Abstract
Despite significant advances in our knowledge of how testosterone mediates life-history trade-offs, this research has primarily focused on seasonal species. We know comparatively little about the relationship between testosterone and life-history stages for non-seasonally breeding species. Here we examine testosterone profiles across the lifespan of males from three non-seasonally breeding primates: yellow baboons (Papio cynocephalus or P. hamadryas cynocephalus), chacma baboons (Papio ursinus or P. h. ursinus), and geladas (Theropithecus gelada). First, we predict that testosterone profiles will track the reproductive profiles of each taxon across their respective breeding years. Second, we evaluate age-related changes in testosterone to determine whether several life-history transitions are associated with these changes. Subjects include males (>2.5 years) from wild populations of each taxon from whom we had fecal samples for hormone determination. Although testosterone profiles across species were broadly similar, considerable variability was found in the timing of two major changes: (1) the attainment of adult levels of testosterone, and (2) the decline in testosterone after the period of maximum production. Attainment of adult testosterone levels was delayed by one year in chacmas compared with yellows and geladas. With respect to the decline in testosterone, geladas and chacmas exhibited a significant drop after three years of maximum production, while yellows declined so gradually that no significant annual drop was ever detected. For both yellows and chacmas, increases in testosterone production preceded elevations in social dominance rank. We discuss these differences in the context of ecological and behavioral differences exhibited by these taxa.
Keywords: androgen, fecal steroid, hormone, life-history, maturation, method validation
Introduction
The steroid hormone testosterone (T) is known to affect many vertebrate life-history traits and has been implicated as a mediator of life-history trade-offs (Hau, 2007; Ricklefs and Wikelski, 2002) (reviewed in Zera and Harshman, 2001). For example, the increase in production of T when males reach puberty and begin to seek out mating opportunities is at the same time associated with costs, such as reduced immune function (McGlothlin et al., 2007).
One model for T-behavior trade-offs, known as the “challenge hypothesis”, proposes that variation in male T across life-history stages reflects differential allocation to mating and parenting behavior (Wingfield et al., 1990). Specifically, the challenge hypothesis predicts that high T facilitates inter-male competition at times in the life cycle when males need to compete for receptive females or the resources necessary to attract such females. However, because high levels of T may interfere with paternal behavior (e.g., Goymann et al., 2007; Gray et al., 2006; Muller et al., 2009; Nunes et al., 2000; Nunes et al., 2001), T levels should decrease when males care for offspring. Formulation of the challenge hypothesis was based on monogamous, seasonal birds with a high degree of paternal care and cycles of mating and care within each year. For such seasonal species with relatively short and intense cycles of mating, the period of interest for investigating T-mediated trade-offs is within each breeding season (temperate birds: (e.g., McGlothlin et al., 2007; Wingfield et al., 1990), tropical birds: (Hau et al., 2008), reptiles: (e.g., Wack et al., 2008), and mammals: (e.g., Brockman et al., 2001; Cavigelli and Pereira, 2000; Malo et al., 2009; Moss et al., 2001; Ostner et al., 2002, 2008).
More recently, the hypothesis has been modified to apply to non-seasonally breeding species as well (Archer, 2006; Muller and Wrangham, 2004). Non-seasonal species experience changes in T as it relates to changes in several life-history stages across the lifespan rather than exhibiting relatively short and discrete elevations of T and mating behavior each year, (e.g., Bribiescas, 2006; Crawford et al., 1997; Ellison et al., 2002; Martin et al., 1977). Therefore, it seems reasonable to expect that, for non-seasonal species, T should be up-regulated at the start of reproductive maturity, maintained throughout the breeding years, and down-regulated once males are no longer breeding or when they focus on paternal behaviors. For non-seasonal species, many of the potential trade-offs extend across life-history stages that, for long-lived organisms, can take years. With the exception of several studies of human and captive non-human primate (e.g., Bribiescas, 2001, 2005, 2006; Crawford et al., 1997; Ellison et al., 2002; Martin et al., 1977), investigation of T profiles across the entire lifespan are rare (but see chimpanzees (Pan troglodytes): Seraphin et al., 2008; and mandrills (Mandrillus sphinx): Setchell and Dixson, 2002).
Here we examine T profiles across the lifespan of males in wild populations of three long-lived, non-seasonally breeding primate taxa (see Methods below): yellow baboons (Papio cynocephalus or P. hamadryas cynocephalus), chacma baboons (Papio ursinus or P. h. ursinus), and geladas (Theropithecus gelada). Specifically, we evaluate age-related changes in T and examine whether several life-history transitions (or “maturational milestones”) are associated with these changes. Despite the rarity of mammalian paternal care, baboon and gelada males are known to invest in some degree of paternal care (Beehner and Bergman, 2008; Buchan et al., 2003; Dunbar, 1984; Moscovice et al., 2009; Palombit et al., 2000). Therefore, we expect that trade-offs between the high levels of T optimal for mating and the low levels of T optimal for parenting, will result in T modulation for these three taxa, and that this modulation will reflect differences among them in their respective life histories.
We have three lines of inquiry. First, as a physiological validation and in accordance with T profiles from other vertebrate species, we test the prediction that juvenile males have significantly lower fecal T metabolites than adult males. Further, based on profiles of T across the human male lifespan, T for all three taxa should exhibit an inverse U-shaped pattern, exhibiting a rise at or around maturity and a decline as the animals senesce.
Second, we make the general prediction that the T profiles for males will follow the reproductive profiles of each taxon across their respective breeding periods. In particular, we expect T to remain elevated during ages when males are reproductively active and to return to pre-reproductive levels when mating activity declines and parental care increases. Although all three taxa are non-seasonal breeders, they differ in the timing of reproductive and paternal behavior. At one extreme, geladas have a unimale system (polygynous), in which one male (“leader male”) has sole reproductive access to females that make up a one-male unit. Gelada males have (1) a single tenure as a leader, (2) no reproductive access to females before obtaining a unit (when they live as “bachelor males” in all-male groups), and (3) no reproductive access to females after losing their unit (when they live as “follower males” in their former unit). For gelada males, changes in mating activity across the lifespan are qualitative and reproductive tenure is discrete. Furthermore, once leader males relinquish their unit and become follower males, then and only then do they engage in protective parenting behavior (Dunbar, 1984) – presumably to protect their offspring from infanticide (Beehner and Bergman, 2008).
In contrast, yellow and chacma baboons both exhibit a multimale social structure with a mating system that is to varying degrees polygynandrous, in which males compete for temporary reproductive access to fertile females – a competition that is mediated by dominance rank. Specifically, dominance rank functions as a queue for mating opportunities (Alberts et al., 2003; Altmann, 1962; Bulger, 1993; Weingrill et al., 2003; Weingrill et al., 2000). For yellow and chacma males, changes in mating activity across the lifespan are quantitative, such that mating activity rises during early adulthood and falls in late adulthood.
Although high dominance rank facilitates reproductive access to females in both baboon taxa, behavioral data and paternity determination indicate that non-alpha males are thought to be more viable competitors for females in yellow baboon groups than they are in chacma groups (Alberts et al., 2003; Alberts et al., 2006; Bulger, 1993; Cheney and Seyfarth, unpublished data). Mating in some chacma populations may therefore at any one time be relatively more unimale than yellow baboons due to higher reproductive skew. Furthermore, the timing of paternal care for chacma males may also parallel that described for geladas because (1) paternal care serves to protect offspring from infanticide (Palombit et al., 2000), (2) infanticidal males are generally newly immigrant males that have attained the alpha position in the dominance hierarchy (Bulger and Hamilton, 1987; Busse and Hamilton, 1981; Collins et al., 1984; Palombit et al., 2000; Tarara, 1987), and therefore, (3) paternal behavior generally occurs after a father has fallen from the alpha position.
