Abstract
OBJECTIVE
Hydrogen inhalation was neuroprotective in several brain injury models. Its mechanisms are believed to be related to anti-oxidative stress. We investigated the potential neurovascular protective effect of hydrogen inhalation especially effect on mast cell activation in a mouse model of intracerebral hemorrhage (ICH).
DESIGN
Controlled in vivo laboratory study.
SETTING
Animal research laboratory
SUBJECTS
171, 8 weeks old male CD-1 mice were used.
INTERVENTIONS
Collagenase-induced ICH model in 8 weeks old, male, CD-1 mice was used. Hydrogen was administrated via spontaneous inhalation. The blood-brain barrier (BBB) permeability and neurological deficits were investigated at 24 and 72 hours after ICH. Mast cell activation was evaluated by Western blot and immuno-staining. The effects of hydrogen inhalation on mast cell activation were confirmed in an autologous blood injection model ICH.
MEASURMENT AND MAIN RESULTS
At 24 and 72 hours post-ICH, animals showed BBB disruption, brain edema, neurological deficits, accompanied with phosphorylation of Lyn kinase and release of tryptase, indicating mast cell activation. Hydrogen treatment diminished phosphorylation of Lyn kinase and release of tryptase, decreased accumulation and degranulation of mast cells, attenuated BBB disruption and improved neurobehavioral function.
CONCLUSION
Activation of mast cells following ICH contributed to increase of BBB permeability and brain edema. Hydrogen inhalation preserved BBB disruption by prevention of mast cell activation after ICH.
Keywords: Intracerebral hemorrhage, brain edema, mast cells, hydrogen, ROS, neuroprotection
INTRODUCTION
Intracerebral hemorrhage (ICH) accounts for 10–15% of strokes with a mortality rate greater than 40%.Many survivors are permanently disabled(1). In experimental ICH animal models, neurological deficits were associated with an increase in production of reactive oxygen species (ROS) and reactive nitrogen species (RNS)(2), which damage the BBB and increase brain edema(3, 4). Hydrogen therapy neutralized ROS and diminished oxidative stress in several brain injury models(5–7). However, the mechanism underlying the beneficial effects of hydrogen therapy remain poorly understood.
Mast cells are resident inflammatory cells that are present in various tissues including the central nervous system(8). They contain granules with vasoactive substances such as histamine, proteolytic enzymes (tryptase), and anticoagulants (heparin). Mast cell activation and degranulation, which contribute to BBB disruption and increased bleeding(8) following brain injury, have been observed in several experimental models. In an autologous blood injection ICH model, mast cell stabilization reduced brain edema and improved neurological deficits(9). Similarly, mast-cell deficient animals had shown significantly less brain edema than wild type after ICH(9). Mast cell activation was accompanied by increased ROS(10) and RNS(11) production. Hydrogen suppressed FcεR-mediated signal transduction and prevented degranulation of mast cells (12).
Interestingly, activation of mast cells was accompanied by increased production of ROS(10) and RNS(11), and hydrogen suppressed FcεR-mediated signal transduction and prevented degranulation of mast cells (12). Therefore, we tested the hypothesis that firstly ICH produced mast cell activation by increased phosphorylation of Lyn kinase in the brain tissues. Activated mast cells degranulated, enhanced tryptase release, and augmented redox stress. Secondly, hydrogen inhalation diminished the phosphorylation of Lyn, which in turn decreased mast cell activation and redox stress. Thirdly, hydrogen inhalation preserved BBB integrity, decreased brain edema, and ameliorated neurological deficits.
MATERIAL AND METHODS
Experimental animals
All procedures and methods for these studies were approved by the Animal Care and Use Committee at Loma Linda University and complied with the Guide for the Care and Use of Laboratory Animals (animals protocol number 8100024). A total of 171 male CD-1, 8 weeks old mice were used.
Animals group
Details were listed in Supplementary Text (S.Text).
Intracerebral hemorrhage induction
Experimental ICH was induced by intrastriatal injection of either bacterial collagenase or autologous blood as we previously described (13, 14). Details were listed in S.Text.
Hydrogen inhalation treatment
One hour after operation, animals were placed in chamber connected to a hydrogen gas tank. The hydrogen treatment chamber was continually flushed with gas mix 2.9% hydrogen and 21% oxygen balanced with nitrogen, flow rate 8 l/minute. Hydrogen concentration was controlled at outlet using a Hydrogen-meter (H2 Scan, Valencia, CA, USA). Chamber was warmed by using warming path (37°C). No hydrogen toxicity was observed.
Vehicle (medical air inhalation) and sham (needle trauma, medical air inhalation) animals were placed in a chamber. Chamber was warmed and continually flushed with medical air (flow rate 8 l/minute).
3-amino-1,2,4-triazole treatment
3-amino-1,2,4-triazole (AT) was purchased by Sigma Aldrich (St. Louis, MO, USA). AT was dissolved in saline and administrate intraperitoneally five hours before ICH induction.
Evaluation of brain edema and neurological deficits
The brain water content and neurological deficits were evaluated as previously described (13). Details were listed in S.Text.
Evaluation of BBB permeability (Evans Blue Assay) and hematoma volume (hemoglobin assay)
BBB permeability and hematoma volume were evaluated as previously described (15) Details were listed in S.Text.
Western blot analysis
Mice were perfused transcardially with 40 ml of cold PBS. Hemispheres were isolated and stored at −80°C until analysis. Protein extraction and Western blots were performed according to the manufacture recommendation as described before(16). Details were listed in S.Text.
Histological study
Details were listed in S.Text.
Immunohistochemistry study
Immunohistochemistry was performed as described previously(17) using the following primary antibodies: rabbit anti-tryptase 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Evaluation of hydrogen peroxide production
For evaluation of hydrogen peroxide (H2O2), commercial available Amplex® Red Hydrogen Peroxide Assay Kit (Life Technologies, NY, USA) was used. Details were listed in S.Text.
Statistical analysis
Quantitative data are expressed as the mean ± SEM. Analysis were performed by independent statistician. Statistical significance was verified by analysis of variance (ANOVA) (Tukey test). P < 0.05 was considered statistically significant.
RESULTS
Effect of Hydrogen Inhalation on Brain Edema and Neurological Deficits at 24 hrs and 72 hrs
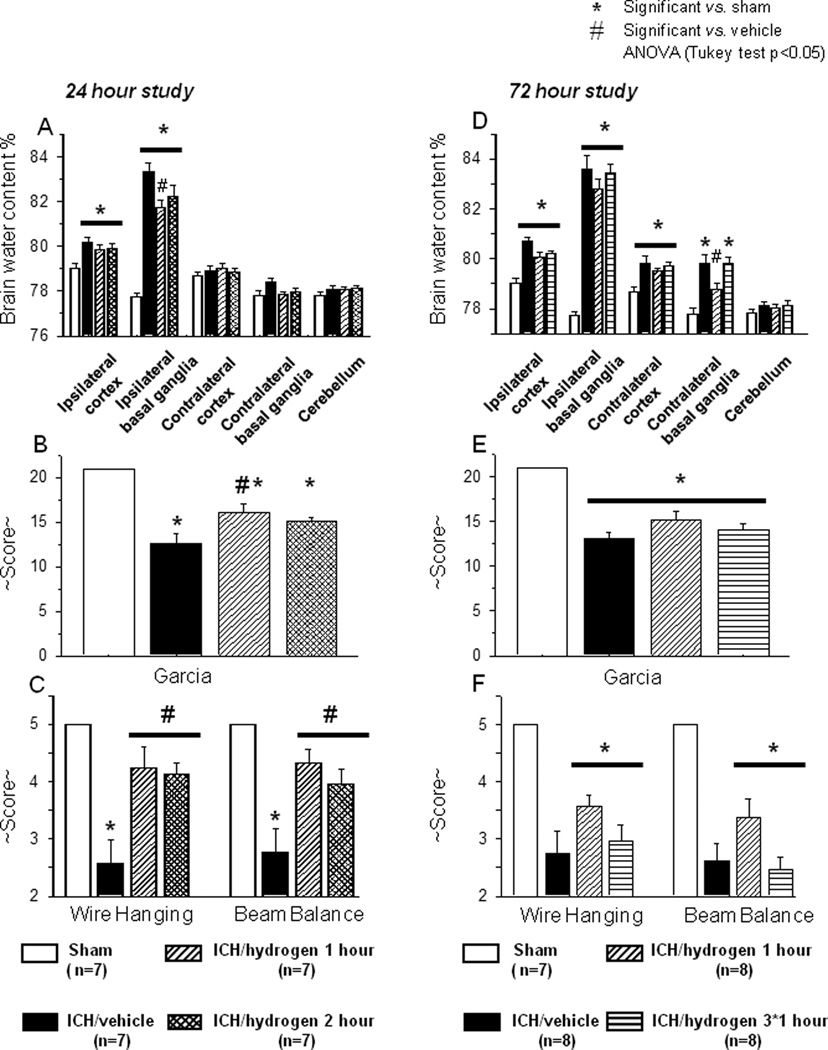
Animals were divided into sham operated and ICH groups. ICH animals were subsequently divided into vehicle, one, and two hours of hydrogen inhalation therapy. At 24 hrs post-ICH, collagenase treated animals had significantly increased edema in the ipsilateral basal ganglia than sham operated animals (83.34+/−0.38 vs. 77.75+/−0.15, p<0.05, Fig. 1A). One hour of hydrogen inhalation treatment significantly decreased brain water content vs. medical-air treated animals (81.76+/−0.31 vs. 83.34+/−0.38, p<0.05), while two hours of hydrogen inhalation treatment tended to decrease edema (82.25+/−0.48 vs. 83.34+/−0.38, p=0.16 Fig. 1A).
