Abstract
Recruitment of immune cells to tumor cells targeted by a therapeutic antibody can heighten the antitumor efficacy of the antibody. For example, p185her2/neu-targeting antibodies not only downregulate the p185her2/neu kinase (ERBB2) but also trigger complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) through the antibody Fc region. Here we describe a generalized strategy to improve immune cell recruitment to targeted cancer cells, using a modified scFv antibody we call a “grababody” that binds the target protein and endogenous immunoglobulins. The model system we used to illustrate the utility of this platform recognizes p185her2/neu and includes an IgG binding domain. The recombinant scFv grababody that was created recruited circulating human IgGs and attracted immune cells carrying Fc receptors to tumor cells that expressed p185her2/neu. The presence of the IgG binding domain significantly enhanced CDC and ADCC activity and improved anti-tumor activity in vivo. Our results illustrate a novel general approach to improve antibody-like proteins for therapeutic applications.
Keywords: Grababody, HER2/neu, antibody engineering, ADCC, effector cell function
Introduction
Antibody-based cancer therapies, which in general target cell surface tumor antigens with recombinantly engineered monoclonal antibodies, have changed the paradigm of treatments for many types of tumors (1). These monoclonal antibodies (mAb), either as humanized antibodies or chimeric molecules, contain the Fc region of human IgG molecules that is required to induce cytotoxic mechanisms, such as ADCC and CDC. Recombinant antibodies have to be expressed in mammalian cells to obtain proper glycosylation, which is required to keep the Fc region of the IgG molecule in an “open” conformation to interact with Fc receptors (2). However, glycosylation increases the complexity of mAb production for therapeutic use. Depending on cell culture conditions, the produced monoclonal antibodies can have varied glycosylation (3), which can either extend or shorten the serum half-life of the antibodies (4–6), and cause side effects in some antibody –based treatments (7).
p185her2/neu belongs to the ErbB family of receptor tyrosine kinases (RTK), which includes four members: ErbB1/EGFR (epidermal growth factor receptor), ErbB2/p185her2/neu (also known as Neu, HER2/neu), ErbB3/HER3, and ErbB4/HER4 receptors. When activated by extracellular ligands, ErbB receptors will form catalytically active homodimeric, heterodimeric or oligomeric complexes. These complexes can lead to alterations of cellular growth and differentiation status. ErbB ligands and subsequent receptor-mediated signalings have been implicated in survival, proliferation and differentiation in a variety of cell types (reviewed in (8–10)). After p185her2/neu was identified as the oncoprotein in the neu oncogene transformed cells (11), monoclonal antibodies to this oncoprotein and subsequently to the human homologues have been developed to reverse the transformed phenotype of cancer cells (12–15).
The humanized anti- p185her2/neu antibody, h4D5 (trastuzumab, Herceptin), is approved to treat breast and stomach cancers. We have defined a constrained peptide, AHNP, based on the CDR3.H loop from h4D5 (16). Binding of AHNP to p185her2/neu suppresses the proliferation of p185her2/neu transformed cells in vitro and in vivo. We have also grafted AHNP to the tetrameric scaffold of streptavidin and established a recombinant protein, ASA (17), which possesses significantly improved biological activity. ASA demonstrates higher association rate binding to p185her2/neu (Kon), a feature explained by the avidity contributed by the tetrameric structure. However, both the AHNP peptide and the ASA protein lack the capability to trigger Fc dependent effector functions.
Here we report an approach to enlist CDC/ADCC functions to these small recombinant proteins by incorporating the Z domain derived from Protein A. This class of novel proteins are termed “Grababody”, as they are able to capture circulating IgGs while binding to target antigens. The captured IgG can further direct complement complexes and immune effector cells carrying Fc receptors to tumor cells expressing targeted receptors. This approach bypasses the need for Fc region and thus avoid glycosylation issues, allowing the facile production of the protein in bacteria.
Materials and Methods
Cell lines and reagents
T6–17, a gift from Dr JH Pierce, was derived from NIH3T3 by overexpressing the p185her2/neu receptor (18). SKBR3 and BT474, which we obtained originally from the American Type Culture Collection, are breast cancer cell lines with p185her2/neu expression. Authenticity of these cells was determined by confirming their known expression profiles for receptors using fluorescence-activated cell sorting (FACS) periodically. Cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat inactivated fetal calf serum, L-glutamine (2mM), penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37°C in a humidified 5% CO2 atmosphere. All cell lines were routinely checked for mycoplasma.
The pIG6-4D5noSS plasmid, which contains the scFv cDNA of h4D5, was obtained from Prof. A. Plückthun, University of Zürich, Zürich, Switzerland (19). The plasmid pEZZ18 that contains the protein A cDNA sequence was purchased from GE Healthcare Life Sciences.