Based on these differences, two predictions emerge. If, as in many bird species, (Wingfield 1990), T profiles are linked primarily to demographically defined mating systems, gelada T profiles will exhibit a more discrete period of elevation while those of yellow and chacma baboons will exhibit a more extended period of adult T levels. Alternatively, if T profiles track the actual differences among taxa in reproductive access to females and parenting behavior, geladas and chacmas will exhibit a discrete period of T elevation, and yellow males will exhibit a more extended period of adult T levels, characterized by a gradual fall in T after peak reproductive years.
Third, we describe the relationship between the maturational rise in T levels and several male maturational milestones. One visible maturational marker, (1) enlargement of testes, is available for only one taxon (yellow baboons) and has been shown to precede significant increases in fecal T metabolites (Gesquiere et al., 2005). Therefore, we examine the relationship in these three taxa between T profiles and four additional maturational markers: (2) the timing of natal dispersal, (3) the acquisition of adult dominance rank, (4) the acquisition of highest rank, and (5) first sexual consortship.
Methods
Because the taxonomic level of the different Papio groups remains uncertain (Jolly, 1993), we avoid this debate altogether by referring to yellow and chacma baboons throughout as different “taxa” (see also Barrett and Henzi, 2008) – whether they are considered different species or subspecies does not affect the results presented here. Subjects for this study include all males aged 2.5 years and older from each study population from whom we had fecal samples for hormone determination.
Yellow baboons and data collection
The data for yellow baboons come from multiple groups in the Amboseli Basin, Kenya. Because individual life-history data for members of these study groups cover more than three decades (Alberts et al., 2003; Alberts and Altmann, 1995a; Alberts et al., 1996; Altmann and Alberts, 2003; Altmann et al., 1988; Pereira, 1988; Shopland, 1987), birthdates are known within a few days for all immature and many mature males. Ages of immigrant males for whom birthdates are not known were estimated using an established protocol based on body size and other age-related physical characteristics when these males first appear in one of the study groups. Timing of male maturational milestones for yellow baboons used in this study are taken from previous studies on the population (see summary in Charpentier et al., 2008). Male dominance ranks were determined by assigning wins and losses for all dyadic agonist encounters between males, as described in Hausfater (1975) and Alberts et al. (2003).
As part of the continuing Amboseli baboon research, repeated fecal samples are collected opportunistically from all group members. Because the testosterone RIA kit previously used in our laboratory (Equate 125I Testosterone RIA kit, SolidPhase, Portland, ME) was discontinued, we validated a subset of our samples using a new T RIA kit (Diagnostics Systems Laboratories). For this validation we used 2570 samples from 125 different males, for an average of 21 fecal samples per male, with at least 20 different males per age category (see age categories below). All data collection procedures adhered to the Institutional Animal Care and Use Committee guidelines of Princeton University and the laws of Kenya.
Fecal sample collection, storage, and extraction were performed as described previously (Beehner et al., 2006b; Gesquiere et al., 2005; Gesquiere et al., 2007; Khan et al., 2002; Lynch et al., 2003). In brief, freshly deposited samples were mixed thoroughly, placed in 95% ethanol and stored in a charcoal refrigerator (∼20-25°C) until shipped to the University of Nairobi (every two weeks), where the ethanol was evaporated, and samples were freeze-dried. Following freeze-drying, samples were stored at -20°C until shipped to Princeton University. After transport, each fecal sample was sifted to remove vegetative matter, and 0.2 g of fecal powder was extracted into 2 ml 90% methanol using a multipulse vortexer for 30 minutes. Following extraction, samples were run through a prepped Oasis cartridge (Waters, Milford, MA) for further purification. Prior to assay, all samples were stored at −20°C.
Chacma baboons and data collection
The data for the chacma baboons come from one wild-feeding group in the Moremi Game Reserve, Botswana. This group has been studied almost continuously since 1982 (Bulger and Hamilton, 1987; Cheney et al., 2004), and the ages of all natal males are known. The ages of immigrant males were estimated based on body size and tooth wear (Kitchen et al., 2003). If newly-immigrated males appeared young and in their prime, they were assigned the median age at dispersal for this population (9.25 years, N=26) at the time they entered the study group. Only natal emigrants who were later seen in a neighboring group were used to calculate age at first dispersal. Dominance ranks of all males were calculated monthly based on the outcomes of dyadic interactions. Males were assigned an adult rank after achieving dominance over another adult male. First consortships were recorded after a male exhibited his first mate-guarding episode (see also Alberts and Altmann, 1995a).
As part of a two-year study from 2001-2003, repeated fecal samples were collected from all adult males. Additionally, as part of a short-term study to target all age groups, repeated fecal samples were collected from males of all ages during August 2007. As for samples from the yellow baboons, chacma fecal samples had previously been analyzed with the Equate T RIA kit. Therefore, we validated chacma fecal samples with the new DSL T RIA kit as well. For the validation, we used 726 samples from 41 different males, for an average of 18 fecal samples per male. All data collection procedures adhered to the Institutional Animal Care and Use Committee guidelines of the University of Pennsylvania and the University of Michigan and the laws of Botswana.
Hormones were extracted from feces in the field using the method described by Beehner and Whitten (2004). Specifically, fresh fecal samples were mixed thoroughly, an aliquot of the sample (∼ 0.5 g) was placed in 10 ml of a methanol/acetone solution (4:1), and the solution was immediately homogenized for 1 min using a battery-powered vortexer (BioVortexer, BioSpec Products, Inc., Bartlesville, OK). The dry weight of all fecal samples was later determined (±0.001 g) using a battery-powered, portable scale (Ohaus Scout Pro, Pine Brook, NJ). Approximately 7 h later, 4.0 ml of the fecal homogenate was filtered through a 0.2μm polytetrafluoroethylene (PTFE) syringless filter (Whatman, Florham Park, NJ), and the filter was subsequently washed with 1 ml of methanol/acetone (4:1). We then added 7 ml of distilled water to the filtered homogenate, mixed the solution (by inverting it 10 times), and loaded it onto a prepped, solid-phase extraction cartridge (Sep-Pak Plus, Waters, Milford, MA), followed by 2 ml of a sodium azide solution (0.1%) as a wash and preservative. All samples were stored dry on cartridges in separate sealed bags with silica beads (∼2 g) at subzero temperatures (-10°C) until transported to the University of Michigan for analysis. In the laboratory, steroids were eluted from cartridges with 2.5 ml 100% methanol and subsequently stored at -20°C until the time of RIA.
Geladas and data collection
The data for the geladas come from two bands of wild-feeding geladas in the Simien Mountains National Park, Ethiopia. Because daily observations on this gelada population began in January 2006, all ages of gelada males are necessarily estimated. We placed males in age categories based on (1) a combination of published descriptions of gelada age characteristics based on morphological traits (Dunbar and Dunbar, 1975) and on (2) our observations of physical size and developmental markers (e.g., canine eruption) as compared to baboons (Papio) of known ages. Age categories were assigned independently by two observers, and both sets were in close agreement (age categories, estimated ages, and general characteristics describing each category can be found in supplementary material). No overt dominance dominance hierarchy among leader males has been reported (Mori, 1979). Juvenile males may form temporary hierarchies with their peers and these hierarchies might later extend to all-male groups (Dunbar, 1984); however, no dominance data are available for either of these groups. Timing of first sexual consortships for gelada males were recorded as the first time a new leader male mated with one of the females in his new unit.
