Abstract
The increasing percentage of obese individuals in the population and its independent association of increased risk for the development of cancer have heightened the necessity to understand the molecular mechanisms that underlie this connection. The deregulation of adipokines in the setting of obesity and their impact on cancer progression and metastasis is one such area of research. Adipokines are bioactive proteins that mediate metabolism, inflammation, angiogenesis, and proliferation. Altered levels of adipokines or their cognate receptors in cancers can ultimately lead to an imbalance in downstream molecular pathways. Discovery of adipokine receptors in various cancers has highlighted the potential for novel therapeutic targets. Leptin and adiponectin represent two adipokines that elicit generally opposing molecular effects. Epidemiological studies have highlighted associations between increased serum leptin levels and increased tumor growth, while adiponectin exhibits an inverse correlation with cancer development. This review addresses the current level of understanding of molecular pathways activated by adiponectin and leptin to identify areas of intervention and facilitate advancement in the field.
Background
A strong correlation between obesity and cancer, coupled with the rising obesity epidemic, has led to a prediction of an increase in forthcoming new cancer cases. Obesity commonly leads to deregulation of adipokines, bioactive proteins primarily secreted from adipocytes, which elicit their biological effects upon binding to cognate receptors. The primary role of adipokines is to help maintain metabolic homeostasis, yet expanded roles for adipokines have demonstrated their ability to modulate inflammation, angiogenesis, proliferation and apoptosis. With these processes in mind, a role for adipokines in cancer progression and metastasis has become apparent. The majority of cancer related studies have focused in vitro on the ability of adipokines to affect the typical hallmarks of cancer including proliferation, evasion of apoptosis, tumor cell migration and invasion, angiogenesis and vascular stimulation, and evasion of immune detection. More pertinent are preclinical studies that have validated the impact of adipokines on cancer progression in vivo, yet the signaling mechanisms through which these adipokines are mediating oncogenic phenotypes still require further elucidation. This review will address the molecular pathways of two prominent adipokines, leptin and adiponectin, and the potential to develop novel cancer therapeutics.
Adipokines: Leptin and Adiponectin
Leptin is a 16kD bioactive protein encoded by the Ob gene, secreted from adipocytes as well as other tissues, which acts as a regulator of energy to control satiety through stimulation in the central nervous system as well as to modulate glucose and insulin homeostasis through activation in peripheral tissues 1. Leptin typically circulates in the blood at a concentration of 5–10ng/mL in healthy patients, yet its level increases in obese and diabetic patients upwards of 50ng/mL 2. Leptin stimulates a specific set of receptors from the extended class I cytokine-receptor family, comprising six isoforms that dimerize with each other, but lack intrinsic kinase activity 3. Leptin receptor isoforms vary with respect to tissue and cell type as well as with respect to ligand stimulation. Auto-regulation of receptor levels as well as ligand-dependent activity may additionally lead to leptin resistance 4, 5.
Adiponectin is part of the complement 1q family of proteins that is primarily secreted by adipocytes as a monomeric protein, which can further oligomerize to form low molecular weight, high molecular weight and multimeric complexes 6. Additionally, adiponectin can be cleaved by leukocyte elastase to generate a globular oligomeric complex 7. Adiponectin is generally maintained between 7–15ug/mL in the plasma of healthy humans and exhibits a negative correlation with body mass index as well as percent body fat 8, 9. Adiponectin activates two main seven transmembrane receptors, adiponectin receptor 1 (adipoR1) and adiponectin receptor 2 (adipoR2) 10. AdipoR1 has a greater affinity for globular adiponectin while adipoR2 binds full length and multimeric adiponectin more avidly 10. Stimulation of either receptor leads to regulation of metabolic effects through the activation and phosphorylation of AMPK, ACC as well as p38 MAPK 10. Knockout of each receptor resulted in an opposition of effects on locomotor activity and metabolism where adipoR1 was shown be associated with increased adiposity and decreased glucose tolerance while adipoR2 is resistant to diet induced obesity 11, 12.
