16 | ||
| 60 to 75 | 12 | 48 | 60 | 120 |
| 40 to 59 | 30 | 120 | 150 | 300 |
| 30 to 39 | 12 | 48 | 60 | 120 |
| Total | 54 | 216 | 270 | 540 |
On the basis of surveys of the participating sites and NAPRTCS data, we anticipate that 40% of the cohort will be female, 62% will be white, 18% will be black, 4% will be Asian or Pacific Islander, 2% will be Native American, and 14% will be other (8). To ensure heterogeneity in the racial/ethnic distribution, the CKiD study will limit enrollment of non-Hispanic white participants to no more than 65% of the cohort.
Multicenter Study Coordination
To facilitate standardized data collection with a large number of active clinical sites and a complex study design with age- and gender-dependent measurements (e.g., ABPM cuff-size, intelligence tests, Tanner stage), we use interactive web-based visit schedules. These interactive visit schedules detail the questionnaires, examinations, and biologic samples that are required for a given child at a given study visit. In addition, training DVDs detailing study procedures are sent to study coordinators, and training meetings are held annually.
Study Follow-Up
Participants will be scheduled to return between 3 and 6 mo after study entry and on the anniversary of study enrollment thereafter. Participants will cease study visits after documented initiation of renal replacement therapy but will continue to be followed for clinical events and mortality by telephone contacts. Children with a high probability for ESRD (initiation of dialysis or transplantation) within the calendar year after a study visit (i.e., GFR <15 ml/min per 1.73 m2 or stage 5 CKD) will have their subsequent study visit accelerated to within 3 mo of the documentation of GFR <15 ml/min per 1.73 m2 to capture data before onset of ESRD and will have telephone follow-up every 6 mo to document the date of initiation of renal replacement therapy.
Table 3 depicts information to be obtained at baseline (i.e., follow-up month 0) and at each subsequent study visit. Tests are to be conducted on all cohort members, except when specifically targeted to a subcohort as noted in Table 3. Annually, study investigators conduct a physical examination and collect biologic specimens, and clinical site personnel assist the parent and the child to complete a set of questionnaires. For future studies, CKiD stores samples of serum, plasma, and urine as well as hair and fingernail clippings in a biosamples repository. In addition, to empower the study with a virtually inexhaustible supply of DNA, CKiD has requested that the National Institute of Diabetes and Digestive and Kidney Disease genetics repository create lymphocyte cell lines based on a sample of whole blood taken at the 6-mo visit. From annual blood and urine collection, we obtain centrally determined HPLC serum creatinine, renal panel, urine creatinine and protein, and local complete blood count and pregnancy tests.
Table 3.
Study measurements by visita
| Area | Measurement | Follow-Up Month |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 3 to 6 | 12 | 24 | 36 | 48 | ||
| General | Physical examination/medical history | X | X | X | X | X | X |
| Medication use | X | X | X | X | X | X | |
| Symptoms checklist | X | X | X | X | X | X | |
| Kidney | Iohexol-based GFR | X | X | X | |||
| Central renal panel | X | X | X | X | X | ||
| Central urine creatinine, proteinb | X | X | X | X | X | ||
| Cystatin C–based GFR | X | X | X | ||||
| HPLC serum creatinine | X | X | X | X | X | ||
| Local basic metabolic panel | X | X | X | X | X | ||
| Local urine creatinine, proteinb | X | X | X | X | X | ||
| Local complete blood count | X | X | X | X | X | ||
| Local pregnancy test | X | X | X | X | X | ||
| Cardiovascular | Echocardiography | X | X | ||||
| ABPM | X | X | |||||
| Carotid IMTc (n = 30) | X | X | |||||
| Lipid profile | X | X | |||||
| Clinical BP | X | X | X | X | X | X | |
| Neurocognitive | Pediatric quality of life | X | X | X | X | X | |
| Cognitive and development | X | X | X | ||||
| Behavioral assessment | X | X | X | ||||
| Magnetic resonance imaged (n = 30) | X | X | X | ||||
| Neuropsychological assessmentsd (n = 30) | X | X | X | ||||
| Growth and nutrition | Height or length | X | X | X | X | X | X |
| Weight | X | X | X | X | X | X | |
| Head and mid-arm circumference | X | X | X | X | X | X | |
| Tanner stage | X | X | X | X | X | X | |
| 3-d diet record, food frequency | X | X | X | X | X | ||
| Physical activity | X | X | X | X | X | ||
| Inflammation | Wide-range C-reactive protein | X | X | ||||
| Bone health | Intact PTH | X | X | X | |||
| Bone biopsyc (n = 10) | X | X | |||||
ABPM, ambulatory BP monitoring; IMT, intima-media thickness; PTH, parathyroid hormone.