Samples available for this study derive from the first 6 months of collection. In total, we collected 328 fecal samples for hormone analysis from 100 different males, for an average of 3 samples per male (range: 1-10), with at least 10 individuals in each age category. Hormones were extracted from feces in the field using a method almost identical to that for the chacma baboons described above (with a few modifications to the volume of sample and solutions used). In brief, fresh fecal samples were thoroughly mixed, an aliquot of the sample (∼ 0.1 g) was placed in 3 ml of a methanol/acetone solution (4:1), and the solution was immediately homogenized. The dry weight of all fecal samples was later determined (±0.001 g). Approximately 7 h later, 2.5 ml of fecal homogenate was filtered through a 0.2 μm PTFE filter and washed with and additional 1 ml of methanol/acetone (4:1). We then added 7 ml of distilled water to the filtered homogenate, mixed the solution, loaded it onto a prepped Sep-Pak cartridge, and washed the cartridge with 1 ml of a sodium azide solution (0.1%). All samples were stored dry on cartridges in separate sealed bags with ∼2 g silica beads. Samples were stored at ambient temperatures for up to 10 days until they could be transported to a freezer at a nearby lodge. Once frozen, samples remained at subzero temperatures (-10°C) until transported to the University of Michigan for analysis. In the laboratory, steroids were eluted from cartridges with 2.5 ml 100% methanol and subsequently stored at -20°C until the time of RIA.
Testosterone RIA
For all three taxa, we used the same assay methods and the same T primary antibody. We assayed all samples for T metabolites using a modified protocol from a commercially-available T RIA kit (Diagnostics Systems Laboratories, Beckman Coulter, Webster, TX). All assays used the standards, the primary antibody, the labeled testosterone, and the precipitant solution provided by the kit. Working buffer was a charcoal adsorbed human serum similar to the buffer in which the standards were diluted (American Biological Technologies, Inc., Seguin, TX). The primary antibody from the DSL T kit cross-reacts 100% with testosterone, 6.6% with 5α-dihydrotestosterone, 2.2% with 5-androstane-3β, 17β-diol, 1.8% with 11-oxotestosterone, 0.9% with androstenedione, and 0.6% with 5β-dihydrotestosterone. Cross reactivity of the antiserum with all other steroids is less than 0.5%. All samples were run in duplicate, and the results are expressed as ng/g dry fecal matter.
Method validation
First, to evaluate the effectiveness of extracting T metabolites from primate feces, we determined recovery for each primate taxa with each method. For the yellow baboons, 10,000 cpm of 125I testosterone was added to 0.2 g of dry feces (Lynch et al., 2003). After incubation for 1 hr at room temperature, we proceeded with methanol extraction and solid phase extraction as described above. For the chacma baboons and geladas, we thoroughly mixed a large mass of feces in a plastic cup and measured out 10 aliquots of 0.5 ml wet feces. We then added 18,000 cpm 125I testosterone tracer to each aliquot. After incubating aliquots for 1 h at room temperature, we placed aliquots in 3 ml MeOH/acetone solution (4:1) and extracted each using the same methods described for each taxon above. Following elution, we measured recovery of the radioactivity with a gamma counter. Recovery results for each taxa are listed in Table 1.
Table 1.
Validation results for yellow baboons, chacma baboons, and geladas.
| Yellow baboons | N | Chacma baboons | N | Geladas | N | All assays | N | ||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (125I Testosterone %) | |||||||||
|
| |||||||||
| *76.6 | 5 | 65.1 | 10 | 55.9 | 10 | ||||
|
| |||||||||
| Parallelism | |||||||||
|
| |||||||||
| R2 | 0.97 | 14 | 0.99 | 12 | 0.98 | 24 | |||
| Equation for observed against expected | y = 1.14x - 0.37 | y = 0.81x + 6.12 | y = 0.88x + 20.32 | ||||||
| p | <0.001 | <0.001 | <0.001 | ||||||
|
| |||||||||
| Accuracy | |||||||||
|
| |||||||||
| R2 | 0.98 | 24 | 0.99 | 24 | 0.99 | 24 | |||
| Equation for observed against expected | y = 1.14x - 2.94 | y = 0.99x - 3.06 | y = 0.99x + 8.30 | ||||||
| p | <0.001 | <0.001 | <0.001 | ||||||
|
| |||||||||
| Precision | |||||||||
|
| |||||||||
| Inter-assay CV (%) | |||||||||
|
|
|||||||||
| High kit control | 11.70 | 66 | |||||||
| Low kit control | 14.09 | 66 | |||||||
| Fecal pool (∼70 pg) | 11.66 | 17 | 6.08 | 35 | 10.79 | 7 | |||
| Intra-assay CV (%) | |||||||||
|
|
|||||||||
| High kit control | 7.83 | 18 | |||||||
| Low kit control | 8.17 | 18 | |||||||
| Fecal pool (∼70 pg) | 6.27 | 12 | 4.76 | 10 | 4.29 | 10 | |||
Second, we validated the DSL antibody for each taxon. We ran serial dilutions of baboon and gelada fecal extract pools to check for parallelism with the standard curve. We also determined mean assay accuracy (observed/expected * 100) for each taxon by spiking each standard with an aliquot from the respective fecal pool and running them as samples. We calculated intra- and inter-assay coefficients of variation for the assay for all three taxa. Results for parallelism, accuracy, and precision for each taxon are listed in Table 1.
Third, as a physiological validation to our methods, we compared T concentrations between juvenile and adult males for all three taxa. Although we did not expect age-related changes to be identical for all three taxa, we did expect a consistent difference in which juvenile males of each taxon would exhibit lower concentrations of T metabolites than the fully adult males.
Age categories
Generally, primate juveniles are defined by maturational markers such as independence from their mother (marking the start of juvenility) and puberty (marking the end). Because these maturational markers vary for each taxon in this study, we broadly define “juveniles” as males between the ages of 2.5 years (when males of all three taxa are independent from their mother) and 4.5 years (when none of the three taxa have reached puberty). We define adults using similar criteria; “adults” in this study comprise males older than 8.0 years (when all three taxa have reached full adulthood). Males between juveniles and adults are in various stages approaching adulthood, and these differences are taxon-specific. Thus, for convenience herein, we broadly use the term “subadult” for all male ages between juveniles and adults (i.e., ages 4.5-8.0 years). We recognize that the subadult biological category is different for each of these taxa.
The ages of most yellow and chacma males were known to within a few days (for males born into study groups) and estimated based on physical characteristics and date of immigration (for immigration males). By contrast, the ages of all gelada males were estimated based on developmental stages distinguished by physical markers (see supplementary material). Therefore, to facilitate the comparison of profiles across all three taxa, we placed yellow and chacma males into multi-year age categories corresponding to those for gelada males. We then calculated a mean T value for each category (with each individual contributing only one value). Testosterone profiles thus comprise a combination of longitudinal and cross-sectional data. After comparing T profiles based on the multi-year categories for all three taxa, we then compared T profiles using single-year categories for yellow and chacma males (yearly age categories were not available for geladas).