Antagonistic Signaling Between Leptin and Adiponectin
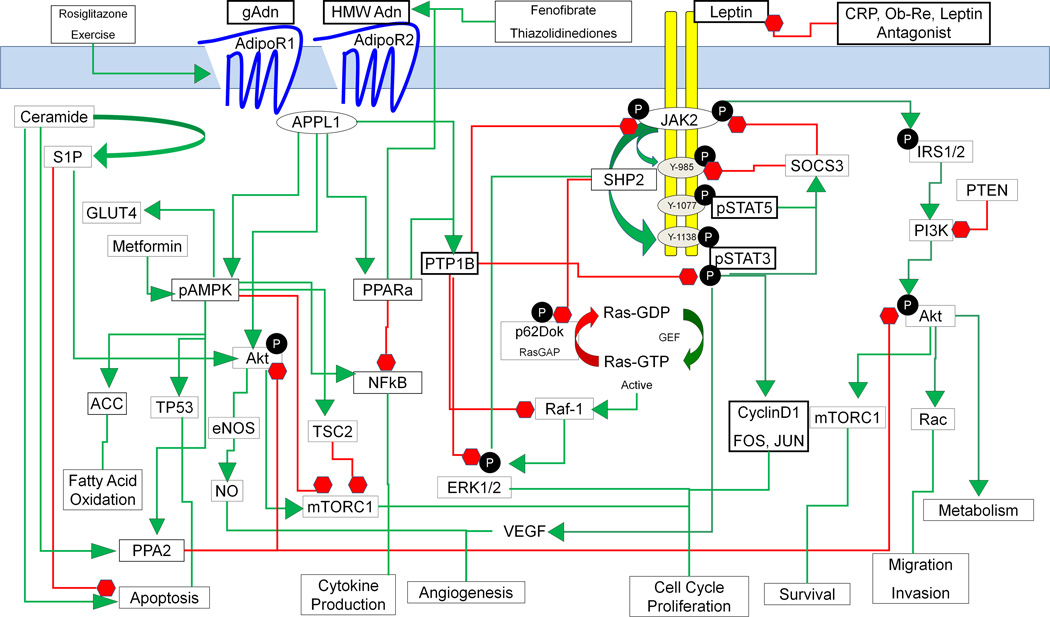
Leptin and adiponectin generally affect cellular behavior in an opposing manner. Highlights of these studies suggest that adiponectin administration in vivo has been shown to decrease growth and proliferation, increase apoptosis, decrease invasion, and decrease vessel density in murine cancer models 13–17. Leptin has been shown to increase proliferation, migration and invasion of cancer cells 18–25 as well as contribute to release of VEGF26. The ratio of leptin to adiponectin was recently described to be a potential key for outcome when assessing plasma levels 27. An important aspect of this consideration is that adiponectin can antagonize the actions of leptin. The molecular mechanisms through which adiponectin and leptin affect cancer cell behavior still require further elucidation. Figure 1 illustrates the dynamic signaling pathways for leptin and adiponectin, which we have combined to ascertain common mediators as potential key components for therapeutic intervention.
Figure 1.
Leptin and adiponectin activate signaling components that integrate PI3K/Akt, RAS/MAPK, and pAMPK/mTor pathways. Green arrows indicate activation of target protein, while red lines indicate inhibitory effects. Leptin stimulation of the long receptor isoform leads to JAK2 phosphorylation and subsequent phosphorylation of tyrosine residues 985 and 1138, which confer PI3K/Akt and STAT3 pathway activation. Leptin stimulation can be prevented with c-reactive peptide, soluble leptin receptor (Ob-Re) or leptin antagonists. Chronic stimulation leads to an increase in SOCS3 which negatively regulates leptin signaling by inhibiting JAK2 activities. Additionally, leptin receptor stimulation activates SHP2 leading to increased Ras/RAF/ERK signaling. Adiponectin receptor 1 (AdipoR1) and receptor 2 (AdipoR2) are preferentially stimulated by the globular (gAdn) and high molecular weight (HMW Adn) oligomers of adiponectin respectively, although both receptors respond with lower affinity to other adiponectin oligomers. Serum levels of adiponectin can be increased through thiazolidinediones or fenofibrates, while the receptor levels can be increased with rosiglitazone or exercise. Adiponectin receptors associate with adaptor protein APPL1 to activate AMPK and PPAR alpha. Adiponectin can antagonize leptin mediated proliferation through activation of phosphatase PTP1B, leading to inhibition of JAK2, dephosphorylation of STAT3, and dephosphorylation of ERK1/2; as well as phosphatase PP2A to decrease phosphor-Akt. Adiponectin also inhibits leptin action through increased AMPK inhibition on mTORC1 directly as well as indirectly through TSC2. Metformin additionally antagonizes leptin action through activation of AMPK. Adiponectin activation also leads to modulation of NFkB, TP53, eNOS, ACC, and ceramidase activity; yet direct antagonism of leptin through these mediators is unclear. Ultimate outcome for a particular pathway in cancer is highly dependent upon genetic integrity and deficiencies in key regulatory mediators will dictate which pathway will dominate.
