Abstract
Background
The role of CD8 T lymphocytes in the pathogenesis of asthma is not well understood. We investigated whether a subset of IL-13-producing BLT1-positive CD8 T lymphocytes is present in asthmatic airways and is associated with impaired lung function.
Methods
Bronchoalveolar lavage (BAL) cells were obtained from asthmatic (n=39) and healthy control (n=28) subjects. Cells were stimulated with phorbol ester and ionomycin in the presence of brefeldin A and stained for CD8, BLT1 and intracellular IL-13. The frequency of IL-13-producing BLT1-positive CD8 T lymphocytes was compared between the two groups and related to lung function, serum IgE levels and reticular basement membrane (RBM) thickness.
Results
A subset of CD8 T lymphocytes expressing BLT1 and producing IL-13 was detected in the airways of all asthmatic subjects. The frequency of this subset among recovered lymphocytes was significantly higher in the airways of asthmatic subjects compared to controls (mean ± SEM: 16.2 ± 1.4 vs. 5.3 ± 0.5, respectively, p < 0.001), and correlated positively with serum IgE levels and RBM thickness. More importantly, the frequency of CD8 T lymphocytes co-expressing BLT1 and IL-13 was inversely related to FEV1 and FEF[25-75] percent predicted values (p<0.001).
Conclusions
A subset of CD8 T lymphocytes expressing BLT1 and producing IL-13 is present in the airways of asthmatics. The accumulation of these cells is associated with airway obstruction, suggesting that they may play a significant pathogenic role in bronchial asthma.
Keywords: Asthma, CD8 lymphocytes, immunoglobulin E, interleukin-13, leukotriene B4 receptor
Introduction
Asthma is characterised by reversible airflow obstruction, persistent airway inflammation, tissue remodeling and bronchial hyperresponsiveness (1). Airway inflammation is thought to be orchestrated mainly by CD4 T cells through type-2 cytokines that mediate bronchial tissue inflammation, mucus hyperproduction and airway hyperresponsiveness (2). However, CD8 T cells can also differentiate into type-2 cytokine producing cells similar to CD4 T cells (3), but their role in the pathogenesis of asthma may be under-recognised. Previous studies have shown evidence for local expression of type-2 cytokines (IL-4 and IL-5 mRNA) by CD8 T cells in asthmatic airways (4). In a short-term follow-up study, CD8 T cells were associated with lung function decline in asthma (5), and patients who died during a severe asthma attack had CD8 T cells outnumbering CD4 T cells in both proximal and distal airways (6).
A potential role for CD8 T cells in asthma was initially supported in experimental animal model studies of allergic airway disease (7), and antigen priming was shown to be important for CD8 T cell-mediated allergic airway inflammation, mucus hyperproduction and airway hyperresponsiveness (AHR) (8). Most importantly, it was a subset of antigen-specific memory CD8 T cells, termed effector memory CD8 T cells, which expressed the high affinity receptor for leukotriene B4 (BLT1) and produced IL-13. This subset of BLT1-positive CD8 lymphocytes accumulated in the lung after allergen challenge and mediated the asthma-like phenotype in an IL-13 dependent manner (9). The present study was carried out to determine if a similar pathogenic subset of CD8 T cells expressing BLT1 and producing IL-13 is present in human asthmatic airways and if these cells are associated with airway obstruction.
Methods
Study Subjects
Thirty-nine subjects with mild to moderate asthma and 28 healthy controls were included in the study. All subjects were non-smokers and none had symptoms suggestive of respiratory infection or asthma exacerbation over the 4 weeks preceding the study. All subjects underwent physical examination, skin testing for atopy, blood testing for IgE, spirometry, and fiberoptic bronchoscopy (10). Methacholine (MCh) responsiveness was assessed at enrollment, 1 week before bronchoscopy. MCh responsiveness was not tested for 5 asthmatic subjects due to lower baseline FEV1 (< 55% predicted) value. An informed signed consent approved by the National Jewish Health Institutional Review Board was obtained from all participating subjects.
Clinical assessments
Skin prick testing (SPT) with a standard allergen panel was performed at baseline (Multi-Test II device, preparations from Greer Laboratories, Lenoir, NC) and was considered positive if the mean length at 20 minutes was ≥3 mm larger than the size of the negative control. IgE was measured in the National Jewish Health clinical laboratory via enzyme immunoassay. Spirometry was performed before and after albuterol, according to standardized techniques recommended by ATS/ERS (11,12). Bronchial reactivity testing to MCh was performed and interpreted according to standard procedures (13). The concentration of MCh (mg/mL) inducing a 20% fall in FEV1 was calculated and reported as the PC20 FEV1. Asthma control and quality of life were assessed using the Juniper Asthma Control Questionnaire (ACQ) (14), and Asthma Quality of Life Questionnaire (AQLQ) (15).