Construction of plasmids
The following primers were used to amplify the cDNAs for 4D5scFV and the ZZ domain: for 4D5scFv, primer4D5s (5′-GGGACCATGGCTGATATCCAGATGACCCAGTCTCCGAGC-3′) and 4D5salI (5′-GGGAGTCGACAGAGCCACCACCGCCAGAAGAAACGGTAACGGT-3′); for ZZ: ZZsal74 (5′-GGGAGTCGACGTAGACAACAAATTCAAC-3′) and ZZrxho (5′-GGGACTCGAGTTTCGGCGCCTGAGC-3′). The amplified cDNAs were digested with restriction enzymes NcoI/SalI (for 4D5scFv) and SalI/XhoI (for ZZ) and ligated together into the bacterial expression vector pET21d to express 4D5scFvZZ. The cDNA for 4D5scFv was also cloned into pET21d to express the scFv as the control.
Recombinant protein production and purification
E. coli BL21 strain BLX harboring pET21d-4D5scFvZZ, pET21d-4D5scFv, or pET15aD4(508-577) were grown in TB medium with 50 μg/ml ampicillin at 37°C overnight. The overnight culture was diluted 1:100 into 2L TB(Amp) medium. When the OD at 600nm reached 1.0, recombinant proteins were induced to express with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) and cultured at 16°C for overnight. Bacterial pellet was resuspended in 150 mL lysis buffer (20 mM sodium phosphate, 0.5 M NaCl, 30 mM Imidazole, 1 mM PMSF, 5 mM 2-ME, pH 7.0) and sonicated. After centrifugation at 12, 000 g for 20 min, the supernatant was incubated for 1 hour at 4°C with 2ml of Ni-Sepharose 6 Fast Flow (GE Healthcare Bio-Sciences, Piscataway, NJ) beads, which had been equilibrated with binding buffer (20 mM sodium phosphate, 0.5 M NaCl, 30 mM Imidazole, pH 7.0). After binding, the resin was washed 3 times with binding buffer and transferred into a column. His-tagged proteins were eluted with lysis buffer containing 200 mM imidazole.
FACS
Cells were first washed with FACS buffer (cold PBS containing 0.5% bovine serum albumin (BSA)). ~3 × 105 cells were then incubated with testing scFv proteins in a total volume of 0.2 mL FACS for 30 min on ice. His-probe antibody and subsequently Alexa-488 conjugated anti-rabbit IgG were used to detect the cell surface captured His-tagged scFv proteins. The mean fluorescence intensity (MFI) of each sample was recorded after FACS analysis of the stained cells.
Surface plasmon resonance (Biacore) studies
To characterize the binding of 4D5scFv and 4D5scFvZZ to p185her2/neu, we performed the surface plasmon resonance-based experiment using the biosensor instrument Biacore 2000, at 25°C. Immobilization of p185her2/neu D4(508-577) on the sensor surface was performed following the standard amine coupling procedure according to manufacturer’s instructions. Briefly, 35 μl of a solution containing 0.2 M N-ethyl-N -(dimethylaminopropyl) carbodiimide (EDC) and 0.05 M N-hydroxysuccinimide (NHS), was injected at a flow rate of 5 μl/min to activate carboxyl groups on the CM5 sensor chip surface. Recombinant p185her2/neu D4(508-577) was flowed over the chip surface at a flow rate of 20 μl/min until the desired level of bound protein (500 RU) was reached. Unreacted proteins were washed out and unreacted activated groups were blocked by the injection of 35 μl of 1 M ethanolamine at 5 μl/min. A reference surface was generated simultaneously under the same conditions but without protein injection and used as a blank to correct instrument and buffer artifacts. Testing antibodies or proteins were injected at a flow rate of 20 μl/min. Binding to D4 immobilized on the chip was monitored in real time as a series of sensorgrams. For simultaneous binding experiments, 4D5scFvZZ was first injected to the chip surface immobilized with D4(508-577), followed by injection of human IgG (C225 or 2C4) or buffer. Kinetic constants were estimated by global fitting analysis of the sensorgram curves to the 1:1 Langmurian interaction model. The D4(508-577) surface was regenerated after each cycle using 10 mM NaOH and 0.1% SDS (w/v). The Kon value was determined from a plot of (ln(dR/dt))/t vs Ab concentration. The Koff was determined from the dissociation part of the sensorgram at the highest concentration of Ab used, with a flow rate of 15 ml/minute to prevent rebinding. The equilibrium dissociation constant (KD) was calculated as the koff/kon ratio.
CDC measured by MTT assay
The CDC activity was measured using mouse serum as the resource for complements as we have reported previously (20). Briefly, T6-17 cells were divided into aliquots (4,000 –5,000 cells/well) into 96-well flat-bottom plates and treated with 4D5scFv, 4D5scFvZZ or humanized h4D5 mAb (positive control) in the presence of 5% FBS (control) or mouse serum. Cells were incubated for 3 days at 37°C in a humidified atmosphere with 5% CO2. Old medium was carefully removed and replaced with 100 μl fresh medium, in which cells were incubated for another 4 hours. A total of 25 μl of MTT solution (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, 5 mg/ml in PBS) was added to each well, and after 2 h of incubation at 37°C, 100 μl of the extraction buffer (20% w/v SDS, 50% N,N-dimethyl formamide, pH 4.7) was added. After an overnight incubation at 37°C, the absorbance at 570nm was measured using an ELISA reader.