Leptin binding to all four forms of the short leptin receptor (Ob-Ra, Ob-Rc, Ob-Rd and Ob-Rf), elicits activation of Janus Kinase (JAK)2 and subsequent phosphorylation of insulin receptor substrates (IRS), initiating activation of the PI3-K/Akt pathway 3. The long form of the receptor (Ob-Rb) contains an intracellular carboxy terminal extension that provides an additional three tyrosine residues (Tyr985, Tyr1077, and Tyr1138), necessary to confer binding and activation of signal transducer and activator of transcription (STAT)3 and STAT5 3, 28, 29. Additionally, a secreted isoform lacking the intracellular signaling domains (Ob-Re) 30, functions to sequester and block leptin induced STAT3 activation 31.
Leptin dependent activation of JAK2 additionally confers phosphorylation of both Tyr985 and Tyr1138 as well as activation of IRS1/2. Phosphorylation of Tyr985 is essential for phosphorylation of Tyr1138 which promotes Src mediated activation of STAT3 28. Additionally, phosphorylation of Tyr985 promotes recruitment of SHP2, a protein phosphatase, and SOCS3, an inhibitor of STAT3 32. Leptin mediated SHP2 binding leads to activation of ERK33, 34 as well as attenuation of p62Dok35, a RasGTPase, leading to activation of Ras and subsequent proliferation. SOCS3 and PPARgamma are upregulated via activation of STAT5 at Tyr1077 subsequent to leptin stimulation 32. Leptin mediated upregulation of SOCS3 is thought to be involved during chronic leptin stimulation, which then acts as a negative regulator to directly bind and block Ob-Rb signaling and well as JAK2 activity 36, 37. Additionally, adiponectin can increase PTP1B, protein tyrosine phosphatase 1B, which then dephosphorylates STAT3 as well as JAK2, further antagonizing leptin signaling 38.
Adiponectin binding can occur through either receptor 1 (AdipoR1) or receptor 2 (AdipoR2), which homodimerize or heterodimerize 6. While gAdN preferentially binds to adipoR1, HMW adiponectin preferentially stimulates adipoR2 10. Knockout studies suggest that adipoR1 is necessary for AMPK activity while adipoR2 is necessary for PPARalpha activity, yet both receptors have been shown to be able to increase phosphorylation of AMPK 11, 12. APPL1, a pleckstrin homology adaptor protein, binds to the intracellular portion of the adiponectin receptors and participates in AMPK activation leading to GLUT4 membrane translocation, p38 MAPK activation and phosphorylation of acetyl-CoA carboxylase, ACC 39. AMPK activation inhibits the mTOR complex via Raptor in the mTorc1 complex as well as activating TSC2, an inhibitor of mTOR 40. Phosphorylation of AMPK further activates TP5341 and pro-apoptotic pathways as well as the activation of PP2A, protein phosphatase 2A, which can negatively regulate Akt in response to adiponectin stimulation42 and therefore antagonize leptin induced Akt. Adiponectin stimulation of APPL1 alternately activates Akt to enhance mTOR in the absence of PTEN43, phosphatase and tensin homolog deleted on chromosome ten, which normally inhibits PI3K activation of Akt. Therefore, crosstalk between leptin and adiponectin as well as the activation of multiple pathways keep proliferative signaling in balance.