Maturational milestones
Male maturational milestones examined in this study include: (1) age at testicular enlargement (signaling puberty and the onset of subadulthood), (2) age at natal dispersal (when males leave their natal group and seek entry in another group), (3) age at attainment of adult dominance rank (signaling the beginning of adulthood), (4) age at first sexual consortship (the best measure available for age at first reproduction in male primates), and (5) age at attainment of highest dominance rank (presumably the time of maximum mating opportunities). Milestones are based on individual life-history data for all except acquisition of highest rank, which, of necessity, is cross-sectional. We report median values for all five of these milestones for yellow males (Alberts and Altmann, 1995a, 1995b; Charpentier et al., 2008; current study), and all but age of testicular enlargement for chacma males (Table 2). For geladas we report age ranges for dispersal and first sexual consortship. Age at testicular enlargement is not known for either gelada or chacma males, and dominance ranks are not available for gelada males because, to our current knowledge, gelada males do not have a formalized dominance hierarchy.
Table 2.
Median age (or age range, in years) that males reach hormonal and life-history milestones for yellow baboons, chacma baboons, and geladas.
| Hormones (testosterone production) | Yellows | N | Geladas | N | Chacmas | N |
|---|---|---|---|---|---|---|
| Onset of adult T levels$ | 6.5-7.5 | - | 6.5-8.0 | - | 7.5-8.5 | - |
| Peak T levels$ | 7.5-8.5 | - | 6.5-8.0 | - | 8.5-9.5 | - |
| Life-history (maturational milestone) | ||||||
|
| ||||||
| Testicular enlargement | 5.38* | 96 | - | - | - | - |
| First dispersal | 7.47* | 93 | 4.5-6.5** | 70 | 9.25 | 26 |
| Acquisition of adult rank | 7.38* | 48 | N/A | - | 8.67 | 15 |
| First sexual consortship | 7.87* | 31 | 6.5-8.0 | 13 | 8.44 | 6 |
| Acquisition of highest rank$ | 8.5-9.5 | - | N/A | - | 9.5-10.5 | - |
Source: Charpentier et al. 2008, known ages
Source: Dunbar & Dunbar, 1975 (p.59), estimated ages
Source: Current dataset, estimated ages
Source: Current dataset, known ages
Based on cross-sectional data (see Figure 1 for sample sizes for each age category) N/A Not applicable
Data analysis
As expected, none of the hormone values for the three taxa were normally distributed. Therefore, we log transformed values prior to all analyses to facilitate the use of parametric statistics. Additionally, to avoid bias from uneven fecal sample distribution across individuals (some males contributed more samples than others), we calculated a mean hormone value for each male for each age category and used this value in our analyses. All analyses were conducted separately for each taxon. Statistical analyses were performed using SPSS (16.0), and the statistical threshold for all analyses was set at p<0.05.
First, for the physiological validation, we compared the T levels of juveniles to adults using a Student's t-test. Second, because T extraction efficiencies were different across the different methods for the three taxa, we standardized the three datasets using Z-scores to facilitate hormone comparisons across taxa. Z-scores represent the number of standard deviations that T values for each category (in this case, age groups) differ from the mean T value for that taxon (with negative Z-scores indicating values lower than the mean and positive Z-scores indicated values higher than the mean). Z-scores were calculated for all three taxa across the multi-year age categories (see supplementary material) as well as the single-year categories for yellows and chacmas. Third, we used one-way ANOVA on the log-transformed T values (not Z-scores) to compare hormone values across multi-year age categories. Because sample sizes were low for chacma juveniles, we increased power by combining juveniles and subadults age 4.5-6.5 years into one category (chacmas only).
Results
Physiological validation
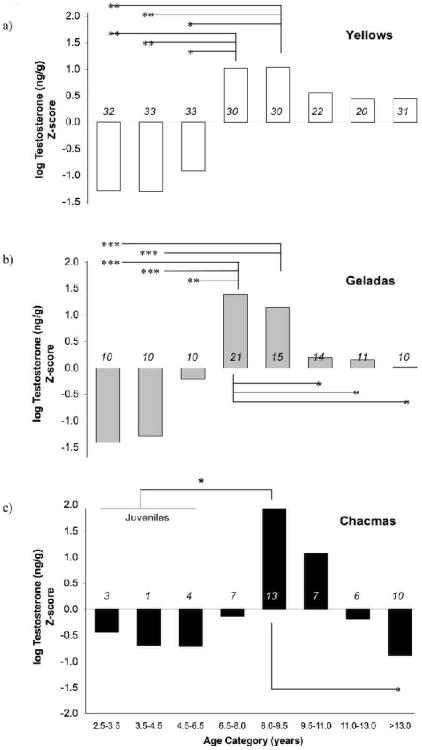
Juvenile males (2.5-4.5 years old) had significantly lower T metabolites than adult males (>8.0 years old) for all three taxa (t-test: yellows, t(111)=−4.20, p<0.001; chacmas, t(39)=−2.17, p<0.05; geladas, t(68)=−5.90, p<0.001). Additionally, T metabolites in all three taxa exhibited an inverse U-shaped pattern across age categories (Fig. 1a-c). In general, juveniles and subadults had the lowest T metabolites (negative Z-scores), adult males had the highest levels of T metabolites (positive Z-scores), and the oldest of the adult males had intermediate levels (Z-scores near zero, with the exception of yellows, see below).
Figure 1.
Testosterone Z-scores for the different age categories of (a) yellow males, (b) gelada males, and (c) chacma males. Although the figure depicts testosterone Z-scores, all statistical analyses were conducted on the log transformed testosterone values rather than on the Z-scores. Due to the small sample size of juveniles for chacma age categories, we pooled juveniles and subadults age 4.5-6.5 years into a single age category for statistical testing. Tukey's post-hoc tests: *p<0.05, **p<0.01, *** p<0.001. The number of males in each age category is indicated above the y-axis.
Three-taxa testosterone comparison
Consistent with the physiological validation, T trajectories were broadly similar across age for all three taxa. Nonetheless, two notable differences were observed. First, yellow and gelada males attained adult T levels at an earlier age than chacma males did (Fig. 1a-c). Testosterone Z-scores for both yellow and gelada males changed from negative to positive between 6.5 and 8.0 years of age, whereas those for chacma males did not do so until 8.0-9.5 years. The ANOVA analysis of log T across age categories further supports this observation (ANOVA: yellows F(7,230)=4.85, p<0.001; geladas F(7,100)=11.13, p<0.001; chacmas F(6,50)=2.98, p<0.05; for post-hoc tests between successive age categories see Fig. 1a-c). None of the juvenile chacma age categories by themselves was significantly different from the 8.0-9.5 y age category; however, this was likely due to insufficient power with this dataset (i.e. sample sizes were low for chacma males < 6.5 years). When we pooled chacma juvenile males and males from the 4.5-6.5 y category, we detected a significant difference from the 8.0-9.5 y age category for chacma baboons.
Second, the duration of elevated T profiles was shorter for gelada and chacma males than for yellow males. Testosterone Z-scores for gelada males dropped quickly after our estimate of 9.5 years (to nearly zero) and scores for chacma males were negative after 11.0 years (Fig. 1b-c). In contrast, Z-scores for yellow males, while dropping slightly after 9.5 years, remain positive even in males 13 years and older (Fig. 1a). Once again, post-hoc tests from the ANOVA support these observations (for post-hoc tests between successive age categories see Fig. 1a-c). Gelada males exhibited significantly lower T levels by 9.5 years, and although chacma males did not exhibit significantly lower T levels until 13.0 years, Z-scores were negative by 11.0 years. By contrast, yellow males maintained high T metabolite levels and did not exhibit a significant drop between any pair of successive adult age categories.
Peak T levels for all three species coincided most closely with age at first sexual consortship (although resolution in geladas was coarse due to our estimated age categories). For both yellow and chacma males, acquisition of adult rank came after the onset of adult T levels and before the onset of peak T levels. Age of highest average rank followed peak T levels by one year.