ADCC
Cytotoxicity was determined by the fluorescent probe calsein AM, using human PBMCs as effector cells and the BT474 human breast cancer cells as target cells at an E:T ratio of 25:1. PBMCs were obtained from the Human Immunology Core of the University of Pennsylvania. Briefly, target cells (0.5 × 104/well) were labeled with calcein AM (final conc., 8μM in 10% FBS-DMEM, Molecular Probes, Eugene, OR) for 30 min at 37°C, washed 2 times and then incubated with 100 μg/ml human IgG, different concentrations of testing proteins and PBMCs (1.25 × 105/well) in a total volume of 200 μl in 96-well F-bottomed plates for 4hr at 37°C with 5% CO2. To achieve total lysis, cells were incubated with 0.1% Triton X-100. After incubation, plate were washed and replaced with PBS. Fluorescence units (FU) were measured in a Microplate reader (TECAN, NC) with 490/515 nm of Ex/Em wavelength. Percentage cytotoxicity was calculated as 100 − (FU of target cells incubated with effector cells − FU of target cells incubated with triton X)/(FU of target cells incubated with medium alone − FU of target cells incubated with triton X) × 100.
Xenograft studies
All mouse procedures were performed according to the guidelines and protocols approved by the IACUC of University of Pennsylvania. NCr Athymic nu/nu (nude) mice (6 to 8-week-old, ~20g) were purchased from the National Cancer Institute. To induce tumor, 5× 104 transformed T6–17 cells were suspended in 100 μl of PBS and injected s.c. into the flank of each animal. Intraperitoneal injection started one day after inoculation at a dose of 10 mg/kg, three times per week. Tumor size was determined by vernier caliper measurements. Tumor volume was calculated by the formula: π*length*width*Height/6.
Result
Construction of a Grababody for p185her2/neu using the scFv of h4D5
Protein A from Staphylococcus aureus has been extensively used to capture immunoglobulin G of different subtypes. Protein A is a 42-kDa staphylococcal cell wall protein with five repeated extracellular domains (E, D, A, B, C) of ~58 residues. All of these domains can bind to the Fc region of IgG. To improve stability against hydroxylamine and cyanogen bromide mediated degradation, a recombinant protein domain called the “Z domain” is generated using the B domain as the template, with only a single amino acid substitution (G29A) (21). The Z domain binds to IgG1 with a very high affinity of 10 nM (22).
Like other IgG binding domains in Protein A, the Z domain consists of three anti-parallel alpha helixes. As determined by structural (23) and mutational studies (24), helix-1 (residues 7–18) and helix-2 (residues 20–38) are involved in the interaction with Fc. However, helix 3 is critical for maintaining the other two helixes in the α helix conformation. Removal of helix 3 drastically reduces Fc binding affinity (> 103 fold) (22).
Crystallographic studies of the IgG-Fc - FcγR complex (PDB: 1T89)(25) and the IgG Fc – domain B complex (PDB: 1FC2) (23) indicate that Fc binds to Fc receptors and Protein A via different regions. While the Fc receptor binds to the hinge region between the CH1 and CH2 domain Fc, Protein A interacts with the elbow region connecting CH2 and CH3 (Figure S1). Based on these structural studies, we determine that Fc can interact with both Fc receptor and the Z domain simultaneously without steric hindrance.
The Z domain is thus grafted into several tumor antigen- binding proteins to obtain a class of recombinant proteins termed “Grababody”. In this report, we fused the Z domain to the c-terminus of 4D5scFV to obtain the Grababody 4D5scFVZZ, anticipating that the IgG molecule recruited by 4D5scFvZZ would be able to interact with Fc receptors on immune effector cells.