Leptin and Leptin Receptors in Cancer
Tumor associated leptin receptor levels are thought to contribute to tumor growth and progression. Increased detection of ObR in ovarian cancers was correlated with decreased survival 44. Leptin receptor expression is enhanced in 83% of human breast cancers, and 34% of patients with high leptin receptor level and high ligand level had detectable distant metastases 45. In the murine MMTV-TGF-alpha model, deficiency in the long form of the leptin receptor (Db/Db) resulted in failure of mammary tumor formation 46. Knockdown of the ObR through siRNA in MCF-7 breast cancer cells resulted in suppression of tumor volume in a mouse xenograft model 47. Knockdown of the long form of the leptin receptor can abolish integrin dependent migration of chondrosarcoma cells through involvement of IRS-1/PI3K-dependent activation of Akt 48. In addition, pancreatic tumors grown in leptin receptor mutant mice (LepDB) had larger tumors and more metastases when compared to wildtype mice 49. Additionally, mutational status may affect receptor function. Three single nucleotide polymorphisms in the leptin receptor gene (K109R, K656N, and Q223R) showed an association with increased basal-like breast cancer risk 50. These results suggest that tumor leptin receptor levels directly influence growth and progression.
Circulating levels of leptin have been investigated to determine the correlation with cancer and progressive disease. Elevated leptin levels in cancer patients compared to normal or preoperative levels have been reported in hepatocellular carcinoma and prostate cancer, while levels are relatively equivocal in breast cancer patients 51–55. Yet, in pancreatic cancer and colon cancer patients, leptin levels were generally found to be decreased 56–59. Complications such as pancreatic dysfunction, advanced progression of disease, weight loss and/or cachexia might be underlying factors for decreased leptin levels. Leptin produced by adjacent adipose might provide a local increased level of stimulation to tumors 60–62, suggesting the presence of tumor associated adipose represents an important microenvironmental influence. Although normally secreted from adipose, self-sufficiency for leptin has recently been shown to be secreted from glioblastoma and breast cancer cells 63, 64. Further, intra-tumoral mRNA leptin levels in patients with high leptin receptor levels correlated with decreased relapse-free survival 55.
Adiponectin and Adiponectin Receptors in Cancer
Epidemiologic studies show low levels of adiponectin have an inverse association with risk for the development of multiple cancers as well as advanced progression of disease 65. Two adiponectin single nucleotide polymorphisms have been shown to increase prostate, colon and breast cancer risk 66–68. Adiponectin deficiency through the use of knockout mice has shown accelerated hepatic tumor formation 69 and increased colon polyp formation 70, yet it delayed tumor growth in a mammary MMTV-PyV-mT model due to decreased vascularization and increased apoptosis in early stages of disease 71, 72. Tumor promoting effects are likely secondary to initiation, but no clear studies have implicated adiponectin as an initiator of cancer development.
The adiponectin receptors have been detected in gastric, colon, prostate, breast, pancreatic and many other cancers 16, 56, 73–75. Adiponectin receptors detection in gastric cancers was associated with longer overall survival 76. Two single nucleotide polymorphisms of adiponectin receptor 1 associate with prostate cancer risk and one with breast cancer risk 67, 68. Six genetic associations in the adipoR1 and adipoR2 genes have been detected in diabetic patients 77. Deletion of the adipoR1, but not adipoR2, resulted in a promotion of epithelial cell proliferation and increased number of aberrant crypt foci in a murine model 70. Future studies addressing the functional role of each adiponectin receptor in cancer initiation and progression will add a substantial contribution to our understanding and the importance of adiponectin signaling in these diseases.
Clinical-Translational Advances
Preclinical Advances
Currently, preclinical advances modulating adipokines have been limited for cancer therapeutics. Recombinant leptin treatment increased MDA-MB-231 breast tumor xenograft growth22 as well as melanoma 78. In an animal study, female mice in the MMTV-TGF-alpha breast cancer model fail to develop tumors when crossed with leptin deficient mice 46. Conversely; leptin antagonist treatment was shown to decrease the growth of triple negative breast tumors in mice79 as well as decrease 4T1 mouse mammary tumor growth in vivo through reduced VEGF, pSTAT3 and Cyclin D1 80. Recent evidence suggests that C reactive protein as well as soluble leptin receptor can act to bind circulating leptin and attenuate its activity 81, 82. This provides insight into novel mediators of leptin action that may mediate its activity in cancer patients. Anti-leptin therapy could potentially be used to decrease circulating levels of leptin or to alter the adiponectin:leptin ratio in cancer patients, although additional preclinical studies will be needed to test the impact of altered leptin and adiponectin signaling in vivo.