Bronchoalveolar lavage and sample processing
Bronchoalveolar lavage (BAL) was performed under fiberoptic bronchoscopy. A total of 180 to 300 ml, depending on subject tolerance, of pre-warmed non-pyrogenic sterile saline was instilled in 60-ml aliquots into the right middle lobe. After each instillation, BAL fluid was gently recovered by aspiration and placed on ice in 50-ml centrifugation tubes. The recovered fluids were centrifuged to sediment cells at 4°C for 10 min at 480× g. Cells in the pellets were pooled and resuspended in 5 ml of Hank's balanced salt solution. Total cell counts were determined by counting in a hemacytometer. Differential cell counts were determined by counting numbers of macrophages, lymphocytes, eosinophils, and neutrophils on cytospin preparations stained with Diff Quick®. A total of 500 cells was counted on cytospin slides to determine the proportion of each cell population in the BAL.
Detection of CD8, BLT1, and IL-13 by Immunofluorescence
With limited numbers of cells available, immunofluorescence was used instead of flow cytometry due to experienced loss of lymphocytes after fixation of cells and permeabilisation in suspension with Triton X-100. CD8, BLT1 and IL-13 were detected simultaneously on cytospin slides by immunofluorescence staining using a monoclonal mouse anti-human CD8 antibody (clone C8/144B; Dako, Carpinteria, CA), a polyclonal rabbit anti-human BLT1 antibody directed against an epitope in the cytoplasmic domain of this receptor (Cayman Chemical, Ann Arbor, MI), and a monoclonal rat anti-human IL-13 antibody (clone JES10-5A2, Biolegend, San Diego, CA), respectively. Briefly, BAL cells were stimulated for 5 h in culture with phorbol 12-myristate 13-acetate (PMA, 5 ng/ml) and ionomycin (500 ng/ml), in the presence of brefeldin A (10 μg/ml). After washing, the cells were spun onto slides, fixed in ice-cold acetone, air-dried and rehydrated in TBS. Cells were incubated with anti-CD8 antibody for 1 h, washed with TBS followed by 15-min fixation with paraformaldehyde (4% in PBS), and permeabilised for 15 min with Triton X-100 (0.5% in TBS). This was followed by 1-h incubation with anti-BLT1 and anti-IL-13 antibodies, added simultaneously. Control staining was performed in the same way but using the following control isotype-matched primary antibodies: mouse IgG1 (clone MOPC-21, Biolegend), rat IgG1 (clone RTK 2071, Biolegend), and normal rabbit IgG (Jackson Immunoresearch, catalog# 011-000-002). After washing with TBS, the cells were incubated for 15 min with normal goat serum (5% in TBS), followed by a 30-min incubation with the following affinity purified secondary antibodies, selected based on their suitability for multiple staining with no cross-reaction between species (all from Jackson ImmunoResearch Laboratories, West Grove, PA): rhodamine-labeled goat anti-mouse IgG (catalog# 115-025-166), FITC-labeled goat anti-rabbit IgG (catalog# 111-095-144), and biotin-labeled goat anti-rat IgG (catalog# 112-065-167). After washing with TBS, cells were further incubated for 30 min with Cychrome 5-conjugated streptavidin (Biolegend), washed again, and mounted on slides with FluoroGel (Electron Microscopy Sciences, Hatfield, PA).
Detection of CD8 by immunocytochemistry
CD8 lymphocytes were detected on cytospin preparations by immunocytochemistry using the alkaline phosphatase method with a chromogenic substrate. Briefly, after 15 min incubation with normal goat serum, the cells were incubated with the mouse anti-CD8 antibody for 2 h, washed with TBS, and further incubated for 1 h with a biotinylated goat anti-mouse IgG (Vector laboratories, Burlingame, CA). After washing, the cells were incubated for 30 min with avidin biotinylated alkaline phosphatase complex (Vectastain ABC-AP standard kit, Vector laboratories) and washed again. The phosphatase reaction was developed by incubation with permanent red substrate (Scyteck laboratories, Logan, UT), the cells were counterstained with Mayer's hematoxylin, and the slides were mounted with aqueous mounting media (Scytek laboratories).
