Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is notorious for local recurrence and metastatic spread to regional lymph nodes. Distant spread is uncommon, and brain involvement is rare. Over the past decade there has been a rising incidence of HPV-related HNSCC, but it is not known if this escalation has had any impact on trends relating to brain involvement.
Methods
Cases of metastatic squamous cell carcinoma (SCC) to the brain were identified from a computerized search of the surgical pathology files of The Johns Hopkins Hospital between 1985 and 2012. The medical records were reviewed to document primary site of tumor origin, treatment, and patient outcome. P16 immunohistochemistry and HPV in situ hybridization were performed on those metastases arising from the head and neck.
Results
Of the 38 metastatic SCCs, 7 (18%) originated in the head and neck. HPV-16 was detected in 4 (57%) of the metastatic HNSCCs. All 4 HPV-positive metastases were from oropharyngeal primaries. The time from treatment of the primary to development of the brain metastasis ranged from 19–57 months (mean, 45). Following aggressive treatment (surgery and radiation), 2 patients died of disease progression (7 and 34 months), and 2 are alive with recurrent brain metastases (4 and 10 months).
Conclusions
Although HPV positivity is regarded as a favorable prognostic indicator, it does not safeguard from spread to the brain. In our experience, just over half of the HNSCCs that metastasized to the brain were HPV-related. The potential for developing a brain metastasis long after curative therapy argues for extended patient follow-up. The development of a brain metastasis is an ominous finding signaling rapid clinical deterioration.
Keywords: brain, metastasis, human papillomavirus (HPV), head and neck squamous cell carcinoma, p16, in situ hybridization
Introduction
More than 37,000 head and neck cancers are diagnosed in the United States each year. The vast majority of these are squamous cell carcinomas (HNSCC), and these account for about 11,000 cancer related deaths annually.[1, 2] The natural history of HNSCC is characterized by a propensity for local recurrence and/or metastatic spread to regional lymph nodes. Indeed, failure to control the disease locally and regionally is by far the major cause of cancer related deaths.[3] Distant spread usually occurs late in the disease course, and typically in the setting of advanced local-regional disease.[4–6] Its presence is generally taken as evidence of non-curable disease and a harbinger of rapid clinical deterioration and death.[7, 8] The incidence of intracranial metastases is only about 0.4% overall, and 2–8% for those patients who already have distant spread to the lungs or other extracranial sites.[4, 7]
Over the past 2 decades there has been a dramatic rise in the incidence of a subtype of HNSCC caused by the human papillomavirus (HPV).[9, 10] These HPV-related HNSCCs have an epidemiologic, demographic and clinical profile that deviates from the profile of conventional non-HPV-related HNSCC. [9, 11–13] HPV-associated cancers tend to occur more frequently in younger, male patients; tobacco smoking does not appear to be a strong cofactor in the development of these tumors;[14, 15] they most frequently occur in the oropharynx;[13, 16] and they are associated with improved clinical outcomes.[13, 17–19]
Improved clinical outcome for patients with HPV-HNSCC primarily reflects the success in achieving local and regional control using various combinations of surgery, radiation and chemotherapy.13,17–19 Success in achieving local and regional control is shifting patterns of failure and changing the natural history of HNSCC. Patients seemingly cured of their disease - based on complete tumor eradication in the head and neck – are at risk of succumbing to metastatic spread to distant and sometimes unusual sites.[20, 21] The purpose of this study was to determine whether the brain is targeted as a distant site by HPV-related HNSCC.
Methods
Patient Selection
The electronic surgical pathology files of the Johns Hopkins Hospital were reviewed for all cases of metastatic squamous cell carcinoma to the brain resected between March, 1985 and May, 2012. For all identified cases, the medical records were reviewed to confirm the origin of the primary tumor. For those patients with brain metastases from a head and neck primary, the medical records were further reviewed to determine various other clinical parameters including tumor stage, time to onset of brain metastasis, and clinical outcome. For all cases of metastatic HNSCC, hematoxylin and eosin-stained sections were reviewed to confirm the diagnosis, and an appropriate tissue block was selected for HPV analysis including p16 immunohistochemistry and HPV DNA in situ hybridization. When available, blocks of the corresponding primary HNSCCs were also obtained for HPV analysis.