First morning urine specimen collection.
Subcohort studies for carotid IMT and bone biopsy at select sites with technical capability to perform the specific tests.
High-risk neurocognitive testing and imaging will be performed at three sites with specific imaging capabilities in children who are older than 9 yr and have GFR <40 ml/min per 1.73 m2. High-risk neurocognitive assessment includes Children’s Memory Scale (CMS), Delis-Kaplan Executive Function System (D-KEFS), Benton Judgment of Line Orientation and Form Discrimination (Benton), and Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4).
The physical examination consists of height or length, weight, head (age < 3 yr) and mid-upper arm circumferences, Tanner stage, edema classification, clinical BP, and resting pulse measured at each study visit. Tanner staging is standardized at annual training meetings, and medical illustrations of male and female Tanner stages are included on the study forms. The collection of annual questionnaires elicits information from the parent and the child about medical history, medication use, symptoms, quality of life, dietary intake, and environmental exposures (e.g., smoking, alcohol). Medication use includes prescription and over-the-counter medicines, dietary supplements, nutritional aids, and alternative medicines. Quality of life is measured with the parent and child versions of the Pediatrics Quality of Life Scale, a 23-item generic health status instrument that assesses five domains of health (Physical Functioning, Emotional Functioning, Psychosocial Functioning, Social Functioning, and School Functioning) in children and adolescents ages 2 yr and older (9,10). To assess physical activity, smoking, and alcohol and drug use in participants who are 12 yr old and older, questions from the 2005 Youth Risk Behavior Survey will be used (11,12).
Three-day diet records and a child version of the Willett food frequency questionnaire (13) will be obtained on a yearly basis beginning at the second visit at 3 to 6 months (V1b). Children and parents are instructed on how to complete a consecutive 3-d 24-h recall food diary by study coordinators who have undergone training to standardize instructions.
Clinical BP is measured three times at one sitting during the study visit, and the mean of the systolic and diastolic BP will be used. Hypertension will be defined as outlined in “The Fourth Report on the Diagnosis, Evaluation, and Treatment of High BP in Children and Adolescents” (14). Other CV measurements, including echocardiography, ABPM (SpaceLabs 90217 device, Issaquah, WA), and carotid artery intima-media thickness, will be taken at the same visits as the iohexol GFR beginning in month 12. A battery of neurocognitive tests (Table 4) and measures of nutrition and bone health (e.g., intact parathyroid hormone) will be taken at the 3- to 6-mo follow-up visit and then in alternate years between iohexol-based GFR measurements. The parathyroid hormone assay that will be used is the Elecsys System 2010 (Roche Diagnostics, Mannheim, Germany), a one-step sandwich electrochemiluminescence immunoassay that is based on the streptavidinbiotin technology (15).
Table 4.
CKiD neurocognitive battery by age
| Test | Age Range |
|---|---|
| Core tests for cognitive and developmental assessment | |
| Mullen Scales of Early Learning | 12 to 29 mo |
| Wechsler Preschool & Primary Scale of Intelligence, Third Edition (WPPSI-III) | 30 mo to 5 yr |
| Conner’s Kiddie Continuous Performance Test (K-CPT) | 4 to 5 yr |
| Conner’s Continuous Performance Test II (CPT-II) | 6 to 18 yr |
| Wechsler Abbreviated Scales of Intelligence (WASI) | 6 to 18 yr |
| Wechsler Individual Achievement Test, Second Edition–Abbreviated (WIAT-II-A) | 6 to 18 yr |
| Core tests for behavioral assessment | |
| Adaptive Behavior Assessment System, Second Edition (ABAS-II) Parent Form | 1 to 18 yr |
| Behavior Rating Inventory of Executive Function–Preschool Version (BRIEF-P) | 2 to 5 yr |
| Pediatrics Quality of Life Scale | |
| Parent Form | 2 to 18 yr |
| Child Form | 8 to 18 yr |
| Behavior Assessment System for Children, Second Edition (BASC-2) | |
| Parental Rating Scales (BASC-PRS) | 2 to 18 yr |
| Self-Report of Personality (BASC-SRP) | 8 to 18 yr |
| Behavior Rating Inventory of Executive Function (BRIEF) | 6 to 18 yr |
| Children’s Depression Inventory (CDI) | 7 to 18 yr |
Measurement of GFR
GFR will be measured at baseline, 12 mo, and biannually thereafter. GFR measurements will be based on blood disappearance curves with four time points at 10, 30, 120, and 300 min after infusion of 5 ml of iohexol (Omnipaque; GE Healthcare [formerly Amersham Health], Princeton, NJ). The timing of these four points was determined on the basis of pilot data (16). Concurrent with the iohexol-based measurement of GFR will be a cystatin C–based GFR measurement.