Binding of the Grababody 4D5scFvZZ to p185her2/neu receptor on the cell surface
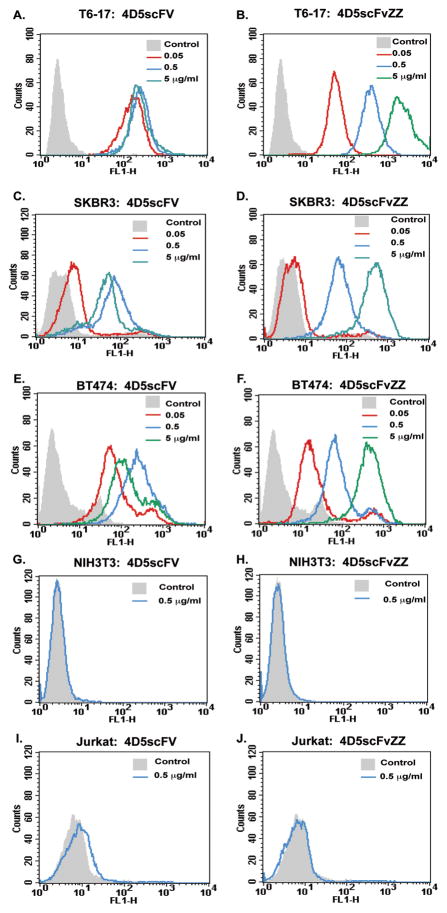
We first tested the ability of 4D5scFvZZ to bind to p185her2/neu on the surface of several cell lines, including the transformed T6-17 cells (NIH3T3 overexpressing human p185her2/neu) and two human breast cancer cell lines, SKBR3 and BT474. As shown in Figure 1, 4D5scFvZZ bound to all these cells in the Fluorescence-activated cell sorting (FACS) analysis in a dose-dependent manner and demonstrated much higher maximal binding activity than 4D5scFv. At 5 μg/ml (143 nM), 4D5scFvZZ reached a maximal binding on T6-17 with a mean fluorescence intensity (MFI) of 2355 (Table 1), a level that is about 8.4 fold higher than the maximal binding of 4D5scFv (MFI = 279, reached at 5 μg/ml or 167 nM).
Figure 1. Grababody 4D5scFvZZ binds to cell surface p185her2/neu.
T6-17 (A, B), SKBR3 (C, D), and BT474 (E, F) cells with different expression levels of p185her2/neu were prepared for Fluorescence-activated cell sorting (FACS) as described under Materials and Methods. Histograms represent staining with different dose of 4D5scFv or 4D5scFvZZ, as indicated in the figure, followed by His-Probe antibody and Alexa488-conjugated goat anti-rabbit antibodies (solid lines). The gray peak represents the control staining with only the His-Probe antibody and the secondary antibody. A mouse cell line NIH3T3 (G, H), which is of the same background of T6-17, and a human cell line Jurkat (I, J) were used as control cell lines, showing no significant binding to either 4D5scFv or 4D5scFvZZ.
Table 1. Comparison of binding of 4D5scFv and the Grababody 4D5scFvZZ to different cell lines in FACS analysis.
Mean fluorescence intensity readouts of each protein construct at indicated concentration were listed. The maximal binding (in BOLD) was the highest mean fluorescence observed in the FACS analysis. Anti-His probe and Alexa-488 labeled anti-rabbit IgG antibodies were used subsequently to detect the His-tagged 4D5scFv and 4D5scFvZZ. To show relative receptor expression levels among these cell lines, binding of the h4D5 antibody was included. h4D5 was detected by a different secondary FITC-labeled anti-human antibody, which had background readout of 3.0–4.3 in these three cell lines.
| 4D5scFv | 4D5scFvZZ | h4D5 | |||
|---|---|---|---|---|---|
| 16.7 nM (0.5 μg/ml) | 167 nM (5 μg/ml) | 14.3 nM (0.5 μg/ml) | 143 nM (5 μg/ml) | 66.7 nM (10 μg/ml) | |
| T6-17 | 255.0 | 279.0 | 406.0 | 2355.0 | 901.2 |
| SKBR3 | 59.2 | 39.6 | 65.2 | 396.2 | 313.8 |
| BT474 | 332.8 | 228.1 | 121.9 | 489.7 | 377.7 |
4D5scFv reached binding saturation at 0.5μg/ml on both SKBR3 and BT474 (Fig. 1, C&E). In contrast, binding of 4D5scFvZZ to these two cell lines did not reach saturation until 5 μg/ml. The maximal binding of either 4D5scFv or 4D5scFvZZ to SKBR3 and BT474 was less than that to T6-17. This is consistent with higher expression of p185her2/neu in T6-17 than the other two human cell lines as determined by the p185her2/neu antibody (15) (Table 1). On the control cell lines NIH3T3 and Jurkat, neither 4D5scFv nor 4D5scFvZZ displayed any significant bindings (Fig. 1 G–J).
Determination of the intrinsic affinity of 4D5scFvZZ to the antigen and IgG
To determine if the incorporation of the Z domain affects intrinsic binding kinetics, we compared 4D5scFv and 4D5scFvZZ for binding to p185her2/neu in surface plasmon resonance (SPR) studies. Previously, we had established an Fc fusion construct containing the p185her2/neu ectodomain (ECD) for SPR studies (17), but it was not ideal for this study since the Fc region by itself could interact with the Z domain. The p185her2/neu fragment had to be expressed without the Fc tag.
The co-crystal structure of h4D5 and p185her2/neu ectodomain (26) reveals that the h4D5 epitope is contained in the domain IV region of p185her2/neu. However, a very short flexible region in the domain IV prevents the expression of the complete domain in bacteria. We expressed a slightly shortened version of domain IV containing a.a. residues 508-577 (Fig. S2). This fragment binds to mAb h4D5 in immunoprecipitation and in SPR assay as well (Fig. S2 and Fig. S3). D4(508-577) also binds to the original mAb 7.16.4 that shares the epitope with h4D5 (15) (Fig. S3).