FIGURE 1.
(A) Intracerebral hemorrhage induced a significant increase of brain water content in ipsilateral basal ganglia and cortex ( N=7) when compared to sham operated (
N=7) when compared to sham operated ( N=7) animals. One hour of hydrogen inhalation starting one hour after inducing of intracerebral hemorrhage (
N=7) animals. One hour of hydrogen inhalation starting one hour after inducing of intracerebral hemorrhage ( N=7) significantly diminished brain edema. Two hours of hydrogen inhalation starting one hour after inducing of intracerebral hemorrhage (
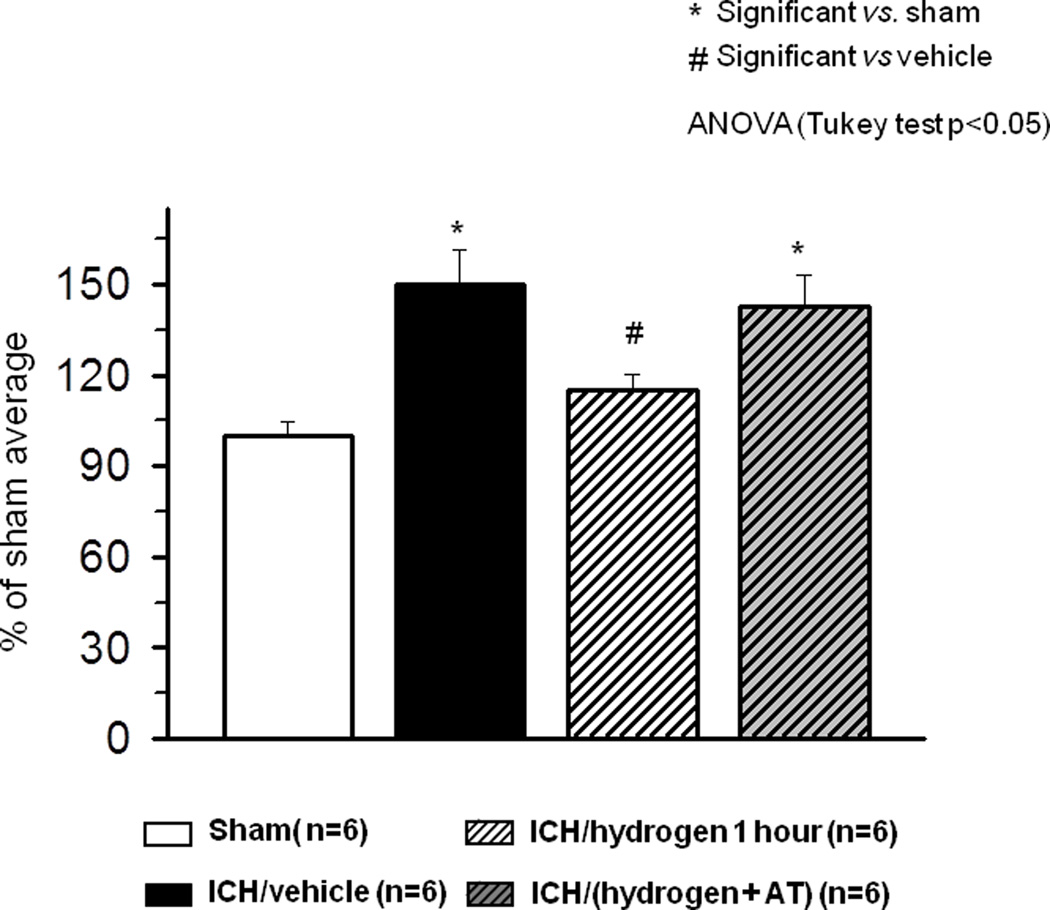
N=7) significantly diminished brain edema. Two hours of hydrogen inhalation starting one hour after inducing of intracerebral hemorrhage ( N=7) showed a strong tendency to ameliorate intracerebral hemorrhage induced increase of brain water content. (B–C) All animals after intracerebral hemorrhage demonstrated significant neurological deficit according to performance on the modified Garcia (B), wire hanging and beam balance (C) tests. One hour of hydrogen treatment ameliorated neurological deficits in all three tests. Two hours of hydrogen treatment significantly decreased the neurological deficits evaluated by wire hanging and beam balance tests and showed a tendency to improvement in the Garcia test (B–C). * Significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
N=7) showed a strong tendency to ameliorate intracerebral hemorrhage induced increase of brain water content. (B–C) All animals after intracerebral hemorrhage demonstrated significant neurological deficit according to performance on the modified Garcia (B), wire hanging and beam balance (C) tests. One hour of hydrogen treatment ameliorated neurological deficits in all three tests. Two hours of hydrogen treatment significantly decreased the neurological deficits evaluated by wire hanging and beam balance tests and showed a tendency to improvement in the Garcia test (B–C). * Significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
At 72 hours after inducing of intracerebral hemorrhage, brain water content in ipsilateral basal ganglia and cortex of ICH ( N=8) remained elevated compared to that of sham operated (
N=8) remained elevated compared to that of sham operated ( N=7) animals. Both a single one hour treatment with hydrogen starting one hour after inducing of intracerebral hemorrhage (
N=7) animals. Both a single one hour treatment with hydrogen starting one hour after inducing of intracerebral hemorrhage ( N=8) and multiple treatments (one hour daily for 3 days
N=8) and multiple treatments (one hour daily for 3 days  N=8) showed a tendency to decrease brain water content (D) and to ameliorate neurological deficits (E-F). * significant vs. sham, p<0.05 ANOVA, Tukey test.
N=8) showed a tendency to decrease brain water content (D) and to ameliorate neurological deficits (E-F). * significant vs. sham, p<0.05 ANOVA, Tukey test.
Statistically significant neurological deficits were evident in all ICH vs. sham-animals at 23 hours after collagenase injection as tested by the modified Garcia test (Fig. 1B), wire hang, and beam balance tests (Fig. 1C). A statistically significant improvement was seen in all three neurobehavioral assessments after one hour of hydrogen inhalation. Two hours of hydrogen inhalation led to statistically significant neurofunctional improvement in the wire hang and beam balance tests and showed a tendency to ameliorate neurological deficits in the Garcia Test.