Primary Outcomes
The primary outcome is the rate of decline of the biomarker GFR, which is measured repeatedly over time in cohort participants. Specifically, iohexol-based GFR will be measured every 2 yr, and Schwartz-based GFR will be assessed every year. A secondary outcome is the time to ESRD, defined by transplantation, dialysis, or a 50% decrease in GFR.
Statistical Analyses
The repeated assessment of growth and development (e.g., physical measures of height, weight, and Tanner staging), cognitive and behavioral function, and health-related quality of life will allow us to assess the trajectories of these measures in children with stable kidney function and in those with declining kidney function in this cohort. The prospective nature of the cohort design will allow us to analyze properly the effect of the exposure (i.e., decline in GFR) on growth rates, sexual development, gain in cognitive and developmental function, etc. This will allow the identification of the effect of progressive kidney disease on slowing growth and development trajectories in children with CKD.
Results
Longitudinal Data Analysis of Biomarkers
The longitudinal data analysis methods described in this section apply with equal force to the analysis of the rate of decline in kidney function as measured by GFR, to the level and rate of changes in standardized neurocognitive function, to the level and rates of change of BP (obtained either by ABPM or in the clinical setting) and fasting lipids, and to the level and rates of growth as measured by changes in height or weight. For analyses that examine risk factors for decline in GFR, the outcome is continuous and may be analyzed as measured, or transformed to the log scale.
An important issue of longitudinal data is the intrinsic dependence of the observations measured over time within individuals (17-19). One approach to handle this dependence is to use generalized estimating equations (20). Alternatively, mixed-effects approaches (21) model the correlation of the repeated observations that are obtained over time for each individual. A key feature of such methods for longitudinal data is the ability to incorporate incomplete and unbalanced data (22). Classical methods of longitudinal analyses that weight individuals according to the number of data points contributed over time can be misleading. In particular, if we censor patients who progress quickly and proceed rapidly to ESRD, then it is possible that those who contribute the most data points would be those who decline the least, and, in turn, fast progressors may be underrepresented. Missing data methods may be tailored to account for such informative censoring (23,24).
Analysis of Time-to-Event Data
In the CKiD study, some time-to-event outcomes of potential interest are (1) stage 5 CKD (ESRD), (2) initiation of renal replacement therapy with dialysis or transplant, (3) a prespecified reduction (e.g., 50%) in GFR, and (4) incident hypertension. To study the natural history of CKD rather than anchor the analysis time scale as zero at the date of enrollment, we will anchor the time scale using birth or date of diagnosis of CKD obtained from medical records. Although children may be enrolled during roughly the same calendar period, construction of risk sets would account explicitly for the time since birth (i.e., age) or CKD diagnosis. Statistical methods to handle late entries will be used (25,26). Semiparametric survival methods, such as Cox proportional hazards regression, will be used to compare groups. However, parametric survival methods (27-29) will be used to provide clinical insight about the underlying disease process.
For a cohort of children with CKD, time to transplantation and time to dialysis may act as competing risks. Common methods for handling competing risks are to (1) construct a combined end point (e.g., ESRD), (2) censor one type of risk while modeling the second type of risk, or (3) fit an explicit model for the competing risks. Although some key analyses will use a combined ESRD end point (option 1), use of competing risk models (option 3) likely will yield insight that is not gleaned from the analysis of the combined end point (30,31). For example, race may have differing effects on the time to transplantation and time to dialysis.
Analytic Methods of Nested Studies
It is anticipated that many hypotheses will involve highly expensive or time-consuming laboratory assays, such that it will not be possible to investigate these hypotheses in the full cohort. In such cases, it will be necessary to either (1) identify case patients and match them to control subjects on the basis of specific characteristics to perform nested case-control analysis or (2) select a subcohort in which to perform a case-cohort analysis (32). An example of a nested case-control study in CKiD would assess a novel biomarker for CKD progression among all case patients who develop ESRD with biosamples that previously were obtained in this cohort compared with a set of age- and gender-matched children who are free of ESRD. The nested design will allow efficient estimation of the association between the novel biomarker and development of ESRD by requiring the biomarker to be measured only in the case patients and a subset of, rather than all, control subjects.