Analysis of CD8, BLT1 and IL-13
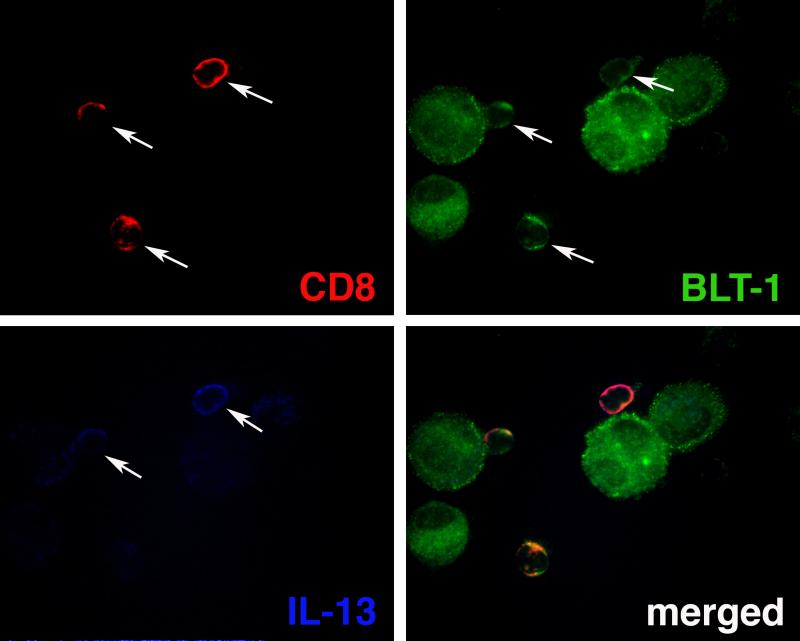
Staining for CD8, BLT1 and IL-13 was analysed under a Leica DMRXA fluorescent microscope (Leica, Wetzlar, Germany) examining multiple fields on the slides and capturing fluorescent images under a 25X objective. Three single-channel fluorescence images (one for each of CD8, BLT1 and IL-13) were taken from every field examined, covering the same cells. Each marker (CD8, BLT1, or IL-13) was identified individually on single-channel fluorescence. The unmerged individual single-channel fluorescence images (as in Fig. 1) were displayed on the computer and cells expressing all three markers (CD8, BLT1, and IL-13) were identified as such and counted among CD8-positive cells. Using these criteria, the cells were reliably identified with less than 8% variability between two observers blinded to the origin of the samples. A total of 200 CD8-positive cells were counted, in a blinded manner, for each BAL sample analyzed.
Figure 1.
Representative illustration of immunofluorescence staining of BAL cells for CD8 (red), BLT1 (green) and IL-13 (blue). Note the co-localisation of IL-13 to BLT1-positive CD8 T lymphocytes. Arrows indicate positive cells.
The proportion of CD8 cells co-expressing BLT1 and IL-13 was determined as percent of CD8+ cells counted on the cytospin slides stained by immunofluorescence. In parallel, the proportion of lymphocytes expressing CD8 was determined on separate cytospin slides stained for CD8 by immunocytochemistry using the alkaline phosphatase method, where lymphocytes can be identified morphologically (Fig. E1, Supplement). The frequency of BLT1+IL-13+ CD8 lymphocytes was determined by dividing the proportion of CD8 cells co-expressing BLT1 and IL-13 by the proportion of lymphocytes expressing CD8. Absolute numbers of these cells were calculated by relating their frequency to the total numbers of lymphocytes recovered in the BAL fluid.
Reticular basement membrane thickness
Reticular basement membrane (RBM) thickness was determined morphometrically on formalin-fixed, paraffin-embedded endobronchial biopsy tissue sections stained with hematoxylin and eosin. RBM thickness was measured on total length of basal lamina present in the biopsy and covered at least by basal cells. The measurements were performed in cross-sectional planes; areas with tangential planes were not included in the measurements.
Data analysis
Data are presented as means ± SEM. Statistical analysis of data was performed using GraphPad Prism version 5.0 for Mac OS X (GraphPad Software, San Diego, CA). Normally distributed data were analysed for statistical differences between the groups using unpaired t-test. Non-parametric two-tailed Mann-Whitney U test was used to determine statistical differences when the variance was different between the groups. Pearson's correlation tests were used to determine relationships between measured parameters. A p value of < 0.05 was considered statistically significant.
Results
Study Subjects
Patients with asthma had significantly lower pulmonary function values including forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced mid-expiratory flow (FEF[25-75])(p<0.001), and increased methacholine reactivity (p < 0.001), when compared to healthy control subjects (Table 1). Most asthmatics enrolled in the study had positive skin prick test (SPT) reactions to at least 3 common aeroallergens, with a median skin positivity of 6 (25% percentile = 3; 75% percentile = 9). In the control group, 6 of 28 subjects had a positive skin reaction to 1 common allergen only, while all other subjects (22 of 28) had negative skin reactions. Total serum IgE levels were significantly elevated in asthmatics (211.7 ± 43.6 U/ml) compared to controls (44.3 ± 14.2 U/ml) (p<0.001). Asthmatics had a significantly higher proportion of eosinophils in the BAL fluid compared to controls (p<0.001), but no significant differences were seen between the two groups in the percentage of macrophages, neutrophils or lymphocytes (Table 2).
Table I.