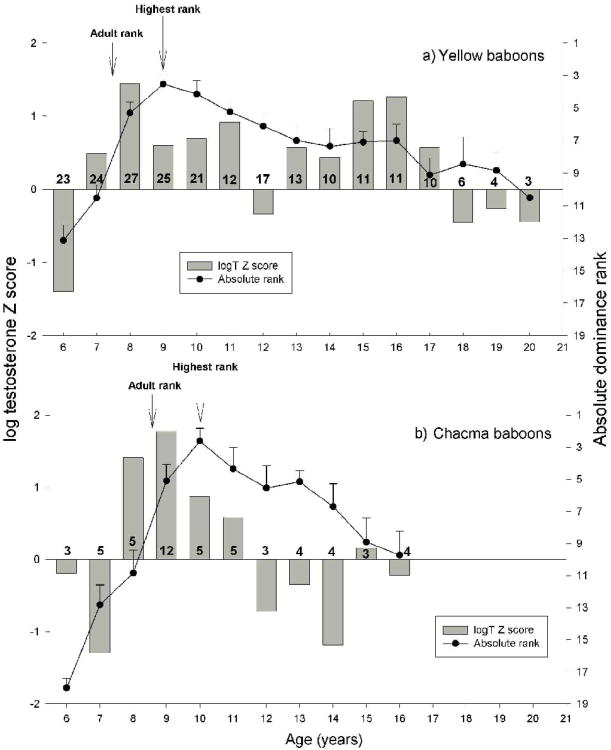
Yearly testosterone comparison: yellows and chacmas
Yellow and chacma males followed a similar pattern of maturation, except that chacma males were approximately one year behind yellow males for both hormonal and maturational markers (Fig 2a-b). Males of both taxa (1) exhibited a significant rise in T (Z-scores changing from negative to positive), (2) followed one year later by lifetime maximum T, which coincided with attainment of adult rank, (3) followed approximately one year later by a decrease in T levels (by at least -0.5 SD), which coincided with males' highest dominance rank. The delayed rise in T for chacma males coincided with delays in all maturational milestones (dispersal, adult rank, and first sexual consortship) as compared to yellow males (or, where relevant, gelada males; Table 2). Chacma males also exhibited a drop in T levels after 11 years (as indicated by negative Z-scores), while yellow males continued to exhibit higher than average T levels until 17 years of age (as indicated by positive Z-scores).
Figure 2.
Testosterone Z-scores (grey bars) and ordinal dominance ranks (±SEM, black dots) across one-year age categories for (a) yellow and (b) chacma males. The number of males in each age category is indicated above the y-axis. Male maturational milestones (see text) are indicated by arrows.
Discussion
Despite broad similarity in testosterone profiles across age groups for males of all three taxa, we observed variability across taxa in the timing of two major testosterone transitions. One of these major transitions was the attainment of adult levels of testosterone. While yellow and gelada males exhibited maximum testosterone between 6.5-8.0 years of age, chacma males did not reach maximum testosterone until 8.0-9.5 years of age. Yearly testosterone profiles indicated that the chacma delay in testosterone production was one year later than that for yellows. The other major transition was the drop in testosterone that males exhibited after an approximately 3-year period of maximal production. This drop was significant for both geladas and chacmas. However, although the testosterone of yellow males decreased slightly, no significant drop between successive years was detected, and testosterone levels did not fall to a consistently lower level until males reached 18 years of age. Consequently, for geladas and chacmas, the period of maximal testosterone production was discrete (i.e., higher than all other age categories by at least one standard deviation) while for yellow males, testosterone production gradually tapered off as males senesced. To understand these differences, we examined testosterone profiles for each taxon in relation to several maturational milestones.
We recall at this point that our main objective in this study was to describe taxon-level patterns in testosterone profiles across the male lifespan. We do not yet have available longitudinal, individual-based data across ages to partition variability into within and between taxa sources of variance, nor can we yet evaluate hormonal responses to specific social situations for the males involved. Rather, as a first step towards comparative endocrinology for wild populations, we take a broad look at overall hormone patterns and how male milestones map onto these profiles. Specific investigations into the relationship between testosterone changes and male developmental markers for individual males are important topics for the future. As such, the present analysis offers a framework for formulating and testing specific hypotheses for such subsequent studies.
Testosterone and maturational milestones
First, the three taxa exhibited considerable variability in the relationship between testosterone and natal dispersal. At one extreme, geladas dispersed well before the attainment of adult testosterone levels. Gelada “dispersal” from natal one-male units, however, may be qualitatively different from dispersal from natal groups in baboons because the first of these dispersal events in geladas occurs during the subadult (and even juvenile) stages (Dunbar and Dunbar, 1975). Juvenile and subadult gelada males repeatedly come and go from all-male bachelor groups, returning to their natal one-male unit each time, making it difficult to establish a final natal dispersal event. At the other extreme, chacmas dispersed about a year after attainment of adult testosterone levels (yellows dispersed at the same time as attainment of adult testosterone levels). Furthermore, natal dispersal for chacma males is delayed not only with respect to testosterone, but also relative to all male milestones for the other two taxa. A model of the ecological effects on dispersal (Alberts and Altmann, 1995a) considers the trade-offs that dispersing males of all ages face between opportunities for mating (which increase with population density) and possibility of mortality (which increases with predation rate). However, the model does not consider the temporal aspect of natal dispersal within the life-history trajectory of a taxon. Indeed, for both the yellow and chacma populations studied here, many males remain to breed for some time in their natal group (Alberts and Altmann, 1995a; Bulger and Hamilton, 1988), possibly because (as per the model) Amboseli yellow baboons live at relatively low densities (Altmann and Alberts, 2003), and Moremi chacma baboons experience relatively high predation (Cheney et al., 2004). Perhaps as a consequence of multiple ecological factors impinging on dispersal timing, we found no clear relationship between the attainment of adult levels of testosterone and dispersal for these taxa.
Second, the age at first sexual consortship was approximately the same time as that of peak testosterone production. However, under the predictions of the challenge hypothesis, sexual activity alone should not stimulate an exponential increase in testosterone production (Wingfield et al., 1990), but rather sexual activity in concert with male-male aggression – which, for baboons, occurs in the context of rank attainment within a dominance hierarchy. Because the age of first sexual consortship and the acquisition of adult rank were temporally similar for yellows and chacmas (<5 months apart for yellows and <3 months apart for chacmas), the present analysis is not fine-tuned enough to sufficiently relate peak testosterone levels to either of these milestones.
Third, yellow and chacma males attained their highest dominance rank one year after peak testosterone production. This supports an earlier finding that baseline testosterone levels predict future dominance ranks, but rank changes in themselves do not affect testosterone levels (Beehner et al., 2006a). Our results support the challenge hypothesis in showing a close link between testosterone and a time period when we expect male-male contests, but the causality of this relationship (if any) is opposite the prediction. The challenge hypothesis was proposed as a feedback loop between the external social environment and the internal physiological one, with a social “challenge” initiating the cycle. However, for both yellow and chacma males, testosterone declines before males fall in rank – sometimes even up to 6 months beforehand (Beehner et al., 2006a). Unless there is an anticipatory decline in testosterone, our data indicate that testosterone production is not necessarily “socially-modulated” (Wingfield et al., 1990) – or, at least not at the broad scale that we use in this study. Individual contests may indeed affect daily fluctuations in testosterone levels (i.e., winner-loser effects; Mazur and Booth, 1998; Mazur et al., 1992). However, we suggest that the more stable baseline testosterone levels that characterize life-history stages do not result from rank changes but, in fact, precede them.