Statistical Power
The primary scientific goal of the study is to determine risk factors for rapid decline of GFR. Combining the measured GFR with the eGFR on the basis of an internally derived formula will result in a database with yearly GFR measurements. Assuming a uniform enrollment rate over the course of 24 mo, we will expect to have 270 children with four visits and 270 with three visits. We assume 10, 5, and 5% will exit follow-up before visits 2, 3, and 4, respectively, as a result of dropout and incident ESRD.
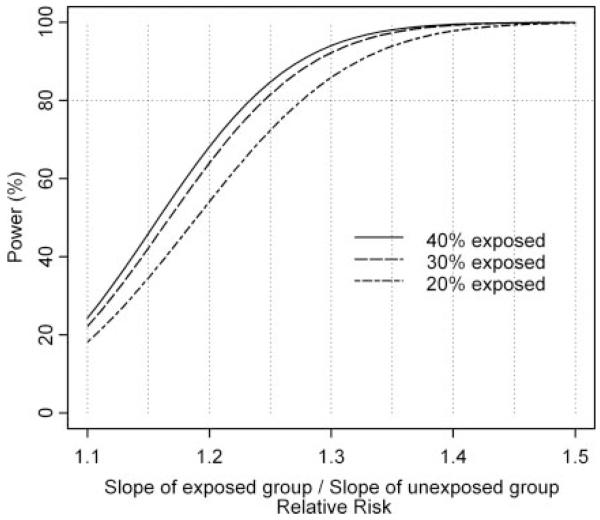
The slope of GFR decline in a group with a particular risk factor, for example hypertension (the exposed group; i.e. Δ1), will be compared with the slope of GFR decline in the unexposed group without hypertension, for example (i.e., Δ0). This ratio Δ1/Δ0 is the primary parameter for which the study is powered. Therefore, the primary hypothesis is whether Δ1/Δ0 = 1 (i.e., there is no risk for an accelerated decline associated with a putative exposure). We present statistical power curves for Δ1/Δ0 to be between 1.1 and 1.5 (i.e., an increase in the rate of decline as a result of the exposure from 10 to 50%), with a two-sided significance level of 5% (Figure 2). We assume that the SD of the baseline GFR is 12 ml/min per 1.73 m2 on the basis of data from the Modification of Diet in Renal Disease (MDRD) and the African American Study of Kidney Disease (AASK). An overall rate of decline of 5 ml/min per 1.73 m2 per year was assumed on the basis of NAPRTCS data (8). The three power curves in Figure 2 depict 20, 30, and 40% of the cohort being exposed. For a risk factor to which 40% are exposed, the study will have 80% power to detect an increase of 24% among the exposed (i.e., ratio of slopes = 1.24) when the overall rate of decline is 5 ml/min per 1.73 m2 per year.
Figure 2.
Statistical power for decline in GFR at 5% two-sided significance level with N = 540 and overall rate of GFR decline of 5 ml/min per 1.73 m2 per year.
Alternative analytical methods will use the time to event, in which the event may be ESRD. Table 5 shows the rate ratios that will be detectable with 80% statistical power at a 5% two-sided significance level according to three possible rates of progression per 100 person-years among the unexposed—3, 6, and 9 per 100 person-years—and by three levels of the prevalence of exposures of interest: 20, 30, and 40%. The total number of person-years will be 1350 (270 × 3 + 270 × 2), and allowing for an attrition of 20%, we use 1080 person-years in these power calculations. Specifically, reading from the center of Table 5, if the incidence is 6 per 100 person-years among the unexposed and 30% are exposed, then we will have 80% power to detect a rate ratio of approximately 1.9. However, if the event were to be defined, as in the AASK study (33), as (1) ESRD, (2) ≥50% decline in GFR, or (3) decline of at least 25 ml/min per 1.73 m2, then a rate of 9 per 100 person-years among the unexposed may be more appropriate and greater statistical power will result under such a composite end point.
Table 5.
Detectable rate ratios for ESRD with 80% statistical power at 5% two-sided significance level (N = 540)
| % Exposed | Incidence among the Unexposed |
||
|---|---|---|---|
| 3 per 100 Person-Years |
6 per 100 Person-Years |
9 per 100 Person-Years |
|
| 20 | 2.61 | 2.06 | 1.84 |
| 30 | 2.37 | 1.91 | 1.72 |
| 40 | 2.26 | 1.84 | 1.67 |
Discussion
CKD is a growing problem in the United States. Previous longitudinal studies of renal disease progression in adults (33,34) have suggested that the annual rate of decline in GFR in patients with CKD is approximately 3 to 5 ml/min per 1.73 m2. Therefore, many young adults who present with ESRD likely developed early stages of CKD in childhood or adolescence. In addition, CKD and its metabolic derangements substantially affect the well-being of children. The CKiD study will focus on risk factors for CKD progression. The study will obtain longitudinal data on 540 children who are aged 1 to 16 yr at study entry and have mildly to moderately impaired kidney function to determine the heterogeneity of rates of decline of renal function.