Subject Demographics.
| Characteristic | Controls (n= 28) | Asthma (n=39) |
|---|---|---|
| Gender, F (M) | 17 (11) | 19 (20) |
| Age, years | 34.2 (22 - 59) | 35.4 (18 - 56) |
| FEV1, % predicted | 99.0 (82 - 123) | 70.2 (37 - 94)* |
| FEV1/FVC ratio | 0.81 (0.61 – 0.99) | 0.65 (0.45 – 0.86)* |
| FEF25-75, % predicted | 93.5 (61 - 134) | 42.1 (16 - 95)* |
| MCh PC20, mg/ml | >25 | 1.7 (0 - 5.7)*, 5 N/T |
| ICS use | 0 | 16/39 |
| LABA use | 0 | 8/39 |
| Reversibility, % | 4.4 (-2.2 - 12.2) | 23.3 (-7.4 - 78.4)* |
| ACQ | N/T | 1.7 (0 - 2.8) |
| AQLQ | N/T | 5.1 (2.4 - 6.9) |
| Atopy | 6/28 | 38/39* |
| SPT reactivity | 0.2 (0 - 1) | 5.9 (0 - 12)* |
| Serum IgE, U/ml | 44.3 (2 - 377) | 211.7 (25 - 1140)* |
Data are presented as mean (range) unless otherwise indicated. Reversibility to β-agonist was tested just prior to bronchoscopy. ACQ: asthma control questionnaire, AQLQ: asthma quality of life questionnaire, F: female, ICS: inhaled corticosteroids, LABA: long-acting β agonist, M: male, MCh: methacholine, N/T: not tested, PC20: provocative concentration of methacholine causing 20% fall in FEV1 value, SPT: skin prick test.
Atopy was assessed by positive skin reactivity (> 3 mm) to one on more allergens.
Denotes statistically significant difference between asthma and control groups (p < 0.001).
Table 2.
Bronchoalveolar lavage data.
| Characteristic | Controls (n= 28) | Asthma (n=39) |
|---|---|---|
| Volume instilled, ml | 270 (180 - 300) | 251 (180 - 300) |
| Volume recovered, ml | 156 (113 - 207) | 125 (50 - 201) |
| Percent BAL recovery | 58.5 (40 – 71) | 48.8 (28 - 68) |
| Total cells recovered (×106) | 9.8 (2.4 – 30.7) | 6.6 (3.0 – 17.6) |
| Cells recovered/ml (×103) | 66.3 (18.7 – 179.9) | 59.4 (15.7 – 222.3) |
| Macrophages, % | 91.6 (70.9 – 99.2) | 89.6 (49.5 – 99.6) |
| Lymphocytes, % | 6.9 (0.8 – 26.5) | 8.5 (0.2 – 47.8) |
| Eosinophils, % | 0.1 (0 – 0.6) | 0.8 (0 – 5.8)* |
| Neutrophils, % | 1.4 (0.2 – 3.6) | 1.1 (0 – 8.2) |
Data are presented as mean (range).
Denotes statistically significant difference between asthma and control groups (p < 0.001).
BAL Cell Evaluation
Figure 1 shows representative staining results for CD8, BLT1 and IL-13. IL-13 was detected in some but not all CD8 lymphocytes. No fluorescence staining was detected using control isotype-matched primary antibodies (not shown). As mentioned above, there was no significant difference between asthmatics and controls in total numbers of lymphocytes recovered in the BAL fluid. Nevertheless, the proportion of lymphocytes stained positive for CD8 was significantly higher in the BAL of asthmatics compared to controls (31.82 ± 1.68% vs. 17.46 ± 1.10%, p<0.0001) (Fig. E2, Supplement). However, the difference in absolute numbers did not reach statistical significance (numbers × 103/ml return: 2.58 ± 1.38 for asthmatics vs. 0.99 ± 0.32 for controls, p=0.442), suggesting that the increased proportion of CD8 cells was restricted to the lymphocyte population in the BAL of asthmatics.
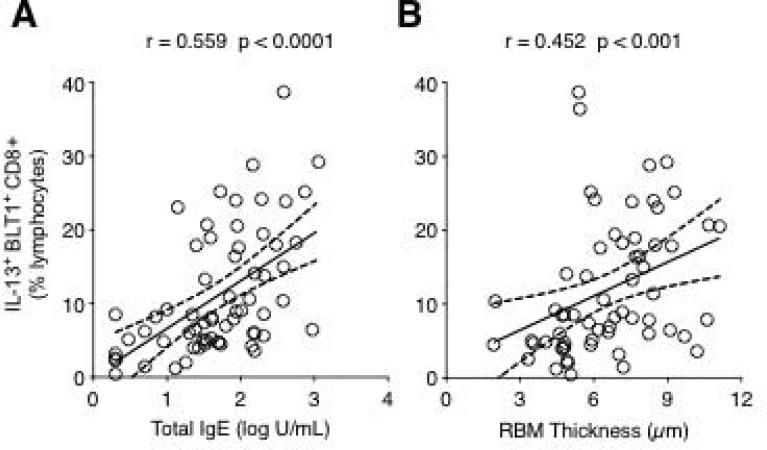
Analysis of BLT1 expression indicated that the majority of recovered BAL CD8 lymphocytes expressed this receptor at a frequency that was similar in both groups (88.60 ± 3.13% for asthmatics vs. 92.76 ± 1.68% for controls, p=0.664). By contrast, when IL-13 expression was analysed, the frequency of IL-13-producing BLT1-positive CD8 lymphocytes was 3-fold higher in the BAL of asthmatic subjects compared with controls (16.15 ± 1.41% vs. 5.27 ± 0.53%, p<0.0001) (Fig. E3, Supplement). This increase was also paralleled by a significant increase in absolute numbers of these cells in the BAL fluid of asthmatics compared to controls (numbers/ml return: 1,170 ± 630 vs. 270 ± 100, p=0.026, respectively). Interestingly, the frequency of these cells correlated positively with serum IgE levels (Fig. 2A) as well as RBM thickness (Fig. 2B).