Using chip-immobilized p185her2/neu domain IV, D4(508-577), the SPR experiment confirmed that the addition of the Fc-binding domain to scFv did not significantly change the intrinsic affinity to p185her2/neu D4(508-577) (KD: 4D5scFv, 145nM; 4D5scFvZZ: 112 nM) (Fig. 2 A&B). The humanized mAb h4D5, which contains human IgG1 Fc region, was also immobilized to chip. Only 4D5scFvZZ showed a very high affinity to this IgG (KD: 1.3 nM). The Z domain may also lead to intermolecular association as a modest affinity was detected between 4D5scFvZZ molecules (Kd = 946 nM).
Figure 2. 4D5scFvZZ binds to immobilized receptor and further capture human IgG molecules.
A & B: Direct binding of 4D5scFv and 4D5scFvZZ to immobilized p185her2/neu subdomain IV, D4(508-577). To show that the 4D5scFvZZ Grababody can simultaneously bind to both the receptor and an human IgG molecule, D4(508-577)-bound 4D5scFvZZ was further exposed to human IgG (green line) or buffer. Two antibodies with human Fc were tested: C. C225 (2 mg/ml); D. 2C4 (0.05 mg/ml). Neither C225 nor 2C4 binds to D4(508-577).
Simultaneous binding of 4D5scFvZZ to p185her2/neu and human IgG
To demonstrate that the Z domain in the Grababody is available to bind IgG after it forms a complex with the receptor, we performed a sequential binding experiment by SPR. The immobilized D4(508-577) was first contacted with 4D5scFvZZ and binding was detected. After the dissociation phase of the first binding, we flowed over the D4(508-577)-4D5scFvZZ complexes with antibodies containing human Fc: mAb C225 (2 mg/ml, Fig. 2C) or 2C4 (0.5 mg/ml, Fig. 2D). C225 is specific to human EGFR. Although 2C4 binds to p185her2/neu, it binds to an epitope in Domain II. None of these two antibodies binds to the D4(508-577) fragment (Fig. S3) but would be able to interact with the Z domain. As expected, binding was observed for the D4(508-577)- 4D5scFvZZ complexes (Fig. 2 C&D).
Induction of immune effector cell function by 4D5scFvZZ
To investigate if the Grababody can lead to antibody mediated effector functions, we performed in vitro experiments to test both CDC and ADCC activities. 4D5scFvZZ-induced CDC was evaluated by MTT assay in the presence of mouse serum, which can be a good source of complements and has been shown to provide higher inhibition activity in this type of assay when compared with human serum (20). As shown in Figure 3, a significant inhibition of T6-17 proliferation was observed with 4D5scFvZZ in a dose-dependent manner in 5% mouse serum, but not in 5% FBS. 4D5scFv also showed some proliferation inhibition activity in mouse serum, but the activity was weak. As the positive control, the humanized antibody h4D5 showed the very strong inhibition (~ 55%) in the presence of mouse serum.
Figure 3. 4D5scFvZZ induces CDC towards cells expressing p185her2/neu.

T6-17 cells were incubated with h4D5, 4D5scFv or 4D5scFvZZ in the presence of either 5% FBS or mouse serum. Cell viability was measured by standard MTT assay. Growth inhibition was determined by this formula: [(control wells−treated wells)/control wells] × 100%. t test was performed to compare statistical difference between groups. Although all treatment groups showed statistical inhibition as compared with control (p < 0.05), we consider >20% inhibition to be biologically significant. Both 4D5scFv and 4D5scFvZZ showed dose dependent inhibition activity in the presence of mouse serum.
ADCC of 4D5scFvZZ against BT474 breast cancer cells was measured in vitro by the calcein release assay, a non-radioactive alternative to the traditional Cr51 assay (27). Human PBMC were used as effector cells. ADCC activity (~65%) can be observed with 10 μg/mL of 4D5scFvZZ, at an effector-to-target cell ratio of 25:1 and in the presence of 100 μg/mL human IgG (Fig. 4). Compared with human IgG only, no significant ADCC activity was observed with 4D5scFv. We could not detect any significant ADCC activity for 4D5scFvZZ towards control cell lines A431 and NIH3T3, or towards BT474 when human IgG antibodies were not supplemented. These studies confirmed that 4D5scFvZZ induced ADCC towards p185her2/neu - expressing tumor cells in an IgG- dependent manner.
Figure 4. ADCC activity mediated by 4D5scFvZZ.