Adiponectin treatment decreases the number of polyps especially those larger in size, in the ApcMin intestinal tumor model 83. Adiponectin treatment induced apoptosis of gastric cancer cells in vitro while in vivo its infusion into mice led to decreased metastasis 16. Additionally, liver tumor growth and lung metastases were lowered by adiponectin overexpression 14. Interestingly, rosiglitazone treatment increased adiponectin serum concentrations84 as well as adiponectin receptor expression 85. Additionally, hypocaloric diet and exercise led to an altered oligomeric distribution of adiponectin as well as it increased adipoR1 and adipoR2 expression 86.
Clinical Advances
The administration of leptin, adiponectin, or direct antagonists of either of these adipokines has not been reported in the literature for the treatment of human cancers. Leptin therapy was shown ineffective for patients with Type II diabetes, yet it did improve insulin sensitivity in leptin deficient patients 87. Currently, clinical applications of adiponectin and leptin therapeutics are more likely to address metabolic disorders, obesity, and diabetes than cancer therapeutics. Yet, the application of anti-leptin therapy or administration of adiponectin could both provide straightforward treatment options in cancer therapeutics through direct interactions in cancer cells or indirectly by reducing obesity and metabolic disorders which have been associated with increased risk for cancer.
Alternately, targeting downstream adipokine signaling mediators are likely to be an advantageous choice. Downstream targeting of the adiponectin with Metformin can lead to activation of AMPK. Metformin is gaining wide attention for its role as an anti-diabetic as well as its anti-tumor effects for breast, prostate, lung, colon, ovarian cancers 88. Metformin therapy preceding cancer diagnosis was associated with better survival in diabetic as well as non-diabetics 89. Use of metformin and thiazolidinediones among a defined patient population of diabetics with either stage 2 to advanced HER2+ breast cancer or those with prostate cancer associated with decreased mortality 90, 91. Thiazolidinediones, which are PPAR gamma agonists and include pioglitazone and rosiglitazone, increase the secretion of HMW adiponectin from adipocytes 92. Recent data from randomized controlled trials indicated that thiazolidinedione use provides a modest decrease in the risk for lung, colorectal and breast cancers 93. Additionally, administration of a cholesterol reducing drug, fenofibrate, increased plasma adiponectin concentration 94. Mechanisms to target the leptin pathway include the use of common pathway inhibitors such as STAT3 inhibitors95, Akt inhibitors 96, and RAF inhibitors 97. Novel mechanisms of adipokine modulation through PTP1B and PP2A may additionally be used to inhibit the leptin receptor. Dual targeted therapies directed toward decreasing response from leptin stimulation and increasing the response from adiponectin pathways have the potential for more efficacious cancer therapy.
Conclusions
Obesity is a growing clinical problem and is independently associated with multiple cancers 98. This review illustrates that adipokines contribute to multiple aspects of cancer progression and elicit a broad range of effects in normal as well as transformed cells. Adipokine stimulation appears not to follow a straightforward direct pathway, but instead contributes to a highly integrated cellular response. Determining circulating levels of adipokines as well as their receptors is equally important in determining which pathways are active and dominant. Additionally, cancers acquire genetic mutations and epigenetic modifications that can result in activation of oncogenes such as Ras, RAF, ERK and Akt or that can result in inactivation of tumor suppressors such as p53 and PTEN. In the future, we will likely have to consider individualized mutational status for cancer as well as in cancer cell lines in order to understand the impact these alterations have on adipokine signaling pathways. Integration of these aspects will then allow for targeted therapeutics and manipulation of adipokine pathways in cancer.
Acknowledgments
Grant Support
Research supported by the 2010 Pancreatic Cancer Action Network-AACR Career Development Award, Grant Number 10-20-25-VANS. M.N.VanSaun is also supported in part by NIH, National Institute of Health, Grant #1U01CA143072-01; Awarded to Lee Gorden.