Figure 2.
Relationship between the frequency of IL-13+ BLT1+ CD8 T lymphocytes and serum IgE levels (A) and RBM thickness (B). The frequency of IL-13+ BLT1+ CD8 T lymphocytes was positively correlated to total serum IgE levels and RBM thickness.
Lung Function Outcomes
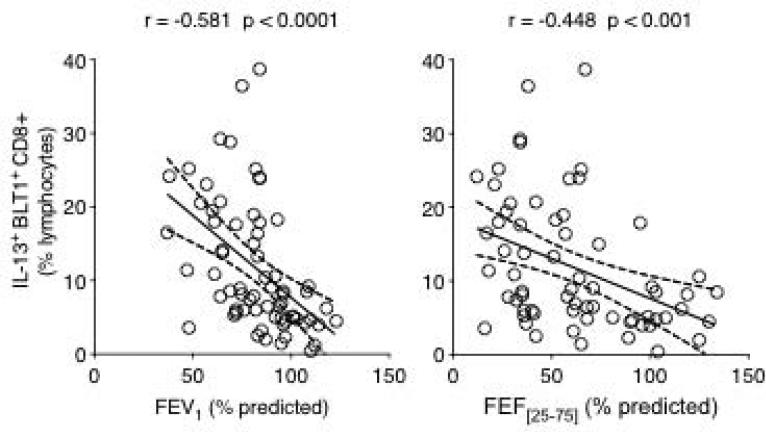
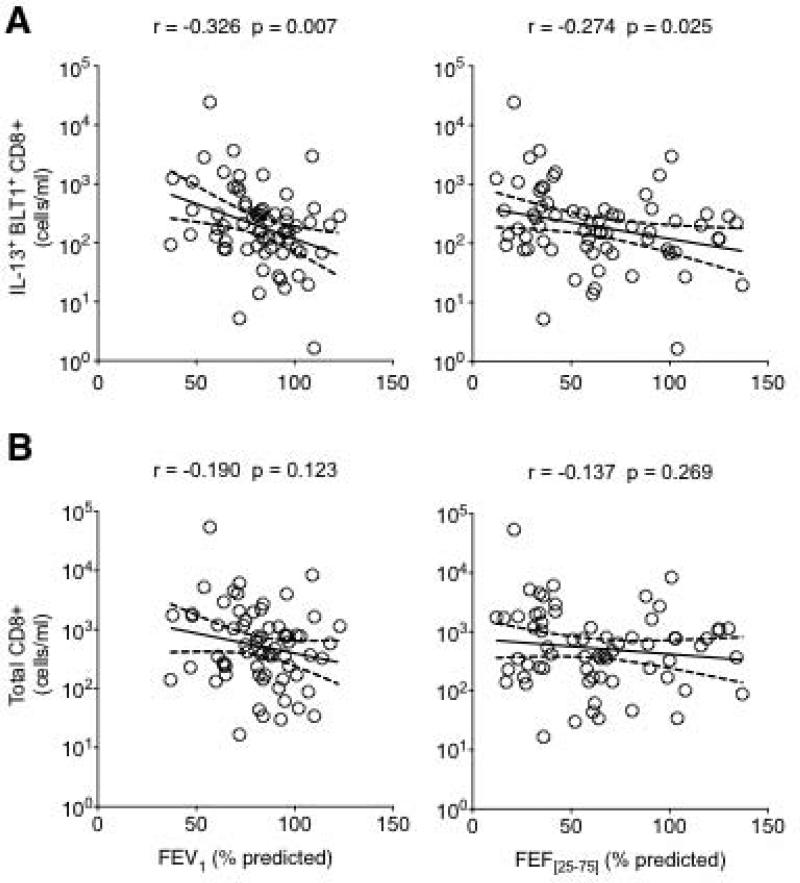
To determine if IL-13-producing BLT1-positive CD8 lymphocytes were associated with asthmatic airway obstruction, we examined the relationship between the frequency of these cells and measured lung function parameters. A significant inverse relationship was observed between the frequency of these cells and both FEV1 and FEF[25-75] % predicted values (Fig. 3). Interestingly, when absolute numbers were considered, only the IL-13-producing BLT1-positive subset of CD8 (Fig. 4A), not total CD8 (Fig. 4B) lymphocytes was inversely correlated to FEV1 and FEF[25-75].