Immunohistochemistry
Five-micrometer sections of formalin-fixed and paraffin embedded tissues were deparaffinized. Antigen retrieval was performed using heat induced (92°C for 30 minutes) epitope retrieval with 10mM citrate buffer. Sections were incubated with a mouse monoclonal antibody against p16 (MTM Laboratories, Heidelberg, Germany) which was visualized using the Ultra view polymer detection kit (Ventana Medical Systems Inc., Tucson, AZ) on a Ventana Benchmark XT autostainer (Ventana). P16 expression was scored as positive if strong and diffuse nuclear and cytoplasmic staining was seen in ≥70% of the tumor.[22]
DNA In situ hybridization
Five-micrometer sections from the formalin-fixed paraffin-embedded tumor blocks were evaluated for the presence of HPV DNA by in situ hybridization (ISH). Two different detection methods were used. Type-specific assays for type 16, 18, and 31/33 were performed using the in situ hybridization-catalyzed signal amplification method for biotinylated probes (DAKO GenPoint, Carpinteria, CA). Briefly, the 5-mm tissue sections underwent deparaffinization, heat-induced target retrieval in citrate buffer, and digestion using Proteinase K (Roche Diagnostics, Indianapolis, IN). Slides were subsequently hybridized with biotinylated HPV type-specific probes for types 16, 18, and 31/33 (DAKO, Carpintera, CA). Signal amplification was performed by consecutive application of a streptavidin-HRP complex and AQ2 biotinyl tyramide. Visualization of hybridization signals was performed by incubation with the chromogenic substrate diaminobenzidine.
For broader high-risk HPV detection, we also used the Ventana Inform HPV III Family 16 Probe (B) kit (Ventana Medical Systems, Tucson, AZ). For this assay, slides were conditioned using Ventana cell conditioner #2 and ISH-protease 3. Hybridization utilized the HPV III Family 16 probe set that captures HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 66. Signals were detected using the ISH iView Blue Plus Detection Kit, which is an indirect biotin-streptavidin system that detects fluorescein-labeled probes. The kit uses an alkaline phosphatase enzyme and NBT/BCIP substrate chromogen reaction that provides an intense blue, permanent color as well as a red counter stain. All reagents are provided prediluted and ready-to-use on BenchMark Series automated slide stainers (Ventana Medical Systems, Tucson, AZ).
For both detection assays, punctate hybridization signals localized to the tumor cell nuclei defined an HPV-positive tumor. HPV–positive controls were cases of HPV-16, HPV-18, and HPV-31/33–positive oropharyngeal cancers, as well as the HPV16-positive SiHa and CaSki cell lines.
Statistical Analysis
All quantitative figures and statistics were completed using GraphPad Prism (Version 5.00 for Mac OS, GraphPad Software, San Diego, CA). Data are presented as Kaplan-Meier curves and data between HPV (−) and HPV (+) cohorts are compared using the log-rank (Mantle-Cox) test.
Results
Over the study period, 39 patients underwent resections of metastatic squamous cell carcinomas to the brain. The clinical demographics of these patients are summarized in Table 1. The brain metastases were from primary carcinomas in the lung (n=25), head and neck (n=7), cervix (n=3), esophagus (n=1), thyroid (n=1) and prostate (n=1). The metastatic thyroid carcinoma was an anaplastic carcinoma showing squamous differentiation, and the metastatic prostatic carcinoma was an adenocarcinoma that had undergone squamous differentiation following hormonal therapy.
Table 1.
Summary of clinical demographics for patients with resected metastatic squamous cell carcinomas to the brain.
| patient characteristic | lung (n=25) | head & neck (n=7) | cervix (n=3) | esophagus (n=1) | thyroid (n=1) | prostate (n=1) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male (%) | 11 (44) | 7 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| Female (%) | 14 (56) | 0 (0) | 3 (100) | 0 (0) | 1 (100) | 0 (0) |
| Race | ||||||
| Caucasian (%) | 18 (72) | 5(63) | 2 (67) | 0 (0) | 1 (100) | 1 (100) |
| AA (%) | 5 (20) | 1 (25) | 1 (33) | 1 (100) | 0 (0) | 0 (0) |
| other (%) | 2 (8) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Age (years) | ||||||
| mean | 60 | 59 | 44 | 76 | 64 | 55 |
| range | 33–78 | 42–65 | 38–78 | - | - | - |
| # of brain mets | ||||||
| mean | 1 | 2 | 1 | 1 | 1 | 1 |
| range | 1–3 | 1–9 | - | - | - | - |
| Brain met free survival | ||||||
| average | 21 | 36 | 50 | 11 | 7 | 110 |
| range | 0–96 | 8–57 | - | - | - | - |
AA, African American; met, metastasis; #, number)
By anatomic subsite, the HNSCCs were from the base of tongue (n=3), tonsil (n=2) and larynx (n=1). One patient presented with metastatic squamous cell carcinoma to a cervical lymph node, but the primary site was clinically occult. HPV was detected in 4 of the 7 metastatic HNSCCs as evidenced by HPV16 in situ hybridization and strong p16 immunohistochemical staining (Figure 1). All 4 HPV-HNSCCs were from the oropharynx (base of tongue = 3, tonsil = 1). A tissue block from the primary HNSCC was available for only one of the patients (patient 1), and the primary HNSCC, like the corresponding brain metastasis, was HPV 16 positive. The primary HPV-positive HNSCCs were all diagnosed between 1997 and 2008 (1997, 1998, 2008, 2009), while the 3 HPV-negative cases were diagnosed from 1990 to 2005 (1990, 1992, 2005).