Footnotes
Disclosure of Potential Conflicts of Interest
The author: Michael Nathan VanSaun has disclosed no potential conflicts of interest.
Figure Permission
The figure submitted in this manuscript was fully conceived and prepaid by Michael Nathan VanSaun and was not borrowed from any other source.
References
- 1.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 2.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 3.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (Lond) 2005;29:1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 4.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Pierre J, Tremblay ML. Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab. 2012;15:292–297. doi: 10.1016/j.cmet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 7.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 11.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 13.Kim AY, Lee YS, Kim KH, Lee JH, Lee HK, Jang SH, et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol. 2010;24:1441–1452. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Man K, Ng KT, Xu A, Cheng Q, Lo CM, Xiao JW, et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res. 2010;16:967–977. doi: 10.1158/1078-0432.CCR-09-1487. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M, Kitayama J, Yamauchi T, Kadowaki T, Maki T, Miyato H, et al. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci. 2007;98:1120–1127. doi: 10.1111/j.1349-7006.2007.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu YC, Shieh DC, Tong KM, Chen CP, Huang KC, Chen PC, et al. Involvement of AdipoR receptor in adiponectin-induced motility and alpha2beta1 integrin upregulation in human chondrosarcoma cells. Carcinogenesis. 2009;30:1651–1659. doi: 10.1093/carcin/bgp156. [DOI] [PubMed] [Google Scholar]
- 18.Han G, Wang L, Zhao R, Yue Z, Zhou X, Hu X, et al. Leptin promotes human glioblastoma growth through activating Signal Transducers and Activators of Transcription 3 signaling. Brain Res Bull. 2012;87:274–279. doi: 10.1016/j.brainresbull.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Lv L, Xiao W, Gong C, Yin J, Wang D, et al. Leptin activates STAT3 and ERK1/2 pathways and induces endometrial cancer cell proliferation. J Huazhong Univ Sci Technolog Med Sci. 2011;31:365–370. doi: 10.1007/s11596-011-0382-7. [DOI] [PubMed] [Google Scholar]
- 20.Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–1371. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 21.Saxena NK, Vertino PM, Anania FA, Sharma D. leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011;18:413–428. doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh WL, Lu DY, Lee MJ, Fu WM. Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia. 2009;57:454–464. doi: 10.1002/glia.20773. [DOI] [PubMed] [Google Scholar]
- 24.Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008;68:6752–6761. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birmingham JM, Busik JV, Hansen-Smith FM, Fenton JI. Novel mechanism for obesity-induced colon cancer progression. Carcinogenesis. 2009;30:690–697. doi: 10.1093/carcin/bgp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleary MP, Ray A, Rogozina OP, Dogan S, Grossmann ME. Targeting the adiponectin:leptin ratio for postmenopausal breast cancer prevention. Front Biosci (Schol Ed) 2009;1:329–357. doi: 10.2741/S30. [DOI] [PubMed] [Google Scholar]
- 28.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 29.Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, et al. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 30.Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Scarpace PJ. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol. 2009;158:475–482. doi: 10.1111/j.1476-5381.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montoye T, Piessevaux J, Lavens D, Wauman J, Catteeuw D, Vandekerckhove J, et al. Analysis of leptin signalling in hematopoietic cells using an adapted MAPPIT strategy. FEBS Lett. 2006;580:3301–3307. doi: 10.1016/j.febslet.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 33.Shi ZQ, Yu DH, Park M, Marshall M, Feng GS. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000;20:1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 35.Ling Y, Maile LA, Badley-Clarke J, Clemmons DR. DOK1 mediates SHP-2 binding to the alphaVbeta3 integrin and thereby regulates insulin-like growth factor I signaling in cultured vascular smooth muscle cells. J Biol Chem. 2005;280:3151–3158. doi: 10.1074/jbc.M411035200. [DOI] [PubMed] [Google Scholar]