The CKiD study has several design elements that are unique. Kidney function will be measured by blood clearance of iohexol annually for the first 2 yr and then every other year. The first two iohexol-based GFR measurements will provide a precise baseline value from which to assess decline in biannual iohexol-based GFR measurements. Because it forgoes the intrinsic variability and limitations of urinary clearances of inulin, iothalamate, and indirect measures of kidney function that are based on serum creatinine, the use of iohexol GFR measurement, as proposed in CKiD, has the potential to become the standard for a precise measurement of kidney function in large population studies (35).
Furthermore, every year, we have the standard creatinine-based GFR estimates that allow for developing equations between iohexol- and creatinine-based GFR measurements. Through comparison with iohexol GFR measurement, the CKiD cohort study will provide a unique opportunity to improve on the current limitations of creatinine-based estimates of GFR in longitudinal follow-up of children with increasing muscle mass as a result of growth and puberty. Currently, creatinine-based estimates of GFR using the Schwartz formula use a different coefficient for pubertal boys; however, this alternative coefficient frequently is applied at a chronologic age cutoff, rather than more specifically associated with pubertal development and muscle mass. Delayed puberty, which is common in adolescents with CKD, may result in striking differences in eGFR depending on which Schwartz coefficient is applied. With the incorporation of age, gender, Tanner staging, height, and creatinine, compared with iohexol GFR in the CKiD study, we expect to find a continuous formula to estimate GFR from a combination of these parameters. Such an equation will be the essential means to complete the iohexol-based GFR data every year. For such purposes, we apply standard statistical procedures of imputation of missing data. Our design of collecting data on all participants every other year is stronger than alternative designs of selecting subsets of the cohort to be measured every year.
There are a number of unique challenges in conducting a multicenter cohort study of children. In contrast to studies of adults, one must obtain consent from parents as proxies for their children, as well as obtain the participating child’s assent. Obtaining consent for banking biologic and genetic materials from children and their parents is complex; for these specimens to remain in the repository, we may have to obtain consent for participation from children and adolescents when they turn 18 yr old.
In children, few large prospective cohort studies have addressed risk factors for CKD progression. High serum cholesterol (5), hyperlipidemia (36-38), hypertension (39), proteinuria (40), and race and ethnicity (41) each have been associated with CKD progression. This cohort study, with standardized prospective measurements and precise measures of GFR, will allow refined assessment of the risk that is associated with these clinical findings in children.
The effects of CKD on neurodevelopment and cognitive function of children remain largely unknown, but age of CKD onset (42,43), duration of kidney failure (44,45), hypertension (46), anemia (47-50), and depression (51-53) have been associated with impairments in cognition and neurodevelopment. Furthermore, systematic assessment of nutritional status by measurement of growth parameters (height, weight, mid-upper arm circumference, pubertal development, and nutritional intake via 3 d diet history) and food frequency questionnaire will be collected to define further the influence of CKD on these parameters and the influence of these parameters on cognition and development.
A key strength of the CKiD study is the measurement of GFR in the entire cohort. Currently, the best clinical estimate of GFR used in children is the Schwartz formula. However, studies that describe the precision of the Schwartz formula show that only approximately 75% of estimates are within 30% of the GFR measured by inulin clearance (54). Formulas such as the Cockcroft-Gault equation (55) and the more recent MDRD equation (56), which are used to estimate GFR in adults, do not generalize to children (57). The study intends to develop a novel estimating equation for GFR in children.
The proposed infrastructure for the CKiD study can serve as a platform for ancillary research grants and career development awards to enhance the scientific output of the study. Serum, plasma, and urine will be collected so that future ancillary studies potentially can examine the role of cytokines and chemokines in CV disease, malnutrition, growth failure, and CKD progression. Lymphocyte cell lines will be generated to facilitate study of genetic predisposition to CKD progression. Successful study implementation will improve understanding of the physiologic, genetic, environmental, and socioeconomic factors that are associated with CKD progression and with the impact of neurocognitive and CV sequelae on the overall well-being of children. Finally, identification of modifiable risk factors for progressive CKD, neurocognitive deficits, and CV disease may lead to intervention trials to improve the health outcomes of children and adolescents with CKD.
Acknowledgments
The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurologic Disorders and Stroke, National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, and UO1-DK-66116).