ADCC activity of human PBMC against breast cancer BT474 cells was tested in the presence of 4D5scFvZZ (1 or 10 μg/ml) and human IgG (100 μg/ml) (A). A significant increase of ADCC activity was observed when 10 μg/ml of 4D5scFvZZ was used (t test, p< 0.05) as compared with human IgG alone. In contrast, no activity was detected when 4D5scFv was used under similar conditions. ADCC activity of 4D5scFvZZ was dependent on the supplemented human IgG (B). In the absence of human IgG, only the h4D5 antibody showed very significant ADCC activity (p < 0.01). No significant ADCC activity was detected for 4D5scFvZZ on control cell lines A431 (C) and NIH3T3 (D).
In vivo activity of 4D5scFv vs. 4D5scFvZZ
To examine if the Z domain in the Grababody actually improves anti-tumor activity, we studied in vivo tumor growth using the transformed cell line T6-17 that expresses human p185her2/neu (17). Athymic nude mice were inoculated with 5× 104 T6-17 cells. Mice carrying tumor received 4D5scFvZZ, 4D5scFv or control buffer at the dose of 10 mg/kg, three times per week via i.p. injection. As shown in Fig. 5, 4D5scFvZZ demonstrated much greater activity than 4D5scFv in the inhibition of tumor growth. The difference of tumor size in control and 4D5scFvZZ treated mice were statistically different. At day 14 and day 18, the average size of tumors in the 4D5scFvZZ treated group was 84.6% and 76.8% smaller, respectively. This level of inhibition is comparable to what the h4D5 antibody could achieve in the same model at the dose of 1 mg/kg (day 15, 76.5% smaller, Fig. S4).
Figure 5. Comparison of in vivo activity of 4D5scFvZZ and 4D5scFv.

5 × 104 T6–17 cells were injected s.c. into nude mice to induce tumor growth. i.p. treatments with 4D5scFv or 4D5scFvZZ were provided to mice (10 mg/kg/dose, three times per week) started one day after inoculation. Error bars represent the standard error of mean. **: The size of tumors was very significantly different from the controls (t test, p < 0.01). No statistical difference was observed for 4D5scFv treatment although more mice were used. Control: n= 9; 4D5scFv: n=11; 4D5scFvZZ: n=4.
Discussions
Conventional approaches to incorporate effector functions to a protein with affinity to cancer cells involve constructing fusion proteins containing either the Fc fragment of IgG or a scFv fragment specific for Fc receptors. For Fc fusion proteins, current clinical data on the use of whole antibody molecules provide information on the expected clinical efficacy and indicate that the haplotype of Fc receptor in patients can also limit the activity of Fc fusion proteins. For example, patients with FcγRIIa 131H/H and/or FcγRIIIa 158V/V genotypes showed better progression free survival (PFS) than 131R and 158F carriers after anti-EGFR cetuximab treatment (28). Although antibody can be engineered to bind the Fc receptors better with optimized glycosylation such as the fucosylation, it is reported that the low-fucosylated anti-EGFR mAbs, which have higher binding to FcγRIIIA and improved ADCC by mononuclear effector cells, actually display reduced tumor cell killing by polymorphonuclear cells (29). Preferential expression patterns of the activation receptor FcγRIIIA on mononuclear cells and the inhibition receptor FcγRIIIB on polymorphonuclear cells might account for the differences.
A class of bispecific molecules have been developed to direct ADCC activity specifically to tumors (Bsab). In these constructs, a tumor specific scFv, such as scFv for p185her2/neu, is linked to another scFv specific for activating Fc receptors (e.g. FcγRIIIA (CD16)) (30). These Bsab constructs demonstrate ADCC against p185her2/neu expression tumor cells, especially when the scFv has nM affinity to p185her2/neu. However, the ADCC induced by scFv Bsab, even the trivalent form which shows the highest potency, is much weaker than that of trastuzumab (31). The full length antibody-based Bsab (2B1) shows ADCC comparable to trastuzumab but it is observed to induce cytokine release syndromes in clinical trials (30).
Our “Grababody” approach utilizes the Z domain to capture circulating IgG which subsequently direct immune effector cells and the complement system to attack tumor cells (Figs. 3 and 4). The Z domain is derived from the B domain of Protein A (SpA), a cell wall protein of Staphylococcus aureus. There are immunogenicity concerns of using non-human sequence in reagents developed for human use. It should be pointed out that Staphylococcus aureus is a very common pathogen and to which most people have been exposed. It is estimated that about 80% of individuals have nasal carriage of Staphylococcus aureus and 20–30% of the human population have persistent S. aureus colonized in the anterior nares (32, 33).
One immunological concern of SpA relates to its binding to Fab region of human immunoglobulins, primarily those of VH3 origin (34). Because of this binding capability, SpA is also expected to bind VH3-encoded B-cell antigen receptors, leading to the selection of these B cells and production of antibodies accordingly (35, 36). According to the solved complex structure of D domain of Protein A and Fab (37), the helix 2 and helix 3 of the D domain form the interface with Fab. However, although the Z domain has intact helixes 2 and 3 as other domains in Protein A, its affinity to VH3 containing Fab or scFv is tremendously reduced (38). Thus, since the Grababody contain only the Z domain instead of other IgG binding domains or the whole Protein A molecule, we should have less concerns on this issue.