- 36.Knobelspies H, Zeidler J, Hekerman P, Bamberg-Lemper S, Becker W. Mechanism of attenuation of leptin signaling under chronic ligand stimulation. BMC Biochem. 2010;11:2. doi: 10.1186/1471-2091-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert M, Myers MG., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 38.Handy JA, Fu PP, Kumar P, Mells JE, Sharma S, Saxena NK, et al. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem J. 2011;440:385–395. doi: 10.1042/BJ20102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 40.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM, et al. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012;72:4394–4404. doi: 10.1158/0008-5472.CAN-12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, et al. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–4026. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
- 43.Barb D, Neuwirth A, Mantzoros CS, Balk SP. Adiponectin signals in prostate cancer cells through Akt to activate the mammalian target of rapamycin pathway. Endocr Relat Cancer. 2007;14:995–1005. doi: 10.1677/ERC-06-0091. [DOI] [PubMed] [Google Scholar]
- 44.Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, et al. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer. 2009;8:74. doi: 10.1186/1476-4598-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 46.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/LepRdb)LepRdb) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 47.Xue RQ, Gu JC, Du ST, Yu W, Wang Y, Zhang ZT, et al. Lentivirus-mediated RNA interference targeting the ObR gene in human breast cancer MCF-7 cells in a nude mouse xenograft model. Chin Med J (Engl) 2012;125:1563–1570. [PubMed] [Google Scholar]
- 48.Yang SN, Chen HT, Tsou HK, Huang CY, Yang WH, Su CM, et al. Leptin enhances cell migration in human chondrosarcoma cells through OBRl leptin receptor. Carcinogenesis. 2009;30:566–574. doi: 10.1093/carcin/bgp023. [DOI] [PubMed] [Google Scholar]
- 49.Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Nyante SJ, Gammon MD, Kaufman JS, Bensen JT, Lin DY, Barnholtz-Sloan JS, et al. Common genetic variation in adiponectin, leptin, and leptin receptor and association with breast cancer subtypes. Breast Cancer Res Treat. 2011;129:593–606. doi: 10.1007/s10549-011-1517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadik NA, Ahmed A, Ahmed S. The significance of serum levels of adiponectin, leptin, and hyaluronic acid in hepatocellular carcinoma of cirrhotic and noncirrhotic patients. Hum Exp Toxicol. 2012;31:311–321. doi: 10.1177/0960327111431091. [DOI] [PubMed] [Google Scholar]
- 52.Arisan ED, Arisan S, Atis G, Palavan-Unsal N, Ergenekon E. Serum adipocytokine levels in prostate cancer patients. Urol Int. 2009;82:203–208. doi: 10.1159/000200801. [DOI] [PubMed] [Google Scholar]
- 53.Aliustaoglu M, Bilici A, Gumus M, Colak AT, Baloglu G, Irmak R, et al. Preoperative serum leptin levels in patients with breast cancer. Med Oncol. 2010;27:388–391. doi: 10.1007/s12032-009-9222-z. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani G, Maccio A, Mura L, Massa E, Mudu MC, Mulas C, et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med (Berl) 2000;78:554–561. doi: 10.1007/s001090000137. [DOI] [PubMed] [Google Scholar]
- 55.Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 56.Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer Causes Control. 2009;20:625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalbec KM, Max Schmidt C, Wade TE, Wang S, Swartz-Basile DA, Pitt HA, et al. Adipokines and cytokines in human pancreatic juice: unraveling the local pancreatic inflammatory milieu. Dig Dis Sci. 2010;55:2108–2112. doi: 10.1007/s10620-009-0977-z. [DOI] [PubMed] [Google Scholar]
- 58.Salageanu A, Tucureanu C, Lerescu L, Caras I, Pitica R, Gangura G, et al. Serum levels of adipokines resistin and leptin in patients with colon cancer. J Med Life. 2010;3:416–420. [PMC free article] [PubMed] [Google Scholar]
- 59.Bolukbas FF, Kilic H, Bolukbas C, Gumus M, Horoz M, Turhal NS, et al. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer. 2004;4:29. doi: 10.1186/1471-2407-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 61.Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208:989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. discussion 94-6. [DOI] [PubMed] [Google Scholar]
- 62.White PB, True EM, Ziegler KM, Wang SS, Swartz-Basile DA, Pitt HA, et al. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg. 2010;14:1888–1893. doi: 10.1007/s11605-010-1349-x. discussion 93-4. [DOI] [PubMed] [Google Scholar]