Testosterone differences across taxa
Why do chacma males delay testosterone production, rank acquisition, and dispersal by at least a full year relative to yellow males? One explanation may relate to the high density of baboons in the Moremi chacma population (24 baboons/km2; Cheney, 1987; Cheney et al., 2004; Hamilton and Bulger, 1992; Hamilton et al., 1976). Males attempting to disperse to neighboring groups may face high resistance from the males already established in these groups. Males might overcome this resistance by achieving full adult body size prior to emigration, since increased body size upon immigration to a new group could facilitate a more rapid ascent in the dominance hierarchy. Additionally, if males rise in dominance within their natal group, they may gain more “practice” at rank contests, whether this involves actual fighting or displaying (Kitchen et al., 2003). High predation may also have delayed Moremi chacma males' life-history variables, since dispersing males are certain to experience elevated mortality during transfer. Thus, pressures related to ecological factors in this area may have selected for a reproductive strategy that maximizes “maturation” in one's natal group prior to dispersal. Chacma males dispersed and attained their highest dominance rank in the same year. This “strategy” is in sharp contrast to yellow males, and possibly even anubis (P. anubis or P. h. anubis) males; both yellow and anubis males dispersed around 8 years of age (Charpentier et al., 2008; Packer, 1979; Packer et al., 1995), yet did not attain highest rank until nearly 2 years later for yellows (Table 2) and 3 years later for anubis (Packer et al., 2000).
At the other end of the life-history trajectory, why do yellow males continue to produce testosterone well beyond the period when they are high ranking? Low-ranking male yellow baboons have comparatively greater access to sexually receptive females than male geladas and chacmas (Alberts et al., 2003; Alberts et al., 2006). Two components of this weak, dominance-based priority are that younger, non-alpha males are able to gain some fertile matings and that older, non-alpha males are also able to mate more than would be expected under a strict queuing scenario. This prolonged maintenance of higher testosterone levels into older ages for yellow males represents a departure, not only from chacma and gelada males, but also from other known mammalian male profiles (Castracane et al., 1986; Crawford et al., 1997; Muehlenbein et al., 2001; Seraphin et al., 2008).
Testosterone and parental care
In many ways, male development for chacma baboons is more similar to that of geladas than it is to that of yellows. Compared to the testosterone profiles of yellow males, those of chacma and gelada males indicate a comparatively discrete increase to adult levels and decrease about 3 years later. This discrete period of elevated testosterone production is what we might expect for a species such as the gelada, with unimale reproductive units. That chacmas also fit this profile suggests that chacma male physiology may be tracking reproductive activity and/or parenting behavior better than the overall mating system. Although yellow and chacma baboons share a multimale, polygynandrous mating system, the prime “mating” versus “parenting” stages for chacmas and geladas may be more compartmentalized than those of yellows. Older, low-ranking chacma males continue to take advantage of mating opportunities (Crockford et al., 2007), and they achieve some reproductive success (Cheney and Seyfarth, unpublished data). However, they also invest considerable time and energy in paternal care (Moscovice et al., 2009; Palombit et al., 2000; Palombit et al., 1997). In many mammalian species, high levels of testosterone have been shown to be incompatible with paternal behavior (Gray et al., 2006; Muller et al., 2009; Nunes et al., 2000; Nunes et al., 2001), and thus the chacma male sharp decline may be related in part to parenting behavior.
Taxon or population differences?
One factor that has not been addressed in this study is whether the male hormone and life-history patterns we report here are taxon-specific or population-specific. This raises the question of whether we are documenting intrinsic or extrinsic sources of variation. The overall hormonal profile may exhibit a fixed pattern within a taxon, and tell us something about a taxon's overall adaptive strategy. Alternatively, different populations may demonstrate facultative responses to environmental changes, and hormones (such as testosterone) can facilitate this behavioral flexibility (Oliveira, 2004). Offering some support to the latter hypothesis, captive yellow or yellow-olive hybrid males exhibited adult levels of testosterone much earlier (Altmann et al.,; Castracane et al., 1986; Muehlenbein et al., 2001) than the wild yellow males in the current study. At present, no comparable hormone data are available from other wild populations of baboons or geladas to test these alternatives. Certainly, a replication of this study in other populations with different population dynamics and/or ecology would help resolve this issue (for details on the ecology of these three sites see: Amboseli (Alberts et al., 2005; Behrensmeyer, 2006), Moremi: (Ellery et al., 1993), Simien: (Nievergelt et al., 1998)). For example, if the delay in testosterone production and other life-history stages observed in chacma baboons is due to the high population density in Moremi, then we predict a very different pattern for chacma baboons at lower densities (e.g., Drakensberg chacma population, South Africa).
A third possibility (which is obscured using the broad-scale approach we use here), is that T variation represents enduring individual variation and thus reflects alternative life-history strategies. For example, a high parental investment strategy may exhibit a long-term commitment to one mate and parental care, accompanied by low mating effort, while a low parental investment strategy may exhibit a low commitment to one mate and no paternal care, accompanied by high mating effort (Gross, 1985, 1996; Thompson and Moore, 1992; van Rhijn, 1974). With respect to the third possibility, future studies that statistically examine the variability in developmental markers (relative to hormonal profiles) between taxa as well as between individuals within each taxa would be extremely useful for understanding the trade-offs between testosterone and life-history traits in wild populations.
Supplementary Material
Acknowledgments
The yellow baboon research was supported by NSF IBN-0322613, NSF BSE-0323553, RO3 MH65294, and the Chicago Zoological Society. We thank the Office of the President, Republic of Kenya, the Kenya Wildlife Services, its Amboseli staff and Wardens, the Institute of Primate Research, the National Museums of Kenya, and the members of the Amboseli-Longido pastoralist communities. In the field, thanks go to the Amboseli team who contributed to sample and data collection (R.S. Mututua, S. Sayialel, and J.K. Warutere). Thanks to T. Fenn for database assistance, and to C. Markham, P. Onyango, for their input on data analysis or for providing comments on earlier drafts. This research was approved by the Animal Care and Use Committee at Princeton University (IACUC protocol #1689, 9 November 2007) and adhered to all the laws and guidelines of Kenya (Kenya Research Permit MOEST 13/001/C351 Vol. II).
The chacma baboon research was supported by NIH MH62249, NRSA, the Leakey Foundation, the University of Pennsylvania and the University of Michigan. We are grateful to the Office of the President of the Republic of Botswana and the Botswana Department of Wildlife and National Parks for permission to conduct this research. In the field, thanks go to T. Bergman, M. Mokupi, and A. Mokupi for sample and data collection; and in the laboratory, thanks go to S. Mohanty for assay work, troubleshooting, and analysis. This research was reviewed and approved by the Animal Care and Use Committee at the University of Pennsylvania (IACUC protocol #09001) and the University Committee on Use and Care of Animals at the University of Michigan (UCUCA protocol #09554), and adhered to all the laws and guidelines of Botswana.
The gelada research was supported by the National Geographic Society (NGS 8100-06), the Leakey Foundation, NSF BCS-0715179, and the University of Michigan. We are grateful to the Ethiopian Wildlife Conservation Department, the Amhara National Regional State Parks Development and Protection Authority, and the Wardens and staff of the Simien Mountains National Park for permission to conduct this research. In the field, thanks go to T. Bergman and H. Gelaye for sample and data collection, and in the laboratory, thanks go to S. Mohanty for troubleshooting and sample analysis. This research was approved by the University Committee on Use and Care of Animals at the University of Michigan (UCUCA protocol #09554), and adhered to all the laws and guidelines of Ethiopia.