Figure 3.
Relationship between the frequency of IL-13+ BLT1+ CD8 T lymphocytes and measured lung function parameters. The frequency of IL-13+ BLT1+ CD8 T lymphocytes was inversely correlated to both FEV1 (A) and FEF[25-75] (B) percent predicted values.
Figure 4.
Relationship between lung function parameters and absolute numbers of IL-13+ BLT1+ CD8 T lymphocytes (A) or total CD8 T lymphocytes (B). Note that the inverse relationship is significant with the IL-13+ BLT1+ CD8 subset (A) but not with total CD8 T lymphocytes.
No significant correlation was seen between IL-13-producing BLT1-positive CD8 or just CD8 and PC20 values in asthmatics (Fig. E4, Supplement), and inhaled corticosteroid did not appear to alter the percentage of these cells in asthmatic airways (Fig. E5, Supplement). However, when related to asthma severity, a correlation approaching significance was detected between the frequency of IL-13-producing BLT1-positive CD8 subset, but not total CD8, and AQLQ scores (r = -289, p = 0.075) as well as ACQ scores (r = 0.274, p = 0.091) (Fig. E6, Supplement).
Discussion
The present study demonstrates that a subset of CD8 lymphocytes expressing the high affinity receptor for LTB4 (BLT1) and producing IL-13 is present in significantly increased numbers in the airways of patients with asthma compared to healthy subjects. The frequency of these cells was inversely related to airflow obstruction determined by measurements of FEV1 and FEF[25-75] percent predicted values, suggesting a potential pathogenic role in asthmatic airway obstruction. These findings extend observations from recent experimental studies that established an important pathogenic role for effector memory CD8 T cells in the development of AHR and allergic airway inflammation (8,9), and preliminary findings of a similar subset of CD8 cells in asthmatic airways (16). Expression of BLT1 was shown to be required for the accumulation of these cells in the lung (17), and IL-13 was central to their effector function mediating AHR and mucus production (8).
Few studies have reported increased CD8 lymphocyte numbers in asthmatic airways (18-21), and after segmental allergen challenge (22). However, the role of these cells in the pathogenesis of asthma remained somewhat controversial. Early studies speculated that CD8 lymphocytes might play a suppressive role in asthma (19,23). In other studies, asthmatics with higher percentages of BAL CD8 lymphocytes had relatively lower FEV1 and PC20 values compared to asthmatics with lower percentages of CD8 lymphocytes (24), suggesting that the abundance of these cells in asthmatic airways might be associated with worse lung function outcomes. Furthermore, the abundance of CD8 lymphocytes in the airways could predict the annual decline of FEV1 in asthmatics (5). Recent transcriptome analyses showed that severe asthma is indeed associated with activation of circulating CD8 not CD4 T cells (25).
In this study, no significant correlation was seen between IL-13-producing BLT1-positive CD8 T cells or just CD8 T cells and PC20 values in asthmatics. However, when related to asthma severity, a correlation approaching significance was detected between frequency of IL-13-producing BLT1-positive CD8 subset, but not total CD8 T cells, and AQLQ scores as well as ACQ scores. A larger study population may be necessary to establish significant correlations.
CD8 T cells recognise antigen presented by major histocompatibility complex (MHC) class-I molecules, and play a major role in host defense against intracellular pathogens such as viruses. However, exogenous allergen that gains access to the MHC class-I processing pathway can also lead to the development of allergen-specific CD8 T cells (26). Since respiratory viruses and allergens are common triggers of asthma exacerbations, both virus-specific and allergen-specific CD8 T cells are expected to contribute to the worsening and decline of lung function in asthmatic subjects. Whether CD8 T cells play a protective or a pathogenic role is likely determined by conditions in the local environment, the signaling events leading to functional activation, timing of recruitment, and persistence of these cells in the airways.
CD8 lymphocytes have previously been implicated in virus-mediated dysfunction of M2 muscarinic acetylcholine receptor and AHR but only in sensitised animals (27), suggesting that an atopic/allergic phenotype is responsible for CD8 T cell-mediated altered airway dysfunction. In our study, the positive correlation observed between serum IgE levels and IL-13-producing BLT1-positive CD8 lymphocytes might suggest a role for mast cells in the recruitment of these cells into the airways. Indeed, mast cells are a potent source of LTB4 that can be released following cell activation by crosslinking of IgE receptors, and mast cell-derived LTB4 appears to be essential for the recruitment of BLT1-positive effector CD8 lymphocytes to the lungs of allergen-exposed mice (28).