- 63.Ferla R, Bonomi M, Otvos L, Jr, Surmacz E. Glioblastoma-derived leptin induces tube formation and growth of endothelial cells: comparison with VEGF effects. BMC Cancer. 2011;11:303. doi: 10.1186/1471-2407-11-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nejati-Koshki K, Zarghami N, Pourhassan-Moghaddam M, Rahmati-Yamchi M, Mollazade M, Nasiri M, et al. Inhibition of leptin gene expression and secretion by silibinin: possible role of estrogen receptors. Cytotechnology. 2012;64:719–726. doi: 10.1007/s10616-012-9452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 66.Kaklamani VG, Wisinski KB, Sadim M, Gulden C, Do A, Offit K, et al. Variants of the adiponectin (ADIPOQ) and adiponectin receptor 1 (ADIPOR1) genes and colorectal cancer risk. JAMA. 2008;300:1523–1531. doi: 10.1001/jama.300.13.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaklamani VG, Sadim M, Hsi A, Offit K, Oddoux C, Ostrer H, et al. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res. 2008;68:3178–3184. doi: 10.1158/0008-5472.CAN-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaklamani V, Yi N, Zhang K, Sadim M, Offit K, Oddoux C, et al. Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metabolism. 2011;60:1234–1243. doi: 10.1016/j.metabol.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 70.Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15:3256–3264. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drew JE, Farquharson AJ, Padidar S, Duthie GG, Mercer JG, Arthur JR, et al. Insulin, leptin, and adiponectin receptors in colon: regulation relative to differing body adiposity independent of diet and in response to dimethylhydrazine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G682–G691. doi: 10.1152/ajpgi.00231.2007. [DOI] [PubMed] [Google Scholar]
- 74.Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochem Biophys Res Commun. 2006;348:832–838. doi: 10.1016/j.bbrc.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 75.Takahata C, Miyoshi Y, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett. 2007;250:229–236. doi: 10.1016/j.canlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Barresi V, Grosso M, Giuffre G, Tuccari G, Barresi G. The expression of adiponectin receptors Adipo-R1 and Adipo-R2 is associated with an intestinal histotype and longer survival in gastric carcinoma. J Clin Pathol. 2009;62:705–709. doi: 10.1136/jcp.2009.066175. [DOI] [PubMed] [Google Scholar]
- 77.Crimmins NA, Martin LJ. Polymorphisms in adiponectin receptor genes ADIPOR1 and ADIPOR2 and insulin resistance. Obes Rev. 2007;8:419–423. doi: 10.1111/j.1467-789X.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 78.Amjadi F, Javanmard SH, Zarkesh-Esfahani H, Khazaei M, Narimani M. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. J Exp Clin Cancer Res. 2011;30:21. doi: 10.1186/1756-9966-30-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Otvos L, Jr, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 81.Zastrow O, Seidel B, Kiess W, Thiery J, Keller E, Bottner A, et al. The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord. 2003;27:1472–1478. doi: 10.1038/sj.ijo.0802432. [DOI] [PubMed] [Google Scholar]
- 82.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 83.Otani K, Kitayama J, Yasuda K, Nio Y, Iwabu M, Okudaira S, et al. Adiponectin suppresses tumorigenesis in Apc(Min)(/+) mice. Cancer Lett. 2010;288:177–182. doi: 10.1016/j.canlet.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 84.Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005;336:747–753. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 85.Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 86.O'Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, et al. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–E427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 87.Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Ther. 2012;133:108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 89.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol. 2011;22:2640–2645. doi: 10.1093/annonc/mdr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100–E1105. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 93.Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2012;38:475–484. doi: 10.1016/j.diabet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 94.Koh KK, Han SH, Quon MJ, Yeal Ahn J, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005;28:1419–1424. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 95.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 97.Kim DH, Sim T. Novel small molecule Raf kinase inhibitors for targeted cancer therapeutics. Arch Pharm Res. 2012;35:605–615. doi: 10.1007/s12272-012-0403-5. [DOI] [PubMed] [Google Scholar]
- 98.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]