Finally, we would like to thank T. Bergman and two anonymous reviewers for their helpful comments on earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts S, Watts H, Altmann J. Queuing and queue-jumping: Long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Animal Behaviour. 2003;65:821–840. [Google Scholar]
- Alberts SC, Altmann J. Balancing costs and opportunities: Dispersal in male baboons. American Naturalist. 1995a;145:279–306. [Google Scholar]
- Alberts SC, Altmann J. Preparation and activation: Determinants of age at reproductive maturity in male baboons. Behavioral Ecology and Sociobiology. 1995b;36:397–406. [Google Scholar]
- Alberts SC, Altmann J, Wilson ML. Mate guarding constrains foraging activity of male baboons. Animal Behaviour. 1996;51:1269–1277. [Google Scholar]
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Animal Behaviour. 2006;72:1177–1196. [Google Scholar]
- Alberts SC, Hollister-Smith J, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long term change in a savannah environment. In: Brockman DK, van Schaik CP, editors. Primate Seasonality: Implications for Human Evolution. Cambridge University Press; Cambridge: 2005. pp. 157–196. [Google Scholar]
- Altmann J, Alberts SC. Variability in reproductive success viewed from a life-history perspective in baboons. American Journal of Human Biology. 2003;15:401–409. doi: 10.1002/ajhb.10157. [DOI] [PubMed] [Google Scholar]
- Altmann J, Hausfater G, Altmann SA. Determinants of reproductive success in savannah baboons, Papio cynocephalus. In: Clutton-Brock TH, editor. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. University of Chicago Press; Chicago: 1988. pp. 403–418. [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Annals of the New York Academy of Sciences. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Barrett L, Henzi SP. Baboons. Current Biology. 2008;18 doi: 10.1016/j.cub.2008.02.074. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ. Infant mortality following male takeovers in wild geladas. American Journal of Primatology. 2008;70:1–8. doi: 10.1002/ajp.20614. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behavioral Ecology and Sociobiology. 2006a;59:469–479. [Google Scholar]
- Beehner JC, Nguyen N, Wango EO, Alberts SC, Altmann J. The endocrinology of pregnancy and fetal loss in wild baboons. Hormones and Behavior. 2006b;49:688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Whitten PL. Modifications of a field method for fecal steroid analysis in baboons. Physiology & Behavior. 2004;82:269–277. doi: 10.1016/j.physbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Behrensmeyer AK. Climate change and human evolution. Science. 2006;311:476–478. doi: 10.1126/science.1116051. [DOI] [PubMed] [Google Scholar]
- Bribiescas RG. Reproductive ecology of the human male: an evolutionary and life history perspective. In: Ellison PT, editor. Reproductive ecology and human evolution. Aldine de Gruyter; New York: 2001. pp. 107–133. [Google Scholar]
- Bribiescas RG. Age-related differences in serum gonadotropin (FSH and LH), salivary testosterone, and 17-beta estradiol levels among Ache Amerindian males of Paraguay. American Journal of Physical Anthropology. 2005;127:114–121. doi: 10.1002/ajpa.20079. [DOI] [PubMed] [Google Scholar]
- Bribiescas RG. On the evolution, life history, and proximate mechanisms of human male reproductive senescence. Evolutionary Anthropology. 2006;15:132–141. [Google Scholar]
- Brockman DK, Whitten PL, Richard AF, Benander B. Birth season testosterone levels in male Verreaux's sifaka, Propithecus verreauxi: insights into socio-demographic factors mediating seasonal testicular function. Behavioral Ecology and Sociobiology. 2001;49:117–127. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Bulger JB. Dominance rank and access to estrous females in male savanna baboons. Behaviour. 1993;127:67–103. [Google Scholar]
- Bulger JB, Hamilton WJ. Rank and density correlates of inclusive fitness measures in a natural chacma baboon (Papio ursinus) population. International Journal of Primatology. 1987;8:635–650. [Google Scholar]
- Bulger JB, Hamilton WJ. Inbreeding and reproductive success in a natural chama baboon, Papio cynocephalus ursinus. Animal Behaviour. 1988;36:574–578. [Google Scholar]
- Busse CD, Hamilton WJ. Infant carrying by male chacma baboons. Science. 1981;212:1281–1283. doi: 10.1126/science.212.4500.1281. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Copeland KC, Reyes P, Kuehl TJ. Pubertal endocrinology of yellow baboon (Papio cynocephalus): plasma testosterone, testis size, body weight, and crown-rump length in males. American Journal of Primatology. 1986;11:263–270. doi: 10.1002/ajp.1350110308. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Pereira ME. Mating season aggression and fecal testosterone levels in male ring-tailed lemurs (Lemur catta) Hormones and Behavior. 2000;37:246–255. doi: 10.1006/hbeh.2000.1585. [DOI] [PubMed] [Google Scholar]
- Charpentier MJE, Tung J, Altmann J, Alberts SC. Age at maturity in wild baboons: genetic, environmental and demographic influences. Molecular Ecology. 2008;17:2026–2040. doi: 10.1111/j.1365-294X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- Cheney DL. Interactions and relationships between groups. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 267–281. [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner JC, Bergman TJ, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. International Journal of Primatology. 2004;25:401–428. [Google Scholar]
- Collins DA, Busse CD, Goodall J. Infanticide in two populations of savanna baboons. In: Hausfater G, Hrdy SB, editors. Infanticide: Comparative and Evolutionary Perspectives. Aldine; New York: 1984. pp. 193–215. [Google Scholar]
- Crawford BA, Harewood WJ, Handelsman DJ. Growth and hormone characteristics of pubertal development in the hamadryas baboon. Journal of Medical Primatology. 1997;26:153–163. doi: 10.1111/j.1600-0684.1997.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Seyfarth RM, Cheney DL. Baboons eavesdrop to deduce mating opportunities. Animal Behaviour. 2007;73:885–890. [Google Scholar]
- Dunbar RIM. Reproductive Decisions: An Economic Analysis of Gelada Baboon Social Strategies. Princeton University Press; Princeton: 1984. [Google Scholar]
- Dunbar RIM, Dunbar P. Social dynamics of gelada baboons. In: Kuhn H, Luckett WP, Noback CR, Schultz AH, Starck D, Szalay F, editors. Contributions to Primatology. S. Karger; Basel: 1975. [PubMed] [Google Scholar]
- Ellery WN, Ellery K, McCarthy TS. Plant distribution in islands of the Okavango Delta, Botswana: determinants and feedback interactions. African Journal of Ecology. 1993;31:118–134. [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Human Reproduction. 2002;17:3251–3253. doi: 10.1093/humrep/17.12.3251. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Altmann J, Alberts SC, Khan MZ, Couret J, Yu JC, Endres CS, Lynch JW, Ogola P, Wango E. Coming of age: Steroid hormones of wild immature baboons (Papio cynocephalus) American Journal of Primatology. 2005;67:83–100. doi: 10.1002/ajp.20171. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: Sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Hormones and Behavior. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness - revisiting the Challenge Hypothesis. Hormones and Behavior. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Gray PB, Yang CJ, Pope HG. Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proceedings of the Royal Society of London Series B: Biological Sciences. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross MR. Disruptive selection for alternative life histories in salmon. Nature. 1985;313:47–48. [Google Scholar]