How CD8 lymphocytes contribute to the pathogenesis of asthma is not completely understood. CD8 lymphocytes can produce cytolytic factors to clear infected cells, but these factors may also damage the airway epithelium, which would then expose sub-mucosal nerve endings to noxious stimuli that trigger AHR. Alternatively, in a Th2 cytokine-rich environment such as the asthmatic lung, CD8 lymphocytes may differentiate into a pathogenic phenotype that can promote immunopathology via Th2 cytokine production. Experimental studies have shown that CD8 T cells can convert to a Tc2 phenotype and mediate airway eosinophilia in the lungs of allergen-sensitised and challenged mice, a plasticity that could be induced in vitro by culture of these cells with IL-4 (29). Other studies have demonstrated that CD4 T cells are essential as a source of IL-4 that is necessary to the development of IL-13-producing CD8 T cells that mediate AHR in allergen-sensitised and challenged mice (30). In humans, airway CD8 lymphocytes recovered in sputum of atopic asthmatics produced more IL-4 and IL-5 compared to airway CD8 lymphocytes from non-atopic control subjects or to their respective peripheral blood CD8 lymphocytes (31), suggesting that this aberrant response may be compartmentalised to the airways in asthma. The observation of increased IL-4-expressing CD8 lymphocyte numbers in the lungs of subjects who died during an acute asthma attack compared to non-asthma death controls further strengthens the notion that an aberrant response by CD8 lymphocytes may be an important underlying cause for increased and uncontrolled disease severity (32).
In summary, this study established that a subset of CD8 lymphocytes, similar to effector memory CD8 T cells in the mouse model (9), expressing BLT1 and producing IL-13 populates the airways of asthmatic subjects. In asthmatics, numbers of these cells correlated with lower lung function and reticular basement membrane thickening and although the relationship between these cells and severity of asthma did not reach statistical significance, most likely due to the small size of the population studied, the data identified a potentially important role for these cells in dictating the severity of asthma. IL-13 is a critical mediator of AHR and mucus hyperproduction (33) and CD8 T lymphocytes are potentially less sensitive than CD4 lymphocytes to conventional anti-inflammatory corticosteroid therapy (34,35). Further studies targeting this subset of effector CD8 lymphocytes or the pathway responsible for their accumulation and effector function in the lung are necessary to establish a causative role in asthma. If confirmed, such strategies offer the potential to achieve better control of the disease and to prevent the accelerated decline of lung function.
Supplementary Material
Acknowledgments
The authors thank Dr. James Good for assistance with bronchoscopy, the Asthma Clinical Core (Jennifer Brandorff, Mary Gill, Christena Kolakowski, Juno Pak, and Allen Stevens) for evaluation and enrollment of participating study subjects, the Morphology Core (Lynn Cunningham and Janet Henson) for assistance with sample processing, and Diana Nabighian for assistance with preparation of the manuscript.
Source of funding: National Institutes of Health grants HL-36577 and AI-070140.
Abbreviations
- ACQ
Asthma Control Questionnaire
- AHR
Airway Hyperresponsiveness
- AQLQ
Asthma Quality of Life Questionnaire
- BAL
Broncho-Alveolar Lavage
- BLT1
Leukotriene B4 receptor 1
- FEF[25-75]
Forced Expiratory Flow between 25% and 75% of FVC (mid-expiratory flow)
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced Vital Capacity
- IL-
Interleukin-
- LTB4
Leukotriene B4
- MCh
Methacholine
- PC20
Provocative Concentration of methacholine causing 20% fall in FEV1
- RBM
Reticular Basement Membrane
- SEM
Standard Error of Mean
- SPT
Skin Prick Test
Footnotes
Authors’ contributions
AD had the primary responsibility for this manuscript. AD and EWG conceived the study and drafted the manuscript. MLC, HO and EG were involved in laboratory data collection and analysis. ES and RJM were involved in clinical data collection. AD, DYML, RA, ES, RM and EWG all played a role in study design, analysis of data and interpretation of results. All authors read and approved the final manuscript.
Conflict of interest
None of the authors have a conflict of interest pertaining to this work.