The CKiD prospective cohort study has clinical coordinating centers (principal investigators) at Children’s Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, PhD), and data coordinating center (principal investigator) at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, PhD).
We acknowledge the tireless efforts of the CKiD study coordinators: Judith Jerry-Fluker, MPH, Anne Carlson, MHS, Julie Starr, RN, Wendy Wantland, RN, Jacqueline Ndirangu, MPH, and Alicia Wentz, BS.
Appendix: CKiD Investigators by Clinical Site
| Site | Principal Investigator |
|---|---|
| Children’s Mercy Hospital CCC | |
| British Columbia Children’s Hospital | Colin White, MD |
| Cardinal Glennon Hospital | Ellen Wood, MD |
| Children’s Hospital and Medical Center–Cincinnati | Fred Strife, MD |
| Children’s Hospital and Medical Center–Seattle | Sandra Watkins, MD |
| Children’s Hospital–Boston | Nancy Rodig, MD |
| Children’s Hospital Central California | Jerome Murphy, MD |
| Children’s Hospital of Alabama | Mark Benfield, MD |
| Children’s Hospital of Los Angeles | Gary Lerner, MD |
| Children’s Hospital of Winnipeg | Tom Blydt-Hansen, MD |
| Children’s Mercy Hospital | Bradley Warady, MD |
| Egleston Children’s Hospital–Emory University | Barry Warshaw, MD, Larry Greenbaum, MD, PhD |
| LeBonheur Children’s Medical Center | Deborah Jones, MD |
| Medical College of Wisconsin | Cynthia Pan, MD, PhD |
| Oklahoma University Health Science Center | Martin Turman, MD, PhD |
| Oregon Health Science University | Robert Mak, MD, PhD |
| Phoenix Children’s Hospital | Bruce Morgenstern, MD |
| Rainbow Babies & Children’s Hospital | Ira Davis, MD |
| St. Louis Children’s Hospital | Paul Hmiel, MD |
| Stanford University Medical Center | Steve Alexander, MD |
| State University of New York–Stonybrook | Ivy Boydstun, MD |
| The Children’s Hospital at Denver | Doug Ford, MD |
| UCSF Children’s Renal Center | Anthony Portale, MD |
| University of California–Los Angeles | Ora Yadin, MD |
| University of California–San Diego | Nadine Benador, MD |
| University of New Mexico | Craig Wong, MD |
| University of South Florida | Alfonso Campos, MD |
| University of Texas–Southwest Medical Center | Mouin Seikaly, MD |
| University of Wisconsin | Sharon Bartosh, MD |
| Johns Hopkins University CCC | |
| Alfred I. Dupont Hospital for Children | Shermaine Dabbagh, MD |
| Children’s Hospital at Montefiore | Joseph Flynn, MD, Frederick Kaskel, MD, PhD |
| Children Hospital of Michigan | Tej Mattoo, MD, Guillermo Hidalgo, MD |
| Children’s Hospital of New York, Columbia University | Martin Nash, MD |
| Children’s Memorial Hospital, Northwestern University | Craig Langman, MD |
| Children’s National Medical Center | Kanwal Kher, MD, Kevin McBryde, MD |
| Columbus Children’s Hospital, Ohio State University | John Mahan, MD, Mark Mentser, MD |
| INOVA Fairfax Hospital for Children | Lynne Yao, MD |
| Johns Hopkins Children’s Center | Susan Furth, MD, PhD, Barbara Fivush, MD |
| Indiana University, Riley Hospital for Children | Sharon Andreoli, PhD |
| Maimonides Medical Center | Juan Kupferman, MD |
| Mount Sinai Medical Center | Jeffery Saland, MD |
| New York Presbyterian Hospital | Valerie Johnson, MD, PhD |
| North Shore University Hospital | Manju Chandra, MD |
| Robert Wood Johnson Medical School–UMDNJ | Lynne Weiss, MD |
| Schneider Children’s Hospital, Long Island Jewish | Manju Chandra, MD |
| Texas Children’s Hospital, Baylor | Eileen Brewer, MD |
| University of Florida | Vikas Dhamidharka, MD |
| University of Maryland | Susan Mendley, MD |
| University of Michigan, Mott Hospital | Susan Thomas, MD, Patrick Brophy, MD |
| University of North Carolina, Chapel Hill | Debbie Gipson, MD, Maria Ferris, MD |
| University of Rochester Medical Center, Goliano Children’s Hospital at Strong |
Marc Lande, MD |
| University of Texas, Houston | Ronald Portman, MD |
| University of Virginia | Victoria Norwood, MD |
| Vanderbilt Children’s Hospital | Kathy Jabs, MD |
| Westchester Medical Center | Robert Weiss, MD |
References
- 1.USRDS 2003 Annual Data Report: Atlas of End Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2003. [Google Scholar]
- 2.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, III, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. Suppl. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed July 6, 2005];Healthy People 2010. 2001 Available online: www.usrds.org/2003/pdf/oc_hp2010_03.pdf.