Since one hour of hydrogen inhalation was effective at 24 hours after ICH, the effect of a single hydrogen treatment (starting one hour after ICH) was compared with multiple treatments (one hour duration daily for 72 hours) and evaluated at 72 hours after ICH. At 72 hours post-ICH, brain water content remained significantly increased in the ipsilateral basal ganglia of collagenase treated animals vs. sham (83.60+/−0.56 vs. 77.75+/−0.15, p<0.05, Fig. 1D). Single hydrogen treatment tended to decrease brain water content (82.79+/−0.404 vs. 83.60+/−0.56, p=0.49). Statistically significant neurological deficits were observed in all ICH vs. sham animals (Fig1 E–F). Single hydrogen treatment tended to ameliorate neurological deficits at 72 hrs after ICH. Multiple hydrogen treatments were ineffective.
Effect of Hydrogen on the Hematoma Volume-hemoglobin assay
One hour of hydrogen inhalation had no effect on hemorrhage volume at 24 or 72 hours (data not shown).
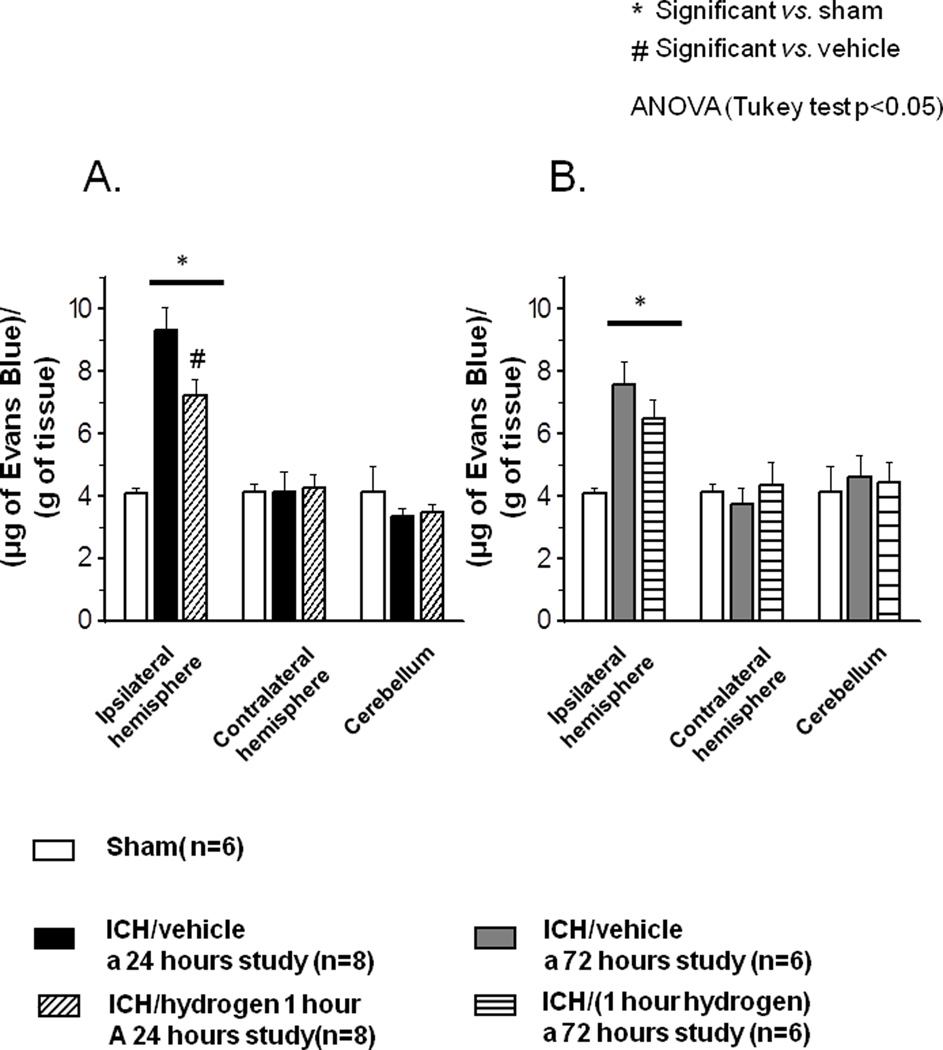
Effect of Hydrogen on Blood-Brain Barrier permeability-Evans Blue assay
Significant accumulation of Evans Blue stain was observed in the ipsilateral hemisphere of ICH compared to sham animals (9.11+/−0.69 vs. 4.09+/−0.14 µg of Evans Blue/g of tissue, p<0.05, Fig 2A). One hour of hydrogen inhalation significantly decreased amount of the stain in the ipsilateral hemisphere of hydrogen-treated compared to vehicle at 24 hour (7.20+/−0.45 vs. 9.11+/−0.69, p<0.05, Fig 2A). However, one hour of hydrogen treatment demonstrated a non significant tendency to preserve BBB at 72 hour (7.55+/−0.76 vs. 6.50+/−0.6, p=0.30, Fig. 2B).
FIGURE 2.
(A) Intracerebral hemorrhage caused significant disruption of blood-brain barrier at 24 hours ( sham operated animals N=6,
sham operated animals N=6,  vehicle-treated animals N=8). One hour of hydrogen therapy (
vehicle-treated animals N=8). One hour of hydrogen therapy ( N=8) significantly reduced intracerebral hemorrhage induced disruption of blood-brain barrier at 24 hrs. (B) One hour hydrogen therapy showed a tendency to preserve intracerebral hemorrhage induced disruption of blood-brain barrier at 72 hours (
N=8) significantly reduced intracerebral hemorrhage induced disruption of blood-brain barrier at 24 hrs. (B) One hour hydrogen therapy showed a tendency to preserve intracerebral hemorrhage induced disruption of blood-brain barrier at 72 hours ( Sham operated animals N=6,
Sham operated animals N=6,  vehicle treated animals N=6,
vehicle treated animals N=6,  hydrogen treated animals N=6). * significant vs. sham, p<0.05 ANOVA, Tukey test.
hydrogen treated animals N=6). * significant vs. sham, p<0.05 ANOVA, Tukey test.
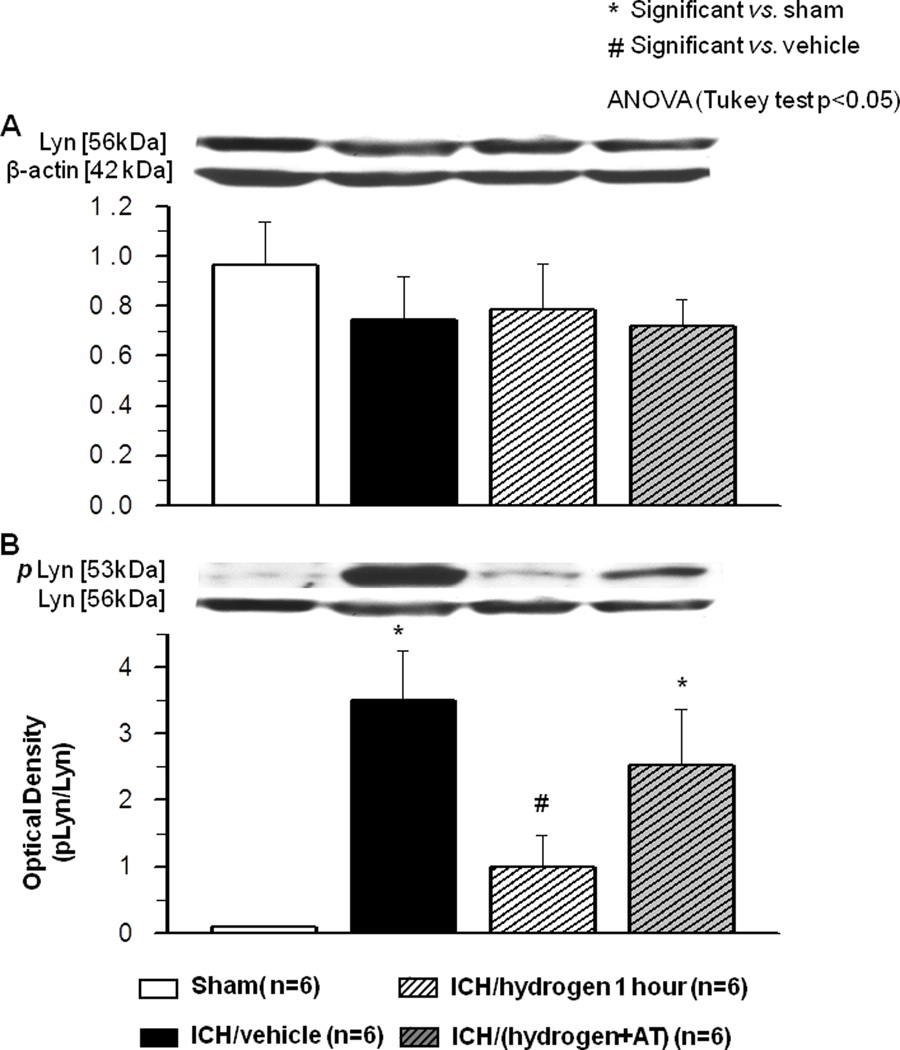
Effect of ICH and Hydrogen on LYN kinase expression and phosphorylation
Lyn expression and phosphorylation was evaluated 6 hours after ICH in both hydrogen treated and untreated groups. Neither ICH nor hydrogen had an effect on LYN kinase expression (Fig. 3A). However, ICH significantly induced Lyn phosphorylation in brain of ICH compared to sham animals. Hydrogen inhalation significantly reduced ICH induced Lyn phosphorylation (Fig. 3B). Catalase inhibition had no effect on Lyn expression (Fig 3A, last column), but increased Lyn phosphorylation (Fig. 3B, last column).