References
- 1.Busse WW, Lemanske RF, Jr., Asthma N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 3.Woodland DL, Dutton RW. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15:336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 4.Ying S, Humbert M, Barkans J, Corrigan CJ, Pfister R, Menz G, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–3544. [PubMed] [Google Scholar]
- 5.van Rensen EL, Sont JK, Evertse CE, Willems LN, Mauad T, Hiemstra PS, et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med. 2005;172:837–841. doi: 10.1164/rccm.200504-619OC. [DOI] [PubMed] [Google Scholar]
- 6.Faul JL, Tormey VJ, Leonard C, Burke CM, Farmer J, Horne SJ, et al. Lung immunopathology in cases of sudden asthma death. Eur Respir J. 1997;10:301–307. doi: 10.1183/09031936.97.10020301. [DOI] [PubMed] [Google Scholar]
- 7.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, et al. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitisation. J Exp Med. 1996;183:1719–1729. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, et al. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol. 2004;172:2549–2558. doi: 10.4049/jimmunol.172.4.2549. [DOI] [PubMed] [Google Scholar]
- 9.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 10.Busse WW, Wanner A, Adams K, Reynolds HY, Castro M, Chowdhury B, et al. Investigative bronchoprovocation and bronchoscopy in airway diseases. Am J Respir Crit Care Med. 2005;172:807–816. doi: 10.1164/rccm.200407-966WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Gardner RM, Crapo RO, Billings RG, Shigeoka JW, Hankinson JL. Spirometry: what paper speed? Chest. 1983;84:161–165. doi: 10.1378/chest.84.2.161. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 16.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117:577–582. doi: 10.1016/j.jaci.2005.12.1340. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, et al. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174:4979–4984. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- 18.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–942. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 19.Kelly CA, Stenton SC, Ward C, Bird G, Hendrick DJ, Walters EH. Lymphocyte subsets in bronchoalveolar lavage fluid obtained from stable asthmatics, and their correlations with bronchial responsiveness. Clin Exp Allergy. 1989;19:169–175. doi: 10.1111/j.1365-2222.1989.tb02360.x. [DOI] [PubMed] [Google Scholar]
- 20.Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150:1038–1048. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Kim SJ, Kwon SS, Kim YK, Kim KH, Moon HS, et al. Distribution and cytokine production of CD4 and CD8 T-lymphocyte subsets in patients with acute asthma attacks. Ann Allergy Asthma Immunol. 2001;86:659–664. doi: 10.1016/S1081-1206(10)62295-8. [DOI] [PubMed] [Google Scholar]
- 22.Bratke K, Bottcher B, Leeder K, Schmidt S, Kupper M, Virchow JC, Jr., et al. Increase in granzyme B+ lymphocytes and soluble granzyme B in bronchoalveolar lavage of allergen challenged patients with atopic asthma. Clin Exp Immunol. 2004;136:542–548. doi: 10.1111/j.1365-2249.2004.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez MC, Diaz P, Galleguillos FR, Ancic P, Cromwell O, Kay AB. Allergen-induced recruitment of bronchoalveolar helper (OKT4) and suppressor (OKT8) T-cells in asthma. Relative increases in OKT8 cells in single early responders compared with those in late-phase responders. Am Rev Respir Dis. 1987;136:600–604. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- 24.Pizzichini E, Pizzichini MM, Kidney JC, Efthimiadis A, Hussack P, Popov T, et al. Induced sputum, bronchoalveolar lavage and blood from mild asthmatics: inflammatory cells, lymphocyte subsets and soluble markers compared. Eur Respir J. 1998;11:828–834. doi: 10.1183/09031936.98.11040828. [DOI] [PubMed] [Google Scholar]
- 25.Tsitsiou E, Williams AE, Moschos SA, Patel K, Rossios C, Jiang X, et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol. 2012;129:95–103. doi: 10.1016/j.jaci.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Seneviratne SL, Jones L, King AS, Black A, Powell S, McMichael AJ, et al. Allergen-specific CD8(+) T cells and atopic disease. J Clin Invest. 2002;110:1283–1291. doi: 10.1172/JCI15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamko DJ, Fryer AD, Bochner BS, Jacoby DB. CD8+ T lymphocytes in viral hyperreactivity and M2 muscarinic receptor dysfunction. Am J Respir Crit Care Med. 2003;167:550–556. doi: 10.1164/rccm.200206-506OC. [DOI] [PubMed] [Google Scholar]
- 28.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader J, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 29.Coyle AJ, Erard F, Bertrand C, Walti S, Pircher H, Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. 1995;181:1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koya T, Miyahara N, Takeda K, Matsubara S, Matsuda H, Swasey C, et al. CD8+ T cell-mediated airway hyperresponsiveness and inflammation is dependent on CD4+IL-4+ T cells. J Immunol. 2007;179:2787–2796. doi: 10.4049/jimmunol.179.5.2787. [DOI] [PubMed] [Google Scholar]
- 31.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma. Am J Respir Crit Care Med. 2005;171:224–230. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan S, Cormican L, Faul JL, Ichinohe S, Johnston SL, Burke CM, et al. Activated, cytotoxic CD8(+) T lymphocytes contribute to the pathology of asthma death. Am J Respir Crit Care Med. 2001;164:560–564. doi: 10.1164/ajrccm.164.4.2102018. [DOI] [PubMed] [Google Scholar]
- 33.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 34.Seki M, Ushiyama C, Seta N, Abe K, Fukazawa T, Asakawa J, et al. Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:823–830. doi: 10.1002/1529-0131(199805)41:5<823::AID-ART8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi H, Miyahara N, Dakhama A, Takeda K, Mathis S, Haribabu B, et al. Corticosteroids enhance CD8+ T cell-mediated airway hyperresponsiveness and allergic inflammation by upregulating leukotriene B4 receptor 1. J Allergy Clin Immunol. 2008;121:864–871. doi: 10.1016/j.jaci.2008.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.