- 4.USRDS 2004 Annual Data Report: Pediatric ESRD. Am J Kidney Dis. 2005;45:S153–S166. [Google Scholar]
- 5.Lin CY, Sheng CC, Chen CH, Lin CC, Chou P. The prevalence of heavy proteinuria and progression risk factors in children undergoing urinary screening. Pediatr Nephrol. 2000;14:953–959. doi: 10.1007/s004679900278. [DOI] [PubMed] [Google Scholar]
- 6.Wingen AM, Fabian-Bach C, Schaefer F, Mehls O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet. 1997;349:1117–1123. doi: 10.1016/s0140-6736(96)09260-4. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 8.North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) [Accessed July 6, 2005];Annual Data Report, 2002. 2002 Available online: web.emmes.com/study/ped/annlrept.html.
- 9.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Varni JW. [Accessed July 6, 2002];PedsQL Pediatric Quality of Life Inventory. 2004 Available online: www.pedsql.org.
- 11.Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, Harris WA, McManus T, Chyen D, Collins J. Youth risk behavior surveillance—United States, 2003. MMWR Surveill Summ. 2004;53:1–96. [PubMed] [Google Scholar]
- 12.CDC . 2005 State and Local Youth Risk Behavior Survey. Centers for Disease Control and Prevention; Atlanta: 2005. [Google Scholar]
- 13.Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 15.Hermse D, Franzson L, Hoffmann JP, Isaksson A, Kaufman JM, Leary E, Muller C, Nakatsuka K, Nishizawa Y, Reinauer H, Riesen W, Roth HJ, Steinmuller T, Troch T, Bergmann P. Multicenter evaluation of a new immunoassay for intact PTH measurement on the Elecsys System 2010 and 1010. Clin Lab. 2002;48:131–141. [PubMed] [Google Scholar]
- 16.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 17.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. 2nd Ed. New York, Oxford: 2002. [Google Scholar]
- 18.Pendergast JF, Gange SJ, Lindstrom ML, Newton MA, Palta M, Fisher MR. A survey of methods for analyzing clustered binary data. Int Stat Rev. 1996;64:89–118. [Google Scholar]
- 19.Ware JH, Lipsitz S, Speizer FE. Issues in the analysis of repeated categorical outcomes. Stat Med. 1988;7:95–107. doi: 10.1002/sim.4780070113. [DOI] [PubMed] [Google Scholar]
- 20.Liang KY, Zegler SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.Munoz A, Carey V, Schouten JP, Segal M, Rosner B. A parametric family of correlation structures for the analysis of longitudinal data. Biometrics. 1992;48:733–742. [PubMed] [Google Scholar]
- 22.Schluchter MD. Analysis of incomplete multivariate data using linear models with structured covariance matrices. Stat Med. 1988;7:317–324. doi: 10.1002/sim.4780070132. [DOI] [PubMed] [Google Scholar]
- 23.Little RJ, Rubin DB. Statistical analysis with missing data. 2nd Ed. Wiley; New Jersey: 2002. [Google Scholar]
- 24.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 25.Detels R, Munoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Schrager LK, Phair JP. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 26.Lamarca R, Alonso J, Gomez G, Munoz A. Left-truncated data with age as time scale: An alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53:M337–M343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 27.Munoz A, Sunyer J. Comparison of semiparametric and parametric survival models for the analysis of bronchial responsiveness. Am J Respir Crit Care Med. 1996;154:S234–S239. doi: 10.1164/ajrccm/154.6_Pt_2.S234. [DOI] [PubMed] [Google Scholar]
- 28.Munoz A, Xu J. Models for the incubation of AIDS and variations according to age and period. Stat Med. 1996;15:2459–2473. doi: 10.1002/(sici)1097-0258(19961130)15:22<2459::aid-sim464>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Piantadosi S, Crowley J. An implicitly defined parametric model for censored survival data and covariates. Biometrics. 1995;51:249–258. [PubMed] [Google Scholar]
- 30.Hoover DR, Munoz A, Carey V, Taylor JMG, VanRaden M, Chmiel JS, Kingsley L. Using events from dropouts in non-parametric survival function estimation with application to incubation of AIDS. J Am Stat Assoc. 1993;88:37–43. [Google Scholar]
- 31.Munoz A, Sabin CA, Phillips AN. The incubation period of AIDS. AIDS. 1997;11(Suppl A):S69–S76. [PubMed] [Google Scholar]