FIGURE 3.
(A) Neither intracerebral hemorrhage nor hydrogen treatment effected expression of Lyn kinase at 6 hours after inducing of intracerebral hemorrhage. (B) Intracerebral hemorrhage significantly increased phosphorylation of Lyn kinase at 6 hours after inducing of intracerebral hemorrhage ( Sham operated animals N=6,
Sham operated animals N=6,  Vehicle N=6). One hour of hydrogen treatment (
Vehicle N=6). One hour of hydrogen treatment ( N=6) significant diminished Lyn kinase phosphorylation when compared to intracerebral hemorrhage animals treated with vehicle. Pretreatment with catalase inhibitor at three hours before intracerebral hemorrhage (
N=6) significant diminished Lyn kinase phosphorylation when compared to intracerebral hemorrhage animals treated with vehicle. Pretreatment with catalase inhibitor at three hours before intracerebral hemorrhage ( N=6) prevented effect of hydrogen on Lyn kinase phosphorylation. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
N=6) prevented effect of hydrogen on Lyn kinase phosphorylation. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
Effect of ICH and Hydrogen Inhalation on the Release of Mast Cells Specific Proteins, Mast Cells Accumulation and Degranulation
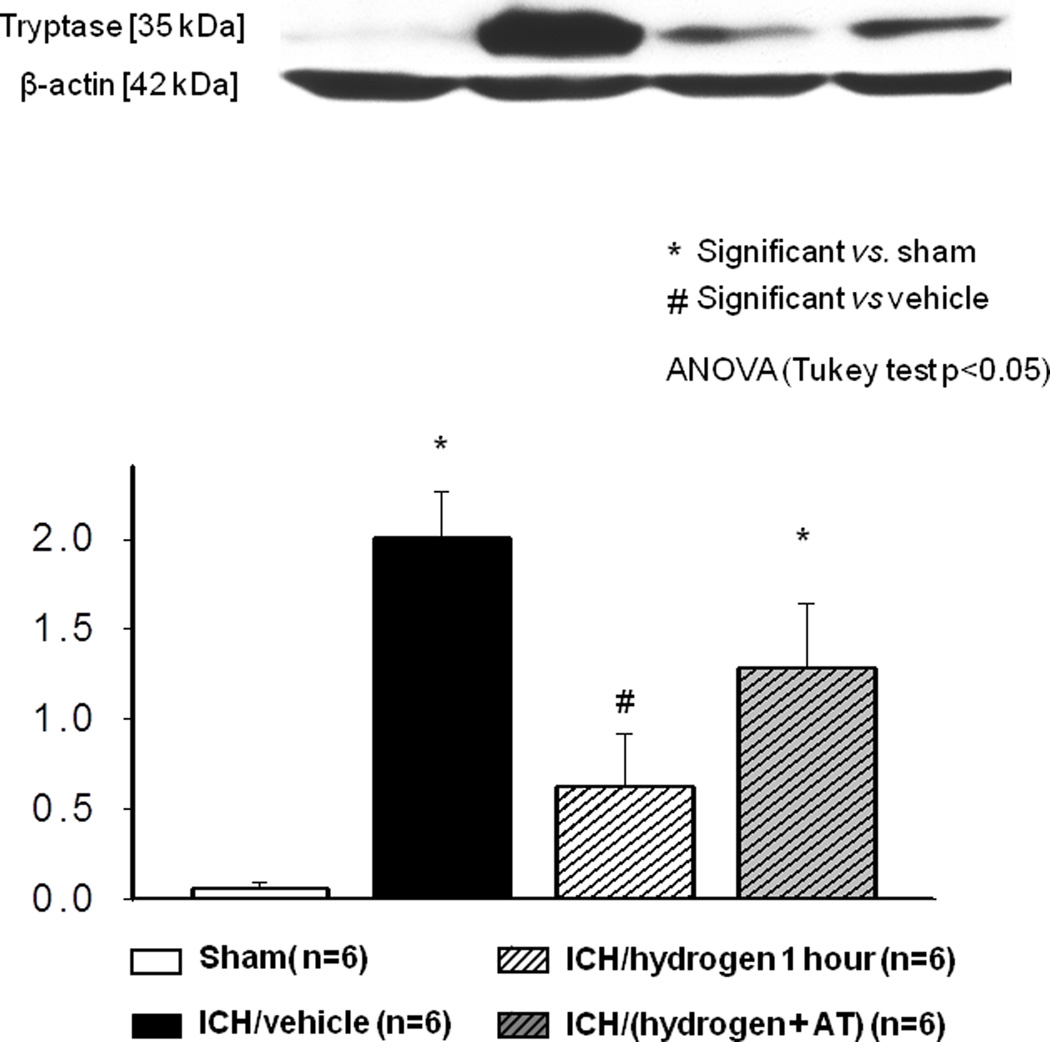
Significant tryptase release was observed six hours after ICH by Western blot analysis. One hour of hydrogen treatment significantly decreased tryptase release (Fig. 4). Catalase inhibition reversed effect of hydrogen on tryptase release (Fig. 4, last column). Similarly, ICH increased amount of tryptase positive cells n the brains of ICH compared to shams animals, visualized by immunostaining against tryptase. Hydrogen inhalation ameliorated this effect (Fig 5).
FIGURE 4.
Intracerebral hemorrhage increased the release of tryptase at 6 hours after induction of intracerebral hemorrhage ( Sham operated animals N=6,
Sham operated animals N=6,  (Vehicle N=6). One hour of hydrogen treatment (
(Vehicle N=6). One hour of hydrogen treatment ( N=6) significant diminished intracerebral hemorrhage induced release of tryptase. Pretreatment with catalase inhibitor at three hours before induction of intracerebral hemorrhage (
N=6) significant diminished intracerebral hemorrhage induced release of tryptase. Pretreatment with catalase inhibitor at three hours before induction of intracerebral hemorrhage ( N=6) reversed effect of hydrogen on the release of tryptase. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
N=6) reversed effect of hydrogen on the release of tryptase. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
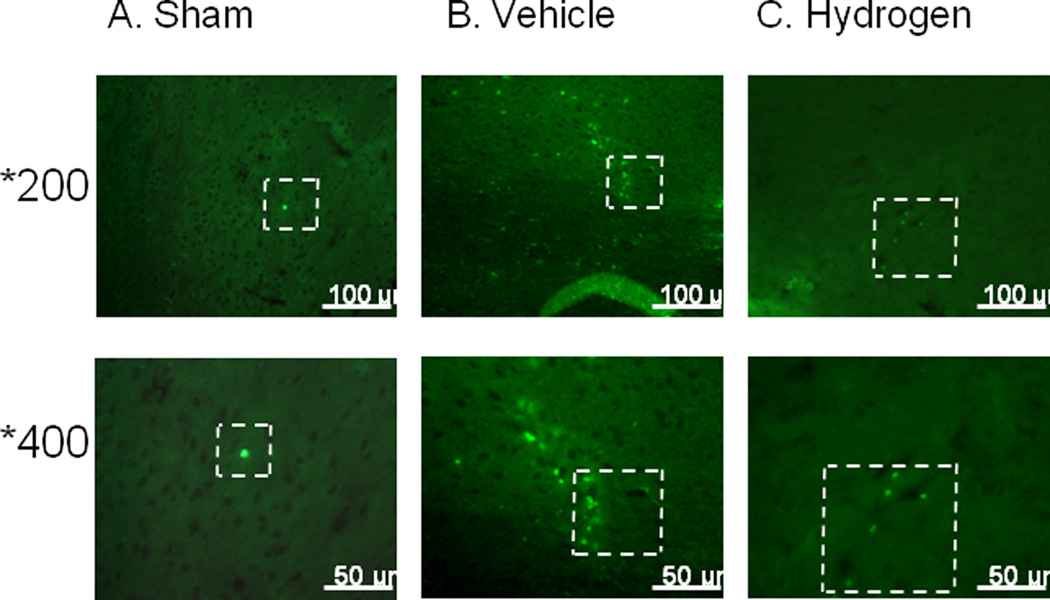
FIGURE 5.