- Gross MR. Alternative reproductive strategies and tactics: diversity within sexes. Trends in Ecology and Evolution. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ, Bulger JB. Facultative expression of behavioral differences between one-male and multimale savanna baboon groups. American Journal of Primatology. 1992;28:61–71. doi: 10.1002/ajp.1350280106. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ, Buskirk RE, Buskirk WH. Defense of space and resources by chacma (Papio ursinus) baboon troops in an African desert and swamp. Ecology. 1976;57:1264–1272. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hau M, Gill SA, Goymann W. Tropical field endocrinology: ecology and evolution of testosterone concentrations in male birds. General and Comparative Endocrinology. 2008;157:241–248. doi: 10.1016/j.ygcen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Hausfater G. Dominance and reproduction in baboons (Papio cynocephalus): A quantitative analysis. Vol. 7. S. Karger; Basel: 1975. [PubMed] [Google Scholar]
- Jolly CJ. Species, subspecies, and baboon systematics. In: Kimbel WH, Martin LB, editors. Species, Species Concepts, and Primate Evolution. Plenum; New York: 1993. pp. 67–107. [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. General and Comparative Endocrinology. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Kitchen DM, Seyfarth RM, Fischer J, Cheney DL. Loud calls as an indicator of dominance in male baboons (Papio cynocephalus ursinus) Behavioral Ecology and Sociobiology. 2003;53:374–384. [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. General and Comparative Endocrinology. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Malo AF, Roldan ERS, Garde JJ, Soler AJ, Vincente J, Gortazar C, Gomendio M. What does testosterone do for red deer males? Proceedings of the Royal Society of London Series B: Biological Sciences. 2009;276:971–980. doi: 10.1098/rspb.2008.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DE, Swenson RB, Collins DC. Correlation of serum testosterone levels with age in male chimpanzees. Steroids. 1977;29:471–481. doi: 10.1016/0039-128x(77)90067-8. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21:353–397. [PubMed] [Google Scholar]
- Mazur AA, Booth A, Dabbs JM., Jr Testosterone and chess competition. Social Psychology Quarterly. 1992;55:70–77. [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. The American Naturalist. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- Mori U. Inter-unit relationships. In: Kawai M, editor. Ecological and sociological studies of gelada baboons. Karger; Basel: 1979. pp. 93–124. [PubMed] [Google Scholar]
- Moscovice LR, Heesen M, Di Fiore A, Seyfarth RM, Cheney DL. Paternity alone does not predict long-term investment in juveniles by male baboons. Behavioral Ecology and Sociobiology. 2009 doi: 10.1007/s00265-009-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AM, Clutton-Brock TH, Monfort SL. Longitudinal gonadal steroid excretion in free-living male and female meerkats (Suricata suricatta) General and Comparative Endocrinology. 2001;122:158–171. doi: 10.1006/gcen.2001.7622. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Campbell BC, Phillippi KM, Murchison MA, Richards RJ, Svec F, Myers L. Reproductive maturation in a sample of captive male baboons. Journal of Medical Primatology. 2001;30:273–282. doi: 10.1034/j.1600-0684.2001.d01-60.x. [DOI] [PubMed] [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proceedings of the Royal Society of London Series B: Biological Sciences. 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘ challenge hypothesis ’. Animal Behaviour. 2004;67:113–123. [Google Scholar]
- Nievergelt B, Good T, Güttinger R, editors. A survey on the flora and fauna of the Simien Mountains National Park Ethiopia. Ethiopian Wildlife and Natural History Society, Walia (special issue); 1998. [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Animal Behaviour. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Hormones and Behavior. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Oliveira RF. Social modulation of androgens in vertebrates: mechanisms and function. Advances in the Study of Behavior. 2004;34:165–239. [Google Scholar]
- Ostner J, Kappeler PM, Heistermann M. Seasonal variation and social correlates of androgen excretion in male redfronted lemurs (Eulemur fulvus rufus) Behavioral Ecology and Sociobiology. 2002;52:485–495. doi: 10.1007/s00265-007-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Kappeler PM, Heistermann M. Androgen and glucocorticoid levels reflect seasonally occuring social challenges in male redfronted lemurs (Eulemur fulvus rufus) Behavioral Ecology and Sociobiology. 2008;62:627–638. doi: 10.1007/s00265-007-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer C. Inter-troop transfer and inbreeding avoidance in Papio anubis. Animal Behaviour. 1979;27:1–36. doi: 10.1016/0003-3472(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Packer C, Collins DA, Eberly LE. Problems with primate sex ratios. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2000;355:1627–1635. doi: 10.1098/rstb.2000.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer C, Collins DA, Sindimwo A, Goodall J. Reproductive constraints on aggressive competition in female baboons. Nature. 1995;373:60–63. doi: 10.1038/373060a0. [DOI] [PubMed] [Google Scholar]
- Palombit RA, Cheney DL, Fischer J, Johnson S, Rendall D, Seyfarth RM, Silk JB. Male infanticide and defense of infants in wild chacma baboons. In: van Schaik CP, Janson CH, editors. Infanticide by Males and Its Implications. Cambridge University Press; Cambridge: 2000. pp. 123–152. [Google Scholar]
- Palombit RA, Seyfarth RM, Cheney DL. The adaptive value of friendships to female baboons: experimental and observational evidence. Animal Behaviour. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- Pereira ME. Agonistic interactions of juvenile savanna baboons. I. Fundamental features. Ethology. 1988;79:195–217. [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends in Ecology and Evolution. 2002;17:462–468. [Google Scholar]
- Seraphin SB, Whitten PL, Reynolds V. The influence of age on fecal steroid hormone levels in male Budongo forest chimpanzees (Pan troglodytes schweinfurthii) American Journal of Primatology. 2008;70:661–669. doi: 10.1002/ajp.20541. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Dixson AF. Developmental variables and dominance rank in adolescent male mandrills (Mandrillus sphinx) American Journal of Primatology. 2002;56:9–25. doi: 10.1002/ajp.1060. [DOI] [PubMed] [Google Scholar]
- Shopland JM. Food quality, spatial deployment, and the intensity of feeding interference in yellow baboons (Papio cynocephalus) Behavioral Ecology and Sociobiology. 1987;21:149–156. [Google Scholar]
- Tarara EB. Infanticide in a chacma baboon troop. Primates. 1987;28:267–270. [Google Scholar]
- Thompson CW, Moore MC. Behavioral and hormonal correlates of alternative reproductive strategies in a polygynous lizard: tests of the relative plasticity and challenge hypotheses. Hormones and Behavior. 1992;26:568–585. doi: 10.1016/0018-506x(92)90023-o. [DOI] [PubMed] [Google Scholar]
- van Rhijn JG. Behavioural dimorphism in male ruffs, Philomachus pugnax (L.) Behaviour. 1974;47:153–229. [Google Scholar]
- Wack CL, Fox SF, Hellgren EC, Lovern MB. Effects of sex, age, and season on plasma steroids in free-ranging Texas horned lizards (Phrynosoma cornutum) General and Comparative Endocrinology. 2008;155:589–596. doi: 10.1016/j.ygcen.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Lycett JE, Barrett L, Hill RA, Henzi SP. Male consortship behaviour in chacma baboons: The role of demographic factors and female conceptive probablilities. Behaviour. 2003;140:405–427. [Google Scholar]
- Weingrill T, Lycett JE, Henzi SP. Consortship and mating success in chacma baboons (Papio cynocephalus ursinus) Ethology. 2000;106:1033–1044. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 1990;136:829–846. [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annual Review of Ecology and Systematics. 2001;32:95–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.