SpA can bind airway epithelial cells and activate TNFR1 signaling (39). Meanwhile, SpA also induces the shedding of sTNFR, which limits TNFα function and is anti- inflammatory (40). The ultimate effect of SpA on TNFα signaling is unclear. Interactions of SpA with EGFR have been reported, which leads to the activation of TACE, a metalloprotease that cleaves TNFR1 and releases sTNFR1 from cell surface (40). In those studies, SpA was used at a very high effective concentration, 2.5 μM (~100 μg/ml). We have not observed any significant binding of EGFR with Protein A in immunoprecipitation studies (41). In addition, as the potency of individual IgG binding domain, such as the B domain and Z domain, to induce sTNFR1 is minimal (39), we do not expect our Grababody to have any significant effect on TNFR1.
Although we only showed a p185her2/neu Grababody constructed from the scFv of h4D5, similar proteins can be generated using smaller antibody derived fragments, e.g. the constrained CDR3 from the heavy chain (data not shown). These shorter antibody fragments in general have a weaker affinity than the whole antibody molecule, but the affinity can be optimized through a variety of techniques. In addition, the half-life of the Grababody may not yet be ideal. In a very recent study, the IgG binding B domain was proposed as a way to extend the plasma half-life of scFv approximately 6 times (42). We expect the p185her2/neu Grababody to have a similar serum half-life of ~12 hours, but that will still be shorter than the half-life of 7–10 days for IgG. The less than ideal affinity as well as half-life suggest that we need to use a higher dosage of Grababody than the antibody to obtain similar levels of in vivo activity (Figs. 5 and S4).
In addition to the future optimization of the affinity and half-life, we recognize that there is still room for improvement for the Z domain in the Grababody. The Z domain could be deimmunized and humanized for future use in human (43). Furthermore, it appears that the IgG binding domain can be shortened. Braisted and colleagues reported a shorter version of the Z domain with slightly reduced affinity to Fc (Kd = 43 nM, vs 10 nM of the Z domain) (22). In a followup study, a disulfide bond was used to constrain the conformation of the mini- Z domain to improve binding to Fc (44).
In summary, we have developed a “proof of concept” class of novel recombinant proteins to direct endogenous antibody to tumor cells. As a result, effector cell functions can be mediated towards tumors. Using a scFv of an anti- p185her2/neu receptor antibody, we have shown much improved in vivo activity of Grababody as compared with the scFv alone. This approach can be quickly adopted for other tumor antigen targeting proteins.
Supplementary Material
Acknowledgments
We appreciate comments from Dr. Mark I. Greene on the manuscript. We also thank the Human Immunology Core of the Path BioResource at UPenn for obtaining human PBMCs.
Grant Support
This work was supported by the grant from the National Institutes of Health to M.I.G. (R01 CA055306).
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
References
- 1.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature reviews Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 2.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. Journal of molecular biology. 2003;325:979–89. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 3.Muthing J, Kemminer SE, Conradt HS, Sagi D, Nimtz M, Karst U, et al. Effects of buffering conditions and culture pH on production rates and glycosylation of clinical phase I anti-melanoma mouse IgG3 monoclonal antibody R24. Biotechnology and bioengineering. 2003;83:321–34. doi: 10.1002/bit.10673. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki E, Niwa R, Saji S, Muta M, Hirose M, Iida S, et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1875–82. doi: 10.1158/1078-0432.CCR-06-1335. [DOI] [PubMed] [Google Scholar]
- 5.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature reviews Drug discovery. 2009;8:226–34. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 6.Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcgammaRs in vivo. Current opinion in organ transplantation. 2010 doi: 10.1097/MOT.0b013e328342538f. [DOI] [PubMed] [Google Scholar]
- 7.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. The New England journal of medicine. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougall WC, Qian X, Peterson NC, Miller MJ, Samanta A, Greene MI. The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene. 1994;9:2109–23. [PubMed] [Google Scholar]
- 9.Pinkas-Kramarski R, Alroy I, Yarden Y. ErbB receptors and EGF-like ligands: cell lineage determination and oncogenesis through combinatorial signaling. Journal of mammary gland biology and neoplasia. 1997;2:97–107. doi: 10.1023/a:1026343528967. [DOI] [PubMed] [Google Scholar]
- 10.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–87. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–6. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 12.Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–8. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- 13.Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci U S A. 1986;83:9129–33. doi: 10.1073/pnas.83.23.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, et al. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother. 1993;37:255–63. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Wang Q, Montone KT, Peavey JE, Drebin JA, Greene MI, et al. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Exp Mol Pathol. 1999;67:15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