6 hours after intracerebral hemorrhage induction, there were more tryptase positive cells in brain of vehicle (B) animals compared to sham-operated animals (A). One hour of hydrogen treatment (C) diminished number of tryptase positive cells.
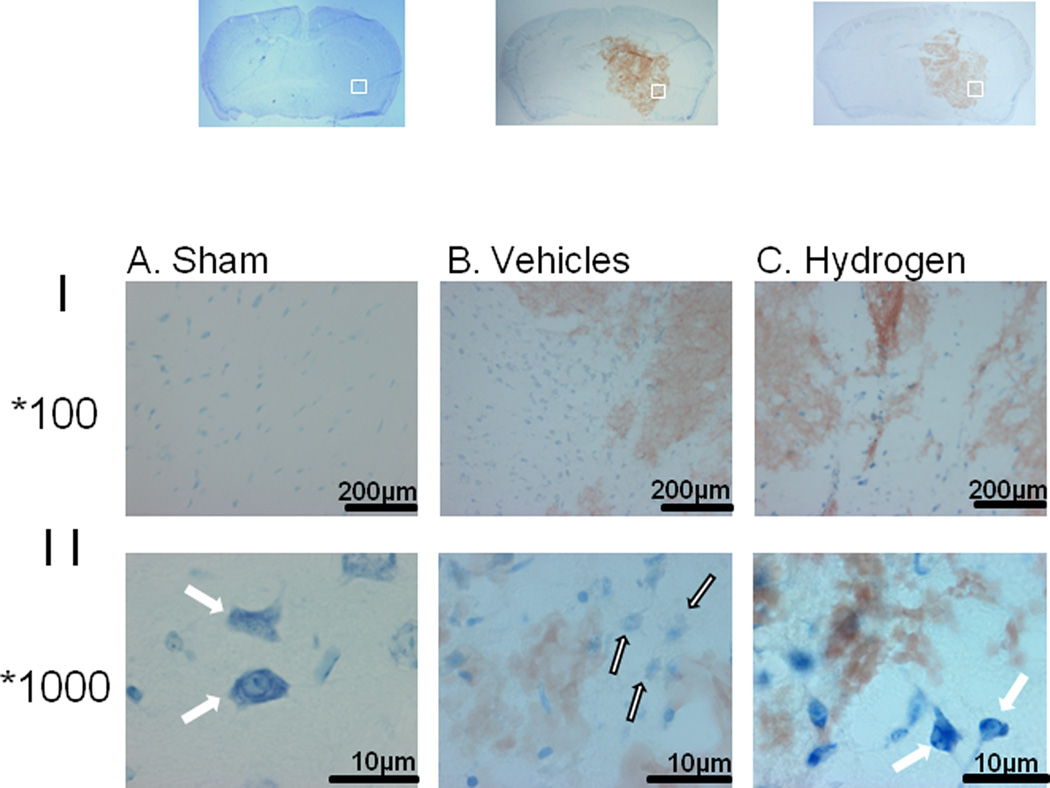
Additionally, ICH increased mast cell accumulation at 6 hours after ICH, evaluated by Toluidine Blue (Fig 6). There were more Toluidine Blue positive cells in peri-hematomal region of ICH compared to the same brain region of sham-operated animals (Fig 6B compared to 6A). Hydrogen inhalation decreased the number of Toludine Blue positive cells (Fig 6C). Moreover most of the mast cells detected in brain of ICH animals ICH showed the clear signs of degranulation with less intensive Toluidine Blue staining compared to granulated intensive stained cells in brain of sham operated animals (Fig 6A, white arrow). Mast cells after ICH had the appearance of “ghost” cells (Fig 6B, white arrow, black outline). Hydrogen inhalation decreased the number of such cells and increased the amount of granulated mast cells (Fig 6C, white arrow).
FIGURE 6.
Effect of intracerebral hemorrhage and hydrogen treatment on mast cells proliferation and activation was investigated by Toluidine Blue staining at 6 hours after inducing of intracerebral hemorrhage. More mast cells were observed in brain of vehicle animals (Panel I-B) compared to sham-operated animals (Panel I-A). One hour of hydrogen treatment reduced the number of mast cells (Panel I-C). Additionally if compared to granulated intensive stained cells in brain of sham operated animals (Panel II-A white arrow), most of the mast cells detected in brain of animals after intracerebral hemorrhage showed the clear signs of degranulation with less intensive Toluidine Blue staining and the appearance of `ghost' cells (Panel II-B white arrow, black outline). Hydrogen inhalation decreased the number of such cells and increased the amount of granulated mast cells (Fig 7C, white arrow).
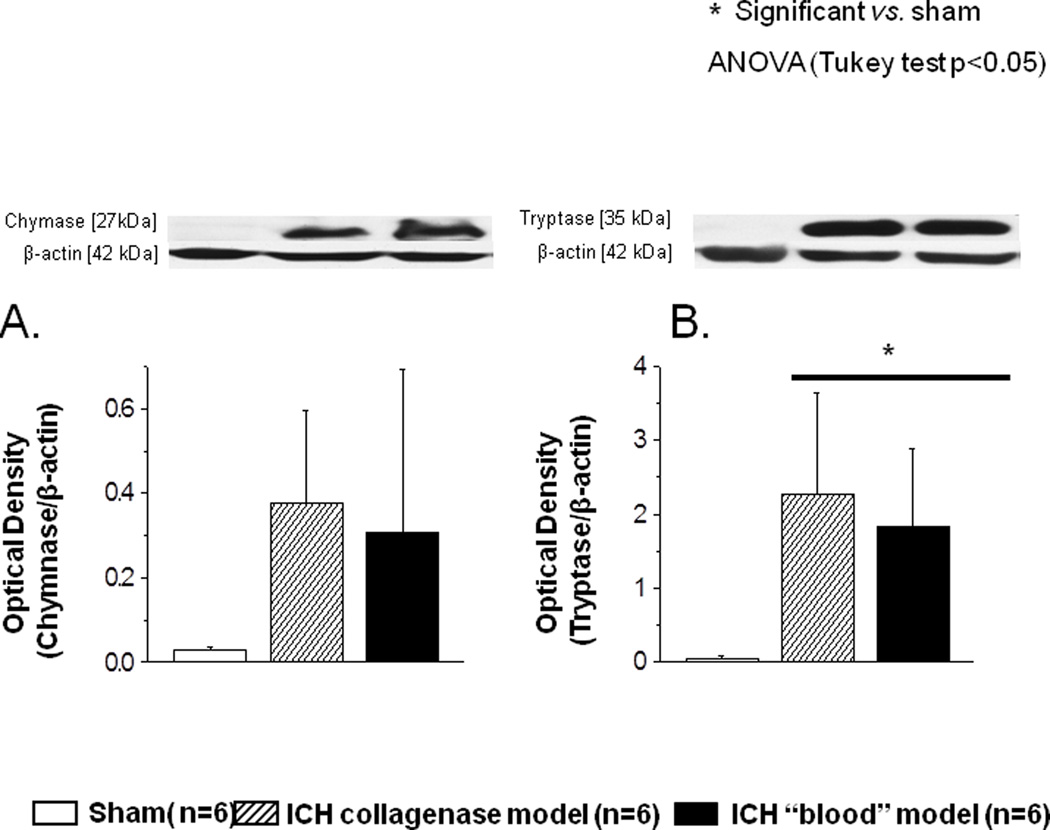
We also tested if the effect on mast cell activation was caused by collagenase or by collagenase-induced intracerebral bleeding. To this end we compared collagenase model with direct injection of autologus blood into the basal ganglia. Hematoma caused by collagenase or autologous blood injection induced the release the proteins tryptase and chymase by mast cells. This release was evaluated by Western blot at 6 hours after ICH and demonstrated no significant difference between the two models (Fig 7).