- 32.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 33.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 34.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GJ, Furth SL, Cole SR, Warady BA, Munoz A. Glomerular filtration rate via plasma iohexol disappearance from: Pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 36.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: A meta-analysis. Kidney Int. 2001;59:260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 37.Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 3):S142–S156. doi: 10.1053/ajkd.1998.v32.pm9820472. [DOI] [PubMed] [Google Scholar]
- 38.Keane WF. Lipids and progressive renal disease: The cardio-renal link. Am J Kidney Dis. 1999;34:xliii–xxlvi. doi: 10.1016/s0272-6386(99)70343-4. [DOI] [PubMed] [Google Scholar]
- 39.Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephrol. 2003;14:2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 40.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 41.Bowden DW. Genetics of kidney disease. Kidney Int Suppl. 2003;83:S8–S12. doi: 10.1046/j.1523-1755.63.s83.3.x. [DOI] [PubMed] [Google Scholar]
- 42.Crittenden MR, Holliday MA, Piel CF, Potter DE. Intellectual development of children with renal insufficiency and end stage disease. Int J Pediatr Nephrol. 1985;6:275–280. [PubMed] [Google Scholar]
- 43.Rasbury WC, Fennell RS, 3rd, Fennell EB, Morris MK. Cognitive functioning in children with end stage renal disease pre- and post-dialysis session. Int J Pediatr Nephrol. 1986;7:45–50. [PubMed] [Google Scholar]
- 44.Fennell RS, 3rd, Rasbury WC, Fennell EB, Morris MK. Effects of kidney transplantation on cognitive performance in a pediatric population. Pediatrics. 1984;74:273–278. [PubMed] [Google Scholar]
- 45.Rasbury WC, Fennell RS, 3rd, Morris MK. Cognitive functioning of children with end-stage renal disease before and after successful transplantation. J Pediatr. 1983;102:589–592. doi: 10.1016/s0022-3476(83)80194-2. [DOI] [PubMed] [Google Scholar]
- 46.Lande MB, Kaczorowski JM, Auinger P, Schwartz GJ, Weitzman M. Elevated blood pressure and decreased cognitive function among school-age children and adolescents in the United States. J Pediatr. 2003;143:720–724. doi: 10.1067/S0022-3476(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 47.Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107:1381–1386. doi: 10.1542/peds.107.6.1381. [DOI] [PubMed] [Google Scholar]
- 48.Lawry KW, Brouhard BH, Cunningham RJ. Cognitive functioning and school performance in children with renal failure. Pediatr Nephrol. 1994;8:326–329. doi: 10.1007/BF00866349. [DOI] [PubMed] [Google Scholar]
- 49.Marsh JT, Brown WS, Wolcott D, Carr CR, Harper R, Schweitzer SV, Nissenson AR. rHuEPO treatment improves brain and cognitive function of anemic dialysis patients. Kidney Int. 1991;39:155–163. doi: 10.1038/ki.1991.20. [DOI] [PubMed] [Google Scholar]
- 50.Sagales T, Gimeno V, Planella MJ, Raguer N, Bartolome J. Effects of rHuEPO on Q-EEG and event-related potentials in chronic renal failure. Kidney Int. 1993;44:1109–1115. doi: 10.1038/ki.1993.356. [DOI] [PubMed] [Google Scholar]
- 51.Craven JL, Rodin GM, Johnson L, Kennedy SH. The diagnosis of major depression in renal dialysis patients. Psychosom Med. 1987;49:482–492. doi: 10.1097/00006842-198709000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Garralda ME, Jameson RA, Reynolds JM, Postlethwaite RJ. Psychiatric adjustment in children with chronic renal failure. J Child Psychol Psychiatry. 1988;29:79–90. doi: 10.1111/j.1469-7610.1988.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 53.Kimmel PL, Thamer M, Richard CM, Ray NF. Psychiatric illness in patients with end-stage renal disease. Am J Med. 1998;105:214–221. doi: 10.1016/s0002-9343(98)00245-9. [DOI] [PubMed] [Google Scholar]
- 54.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2005;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 55.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 56.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 57.Lee CL, Swinford RD, Cerda RD, Portman RJ, Hwang W, Furth SL. Comparison of glomerular filtration rate estimation methods in children with chronic kidney disease (CKD) [Abstract] J Am Soc Nephrol. 2004;15:368A. [Google Scholar]