- 16.Park BW, Zhang HT, Wu C, Berezov A, Zhang X, Dua R, et al. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol. 2000;18:194–8. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 17.Masuda K, Richter M, Song X, Berezov A, Murali R, Greene MI, et al. AHNP-streptavidin: a tetrameric bacterially produced antibody surrogate fusion protein against p185her2/neu. Oncogene. 2006;25:7740–6. doi: 10.1038/sj.onc.1209745. [DOI] [PubMed] [Google Scholar]
- 18.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–82. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 19.Worn A, Pluckthun A. An intrinsically stable antibody scFv fragment can tolerate the loss of both disulfide bonds and fold correctly. FEBS letters. 1998;427:357–61. doi: 10.1016/s0014-5793(98)00463-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Zhang H, Wang Q, Lal P, Carroll AM, de la Llera-Moya M, et al. Suppression of human prostate tumor growth by a unique prostate-specific monoclonal antibody F77 targeting a glycolipid marker. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:732–7. doi: 10.1073/pnas.0911397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson B, Moks T, Jansson B, Abrahmsen L, Elmblad A, Holmgren E, et al. A synthetic IgG-binding domain based on staphylococcal protein A. Protein engineering. 1987;1:107–13. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 22.Braisted AC, Wells JA. Minimizing a binding domain from protein A. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5688–92. doi: 10.1073/pnas.93.12.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20:2361–70. [PubMed] [Google Scholar]
- 24.Cedergren L, Andersson R, Jansson B, Uhlen M, Nilsson B. Mutational analysis of the interaction between staphylococcal protein A and human IgG1. Protein engineering. 1993;6:441–8. doi: 10.1093/protein/6.4.441. [DOI] [PubMed] [Google Scholar]
- 25.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. The Journal of biological chemistry. 2001;276:16469–77. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 26.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 27.Roden MM, Lee KH, Panelli MC, Marincola FM. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology. Journal of immunological methods. 1999;226:29–41. doi: 10.1016/s0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 28.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 29.Schlaeth M, Berger S, Derer S, Klausz K, Lohse S, Dechant M, et al. Fc-engineered EGF-R antibodies mediate improved antibody-dependent cellular cytotoxicity (ADCC) against KRAS-mutated tumor cells. Cancer science. 2010;101:1080–8. doi: 10.1111/j.1349-7006.2010.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, et al. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. Journal of immunology. 2001;166:6112–7. doi: 10.4049/jimmunol.166.10.6112. [DOI] [PubMed] [Google Scholar]
- 31.Shahied LS, Tang Y, Alpaugh RK, Somer R, Greenspon D, Weiner LM. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. The Journal of biological chemistry. 2004;279:53907–14. doi: 10.1074/jbc.M407888200. [DOI] [PubMed] [Google Scholar]
- 32.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical microbiology reviews. 1997;10:505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. The Journal of infectious diseases. 2006;193:172–9. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 34.Hillson JL, Karr NS, Oppliger IR, Mannik M, Sasso EH. The structural basis of germline-encoded VH3 immunoglobulin binding to staphylococcal protein A. J Exp Med. 1993;178:331–6. doi: 10.1084/jem.178.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristiansen SV, Pascual V, Lipsky PE. Staphylococcal protein A induces biased production of Ig by VH3-expressing B lymphocytes. Journal of immunology. 1994;153:2974–82. [PubMed] [Google Scholar]
- 36.Kozlowski LM, Kunning SR, Zheng Y, Wheatley LM, Levinson AI. Staphylococcus aureus Cowan I-induced human immunoglobulin responses: preferential IgM rheumatoid factor production and VH3 mRNA expression by protein A-binding B cells. Journal of clinical immunology. 1995;15:145–51. doi: 10.1007/BF01543106. [DOI] [PubMed] [Google Scholar]
- 37.Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5399–404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansson B, Uhlen M, Nygren PA. All individual domains of staphylococcal protein A show Fab binding. FEMS immunology and medical microbiology. 1998;20:69–78. doi: 10.1111/j.1574-695X.1998.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 39.Gomez MI, O’Seaghdha M, Magargee M, Foster TJ, Prince AS. Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. The Journal of biological chemistry. 2006;281:20190–6. doi: 10.1074/jbc.M601956200. [DOI] [PubMed] [Google Scholar]
- 40.Gomez MI, Seaghdha MO, Prince AS. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. The EMBO journal. 2007;26:701–9. doi: 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Z, Zhang G, Zhou Z, Bembas K, Drebin JA, Greene MI, et al. Differential binding patterns of monoclonal antibody 2C4 to the ErbB3-p185her2/neu and the EGFR-p185her2/neu complexes. Oncogene. 2008;27:3870–4. doi: 10.1038/onc.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutt M, Farber-Schwarz A, Unverdorben F, Richter F, Kontermann RE. Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains. The Journal of biological chemistry. 2012;287:4462–9. doi: 10.1074/jbc.M111.311522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G. Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1272–7. doi: 10.1073/pnas.1014739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starovasnik MA, Braisted AC, Wells JA. Structural mimicry of a native protein by a minimized binding domain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10080–5. doi: 10.1073/pnas.94.19.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.