FIGURE 7.
Collagenase-induced intracerebral hemorrhage and intracerebral hemorrhage induced by direct injection of autologous blood demonstrated comparable release of mast cell proteins. No differences between these two models were detected. * significant vs. sham, p<0.05 ANOVA, Tukey test.
Effect of ICH and Hydrogen Inhalation on the H2O2. Production
We evaluated the effect of hydrogen inhalation on H2O2 production after ICH. ICH significantly increased H2O2 production as early as two hours after ICH insult. One hour of hydrogen inhalation significantly decreased ICH-induced H2O2 production (Fig 8). Catalase inhibition four hours before hydrogen inhalation resulted in H2O2 production augmentation compared to hydrogen treated animals (Fig 8, last column).
FIGURE 8.
Intracerebral hemorrhage ( Vehicle N=6) significantly increased the production of hydrogen peroxide (H2O2) evaluated by Amplex Red peroxidase assay at 2 hours after ICH, when compared to sham operated animals (
Vehicle N=6) significantly increased the production of hydrogen peroxide (H2O2) evaluated by Amplex Red peroxidase assay at 2 hours after ICH, when compared to sham operated animals ( Sham N=6). One hour of hydrogen treatment significantly diminished ICH-induced production of H2O2 (
Sham N=6). One hour of hydrogen treatment significantly diminished ICH-induced production of H2O2 ( N=6). Pretreatment with catalase inhibitor at three hours before inducing of intracerebral hemorrhage (
N=6). Pretreatment with catalase inhibitor at three hours before inducing of intracerebral hemorrhage ( N=6) reversed effect of hydrogen on the H2O2 production. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
N=6) reversed effect of hydrogen on the H2O2 production. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test.
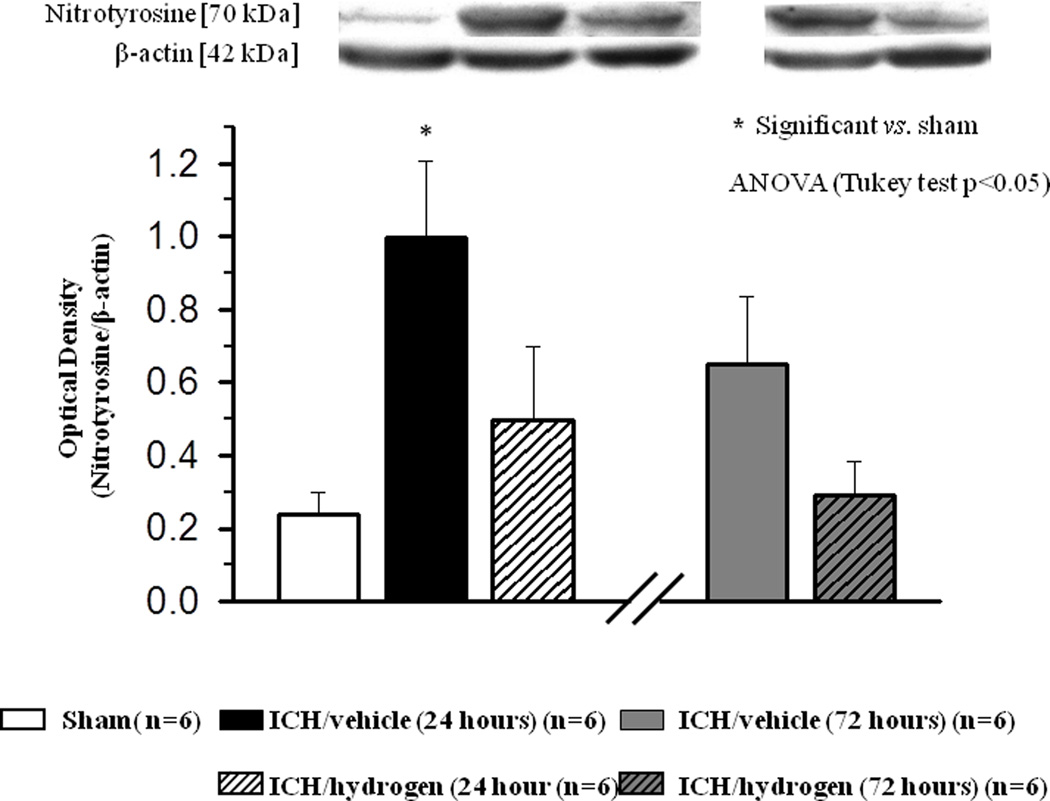
Effect of Hydrogen on Nitrotyrosine Accumulation
Western blot examination demonstrated a significant increase of nitrotyrosine in brain tissue of ICH mice at 24 hour. One hour of hydrogen inhalation reduced ICH-induced accumulation of nitrotyrosine. The hydrogen inhalation tended to decrease nitrotyrosine accumulation at 72 hour (Fig 9).
FIGURE 9.
At 24 hours after inducing of intracerebral hemorrhage, significant accumulation of nitrotyrosine was observed in brain of animals after intracerebral hemorrhage ( N=6) compared to sham operated animals (
N=6) compared to sham operated animals ( N=6). Hydrogen inhalation decreased intracerebral hemorrhage induced accumulation of nitrotyrosine at 24 hours (
N=6). Hydrogen inhalation decreased intracerebral hemorrhage induced accumulation of nitrotyrosine at 24 hours ( ). At 72 hour after inducing of intracerebral hemorrhage, hydrogen treatment showed a strong tendency to diminish nitrotyrosine accumulation in brain of hydrogen treated (
). At 72 hour after inducing of intracerebral hemorrhage, hydrogen treatment showed a strong tendency to diminish nitrotyrosine accumulation in brain of hydrogen treated ( N=6) compared to untreated (
N=6) compared to untreated ( N=6) animals. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test
N=6) animals. * significant vs. sham, # significant vs. vehicle, p<0.05 ANOVA, Tukey test
Effect of Catalase Inhibitor, 3-amino-1,2,4-triazole on the Effects Induced by Hydrogen Treatment
Inhibition of catalase four hours before hydrogen inhalation resulted in H2O2 production enhancement compared to hydrogen treated animals (Fig 8, last column). The enhance of H2O2 production had no effect on Lyn expression (Fig 3A, last column) but resulted in increase of Lyn phosphorylation (Fig 3B, last column) and reversed effect of hydrogen on tryptase release (Fig 4, last column).
DISCUSSION
We investigated hydrogen inhalation therapy’s ability to ameliorate secondary brain injury after ICH in mice. ICH induced mast cell accumulation and degranulation, which increased tryptase release and redox stress in the brain. Those molecular changes were accompanied with BBB disruption, brain edema, and neurological deficits. Hydrogen inhalation diminished ICH-induced mast cell activation, which reduced redox stress, enhanced BBB preservation, and diminished neurological deficits. This is the first study that investigated hydrogen inhalation therapy’s effects on brain mast cell activation and brain injury after ICH.
Hydrogen selectively scavenges hydroxyl radicals (7), which are the dominant reactive oxygen species causing DNA fragmentation, lipid oxidation, and protein nitrification. Several hemorrhagic stroke models investigated the neuroprotective effects of hydrogen therapy (5, 18, 19). Those studies demonstrated that hydrogen therapy reduced ROS levels and postulated that its beneficial effects are linked to its anti-oxidative properties. However, pathways leading to diminished oxidative stress following hydrogen treatment remain to be elucidated. After ICH, ROS causes peroxidation of lipid-rich structures of BBB, resulting in life-threatening BBB disruption and vasogenic brain edema (20–22). It has been recently established that hydrogen, along with its anti-oxidant property, is a biologically active, gaseous signaling molecule(12). Iton et al. demonstrated that hydrogen treatment attenuated mast cell activation (23).
Mast cells are resident cells of several types of tissues including central nervous tissue. They are located perivascularly close to neurons (24). Mast cells include granules that contain substances such as histamine, heparin, tryptase, and TNF-α (25). Release of these substances may increase initial bleeding after ICH and contribute to ICH-induced BBB disruption. Mast cell activation is mediated by the FceRI receptor (26). FceRI receptor stimulation results in downstream phosphorylation of Lyn (27), a Src family kinase that regulates mast cell activation (23). Itoh et al. demonstrated that hydrogen had no effect on FceRI expression, but hydrogen compromised the initial steps of mast cell activation by reducing Lyn phosphorylation (12). In the present study, we used a mixture of oxygen (21%), hydrogen (2.9%), and nitrogen, which is inflammable and safe to use. The neuroprotective effects of this hydrogen concentration after brain injury was demonstrated by others (15, 19, 28).
We observed that hydrogen inhalation ameliorated ICH-induced increase of brain water content and neurological deficits at 24 hours. We were unable to demonstrate that hydrogen inhalation ameliorated ICH-induced brain injury and neurological deficits at 72 hours even though a tendency was observed. Additionally to brain water content, we evaluated BBB integrity by measurement of Evan Blue(13, 15, 16, 29). We observed that hydrogen inhalation preserved BBB integrity at 24 hours after ICH and showed a tendency to protect BBB at 72 hours after ICH.
Mast cell activation after brain injury was observed in several experimental animal studies (30–33). Espositio at al. reported that acute stress activated mast cells in the brains of animals without brain injury (34). Acute stress increased BBB permeability in wild-type but not in mast cell deficient animals. Mast cell stabilization ameliorated stress-induced BBB disruption (34). These results indicated that mast cells are critical in increasing BBB permeability. In an ICH model, Strbian et al. demonstrated that mast cell degranulation inhibition by cromoglycate significantly decreased hematoma volume calculated from T2 MRI images at 24 hours post-ICH (9). Furthermore, hematoma volume was significantly decreased in mast-cell deficient compared to wild type animals. Mast cell stabilization decreased brain swelling, although mast cell activation was not studied directly and cromoglycate was used before ICH induction (9). In the present study, we showed that ICH increased mast cell activation. The mast cell activation was evaluated as a ratio of phosphorylated Lyn divided by unphosphorylated Lyn as well as by the release of mast cell specific protein tryptase in the animals’ brains (12, 35). We observed that ICH significantly increased LYN phosphorylation and the release of tryptase in the brains of ICH compared to sham animals.
We employed the collagenase model of ICH l in most experiments. One limitation of the modelis the inherent inflammatory reaction caused by collagenase. To rule out collagenase as a factor in mast cell activation we employed the autologous blood-injection ICH model and compared the release mast cell specific proteins, between both models. We did not observe a significant difference in the release and postulated that ICH was the causative factor in mast cell activation. Therefore, it was likely that hydrogen inhalation decreased ICH-induced up-regulation of tryptase, as shown by Western blot and immunostaining of tryptase positive cells. Additionally, hydrogen inhalation decreased ICH-induced mast cell degranulation by Toluidine Blue staining, as described previously (36). In the brain of sham operated and hydrogen treated ICH animals, mast cells could be identified by their metachromatic cytoplasmatic granules, whereas in ICH untreated animals most mast cells lost some of the stain and had a "ghost" shape. Moreover, more mast cells were present in the brains of ICH-untreated animals when compared to sham-operated and ICH hydrogen-treated animals. This was in agreement with the other study demonstrating that mast cell stabilization has a beneficial effect after ICH (9).
It has been established that Lyn-mediated signaling pathway activation enhanced NADPH oxidase activity in mast cells (37). NADPH oxidase generates superoxide and is considered a main source of reactive oxygen species in the blood vessels (38, 39). By the use of knock out animals, we previously demonstrated that oxidative stress resulted from NADPH oxidase activation and significantly contributed to ICH induced brain injury (40). Hydrogen attenuated NADPH oxidase activity and consequently reduced H2O2 production (12). We evaluated H202 production by Amplex® Red peroxidase assay as described previously (41, 42). Hydrogen inhalation significantly reduced ICH-induced H2O2 production. Furthermore there is evidence that rodents’ mast cells produce NO (43). Although NO is a weak oxidant, combinations of various NO redox forms induce severe BBB damage (44). Nitrotyrosine, a product of tyrosine nitration, is a common NO-induced cell damage indicator. Nitrotyrosine accumulation after brain injury was reported by others and is regarded as redox stress marker (5, 19, 45). We observed considerable nitrotyrosin accumulation in the brains of ICH-animals that became significantly reduced in hydrogen-treated animals. These findings were in concurrence with prior studies by our laboratory that demonstrated hydrogen inhalation’s anti-oxidant effects in other hemorrhagic stroke models (5, 19).
Because oxidative stress leads to BBB disruption, we investigated the effect of hydrogen inhalation on BBB integrity by measuring brain water content and analysis by Evans Blue assay. We demonstrated that decreased mast cell activation via hydrogen inhalation resulted in BBB preservation. This observation is similar to that of Strbian et al. who demonstrated that pretreatment with a mast cell degranulation inhibitor ameliorated BBB disruption after ICH (9). It is also similar to Zhan et al. (2011), who demonstrated that hydrogen had a protective effect on BBB after subarachnoid hemorrhage (19). However, we were unable to demonstrate any effect of hydrogen inhalation on hematoma volume (9). The discrepancy in the effects of mast cell stabilization on hematoma volume between this study and the results presented by Strbian et al may be due to the use of different ICH models.
Itoh et al. (12) postulated a feed-forward loop between Lyn phosphorylation and NADPH oxidase activity as well as demonstrated that hydrogen-induced attenuation of Lyn phosphorylation led to inactivation of NADPH oxidase and decreased H2O2 production in mast cells. It is presumed that NADPH inhibition and diminished H2O2 production attenuated phosphorylation of Lyn and it's downstream molecules, and H2O2 accumulation counter-acted the effect of hydrogen. To test this hypothesis we used 3-amino-1,2,4-triazole (AT), a catalase inhibitor and extremely potent enzyme that decomposes H2O2 into water and oxygen (46), (47, 48). Consistent with previous publications, we found that giving AT three hours before ICH reversed the protective effect of hydrogen treatment and led to accumulation of H2O2. AT-induced accumulation of H2O2 suppressed phosphorylation of Lyn kinase and increased tryptase release, indicating that mast cells are a potential target for hydrogen therapy.
CONCLUSIONS
We concluded that ICH caused mast cell activation and degranulation. Mast cell activation enhanced the release of mast cell specific proteins and production of reactive oxygen and nitrogen specie, which contributed to BBB disruption and brain edema. Hydrogen inhalation diminished redox stress and BBB disruption by reducing the mast cell activation and degranulation, which ameliorated ICH-induced brain edema increase and neurological deficits in mice.
Hydrogen therapy is easy to use and proven to be neuroprotective. So far, there have been no reported side effects from the therapy. Further studies translating presented results into the clinical setting for ICH management are needed.
Supplementary Material
Acknowledgments
This study was partially supported by grants (NS43338 and NS53407) from the National Institutes of Health to John H. Zhang, MD/PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflicts of interest
REFERENCES
- 1.Woo D, Broderick JP. Spontaneous intracerebral hemorrhage: epidemiology and clinical presentation. Neurosurg Clin N Am. 2002;13(3):265–279. v. doi: 10.1016/s1042-3680(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu B, Ma Q, Suzuki H, et al. Recombinant osteopontin attenuates brain injury after intracerebral hemorrhage in mice. Neurocrit Care. 2011;14(1):109–117. doi: 10.1007/s12028-010-9372-z. [DOI] [PubMed] [Google Scholar]
- 3.Hall NC, Packard BA, Hall CL, et al. Protein oxidation and enzyme susceptibility in white and gray matter with in vitro oxidative stress: relevance to brain injury from intracerebral hemorrhage. Cell Mol Biol (Noisy-le-grand) 2000;46(3):673–683. [PubMed] [Google Scholar]
- 4.Wagner KR, Packard BA, Hall CL, et al. Protein oxidation and heme oxygenase-1 induction in porcine white matter following intracerebral infusions of whole blood or plasma. Dev Neurosci. 2002;24(2–3):154–160. doi: 10.1159/000065703. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Manaenko A, Zhan Y, et al. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169(1):402–414. doi: 10.1016/j.neuroscience.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai J, Kang Z, Liu WW, et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441(2):167–172. doi: 10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 7.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz JC. Histamine as a transmitter in brain. Life Sci. 1975;17(4):503–517. doi: 10.1016/0024-3205(75)90083-1. [DOI] [PubMed] [Google Scholar]
- 9.Strbian D, Tatlisumak T, Ramadan UA, et al. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27(4):795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- 10.Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J Biol Chem. 2004;279(47):48751–48759. doi: 10.1074/jbc.M409738200. [DOI] [PubMed] [Google Scholar]
- 11.Bidri M, Feger F, Varadaradjalou S, et al. Mast cells as a source and target for nitric oxide. Int Immunopharmacol. 2001;1(8):1543–1558. doi: 10.1016/s1567-5769(01)00097-2. [DOI] [PubMed] [Google Scholar]
- 12.Itoh T, Fujita Y, Ito M, et al. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389(4):651–656. doi: 10.1016/j.bbrc.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 13.Manaenko A, Fathali N, Chen H, et al. Heat shock protein 70 upregulation by geldanamycin reduces brain injury in a mouse model of intracerebral hemorrhage. Neurochem Int. 2010;57(7):844–850. doi: 10.1016/j.neuint.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Q, Huang B, Khatibi N, et al. PDGFR-alpha inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann Neurol. 2011;70(6):920–931. doi: 10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manaenko A, Chen H, Kammer J, et al. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195(2):206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manaenko A, Fathali N, Khatibi NH, et al. Arginine-vasopressin V1a receptor inhibition improves neurologic outcomes following an intracerebral hemorrhagic brain injury. Neurochem Int. 2011;58(4):542–548. doi: 10.1016/j.neuint.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q, Manaenko A, Khatibi NH, et al. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011;31(3):881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lekic T, Manaenko A, Rolland W, et al. Protective effect of hydrogen gas therapy after germinal matrix hemorrhage in neonatal rats. Acta Neurochir Suppl. 2011;111:237–241. doi: 10.1007/978-3-7091-0693-8_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan Y, Chen C, Suzuki H, et al. Hydrogen gas ameliorates oxidative stress in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2012 doi: 10.1097/CCM.0b013e31823da96d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128(Pt 7):1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 21.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lekic T, Hartman R, Rojas H, et al. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27(3):627–637. doi: 10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao W, Nishimoto H, Hong H, et al. Positive and negative regulation of mast cell activation by Lyn via the FcepsilonRI. J Immunol. 2005;175(10):6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimitriadou V, Aubineau P, Taxi J, et al. Ultrastructural evidence for a functional unit between nerve fibers and type II cerebral mast cells in the cerebral vascular wall. Neuroscience. 1987;22(2):621–630. doi: 10.1016/0306-4522(87)90358-7. [DOI] [PubMed] [Google Scholar]
- 25.Katsanos GS, Anogeianaki A, Orso C, et al. Mast cells and chemokines. J Biol Regul Homeost Agents. 2008;22(3):145–151. [PubMed] [Google Scholar]
- 26.Dombrowicz D, Flamand V, Brigman KK, et al. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75(5):969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 27.Hutchcroft JE, Geahlen RL, Deanin GG, et al. Fc epsilon RI-mediated tyrosine phosphorylation and activation of the 72-kDa protein-tyrosine kinase, PTK72, in RBL-2H3 rat tumor mast cells. Proc Natl Acad Sci U S A. 1992;89(19):9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckermann JM, Chen W, Jadhav V, et al. Hydrogen is neuroprotective against surgically induced brain injury. Med Gas Res. 2011;1(1):7. doi: 10.1186/2045-9912-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Wu P, Su J, et al. Effects of Aquaporin-4 on edema formation following intracerebral hemorrhage. Exp Neurol. 2010;223(2):485–495. doi: 10.1016/j.expneurol.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Strbian D, Kovanen PT, Karjalainen-Lindsberg ML, et al. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann Med. 2009;41(6):438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- 31.Mattila OS, Strbian D, Saksi J, et al. Cerebral Mast Cells Mediate Blood-Brain Barrier Disruption in Acute Experimental Ischemic Stroke Through Perivascular Gelatinase Activation. Stroke. 2011 doi: 10.1161/STROKEAHA.111.632224. [DOI] [PubMed] [Google Scholar]
- 32.Motawaj M, Peoc'h K, Callebert J, et al. CSF levels of the histamine metabolite tele-methylhistamine are only slightly decreased in Alzheimer's disease. J Alzheimers Dis. 2010;22(3):861–871. doi: 10.3233/JAD-2010-100381. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40(9):3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- 34.Esposito P, Chandler N, Kandere K, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303(3):1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 35.Lozada A, Maegele M, Stark H, et al. Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol Appl Neurobiol. 2005;31(2):150–162. doi: 10.1111/j.1365-2990.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 36.Hu W, Xu L, Pan J, et al. Effect of cerebral ischemia on brain mast cells in rats. Brain Res. 2004;1019(1–2):275–280. doi: 10.1016/j.brainres.2004.05.109. [DOI] [PubMed] [Google Scholar]
- 37.Fukuishi N, Sakaguchi M, Matsuura S, et al. The mechanisms of compound 48/80-induced superoxide generation mediated by A-kinase in rat peritoneal mast cells. Biochem Mol Med. 1997;61(1):107–113. doi: 10.1006/bmme.1997.2594. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24(9):471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 39.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, Liu J, Zhou C, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94(5):1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 41.Mander PK, Jekabsone A, Brown GC. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol. 2006;176(2):1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Irwin RW, Zhao L, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bidri M, Ktorza S, Vouldoukis I, et al. Nitric oxide pathway is induced by Fc epsilon RI and up-regulated by stem cell factor in mouse mast cells. Eur J Immunol. 1997;27(11):2907–2913. doi: 10.1002/eji.1830271124. [DOI] [PubMed] [Google Scholar]
- 44.Boje KM, Lakhman SS. Nitric oxide redox species exert differential permeability effects on the blood-brain barrier. J Pharmacol Exp Ther. 2000;293(2):545–550. [PubMed] [Google Scholar]
- 45.Kuo JR, Lo CJ, Chang CP, et al. Attenuation of brain nitrostative and oxidative damage by brain cooling during experimental traumatic brain injury. J Biomed Biotechnol. 2011;2011:145214. doi: 10.1155/2011/145214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escarabajal D, Miquel M, Aragon CM. A psychopharmacological study of the relationship between brain catalase activity and ethanol-induced locomotor activity in mice. J Stud Alcohol. 2000;61(4):493–498. doi: 10.15288/jsa.2000.61.493. [DOI] [PubMed] [Google Scholar]
- 47.Aragon CM, Spivak K, Amit Z. Effects of 3-amino-1,2,4-triazole on ethanol-induced open-field activity: evidence for brain catalase mediation of ethanol's effects. Alcohol Clin Exp Res. 1989;13(1):104–108. doi: 10.1111/j.1530-0277.1989.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 48.Pastor R, Sanchis-Segura C, Aragon CM. Ethanol-stimulated behaviour in mice is modulated by brain catalase activity and H2O2 rate of production. Psychopharmacology (Berl) 2002;165(1):51–59. doi: 10.1007/s00213-002-1241-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.