Figure 1.
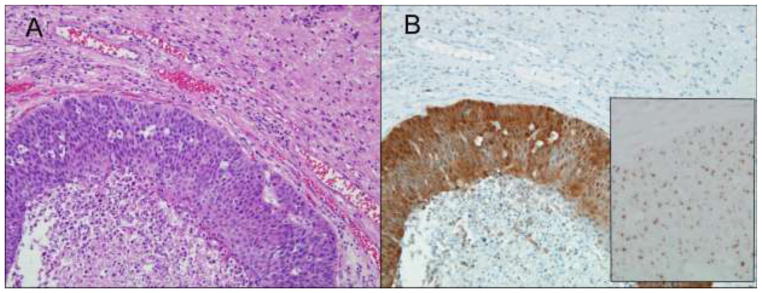
Metastatic head and neck squamous cell carcinoma to the brain (A, hematoxylin and eosin stain). The tumor is strongly p16 positive by immunohistochemistry (B), and it is HPV-16 positive by in-situ hybridization (B, inset).
The clinicopathologic features of the patients with metastatic HNSCC are summarized in Table 2. All 7 patients with metastatic HNSCC to the brain were males. At the time of treatment for their primary HNSCCs, the patients ranged in age from 42 to 65 years (mean 55). Staging information was available for 4 of the patients, and all 4 had regional spread to cervical lymph nodes but no evidence of distant metastases. The primary HNSCCs were all treated with surgical resection and cisplatin-based chemotherapy; and all but one patient also received local-regional radiation therapy. The time from treatment of the primary HNSCC to diagnosis of the brain metastasis (brain metastases free survival) ranged from 8 to 57 months (average, 36 months). The brain metastases free survival was longer for patients with HPV positive HNSCCs, but the difference was not statistically significant (45 months v. 27 months, p=0.24). Distant spread was never restricted to the brain. Instead, brain metastases consistently occurred together with metastatic spread to the lungs in all 7 patients.
Table 2.
Clinical and pathologic features including HPV status for patients with metastatic HNSCC to the brain
| case | gender | age | 1° site | 1° stage | lung mets | # brain mets | brain met free survival (months) | HPV status | |
|---|---|---|---|---|---|---|---|---|---|
| P16 IHC | HPV16 ISH | ||||||||
|
| |||||||||
| 1 | M | 61 | BOT | T3N2bM0 | yes | 1 | 57 | + | + |
| 2 | M | 46 | BOT | T3N1M0 | yes | 1 | 22 | + | + |
| 3 | M | 53 | tonsil | T4N2bM0 | yes | 9 | 53 | + | + |
| 4 | M | 65 | BOT | T2N1MX | yes | 2 | 37 | + | + |
| 5 | M | 59 | larynx | T4aN2cMO | yes | 1 | 8 | − | − |
| 6 | M | 42 | tonsil | unknown | yes | 1 | 27 | − | − |
| 7 | M | 55 | unknown | unknown | yes | 1 | 52 | − | − |
BOT, base of tongue; mets, metastases; IHC, immunohistochemistry; ISH, in situ hybridization
Following the appearance of an intracranial metastasis, all patients underwent a neurosurgical procedure to palliate the associated neurologic decline. As a result of surgery, all patients were able to return to normal independent living. The 3 patients with metastatic HPV negative HNSCCs tumors died 1, 4 and 5 months following their first brain metastasis. Of the 4 patients with HPV positive brain metastases, 2 died due to progression of intracranial metastases at 7 months and 34 months. Two patients with HPV positive brain metastases are currently alive with residual or recurrent brain involvement at 4 months and 10 months after resection of their brain metastasis. Of these two patients, one received whole-brain radiation following a second surgery due to progression of the metastatic tumor. The second patient received stereotactic radiosurgery. Patients with HPV positive brain metastases had a longer median survival than patients with HPV negative brain metastases, but the difference did not reach statistical significant (53 months v. 27 months, p=0.12).
Discussion
The unique biology of HPV induced tumorigenesis of the head and neck is altering the natural history of HNSCC including patterns of clinical behavior. As one important example, HPV positivity confers much improved clinical outcomes for patients with HNSCC.[17, 19, 23] This improved clinical outcome can largely be attributed to dramatic local and regional therapeutic responses. These loco-regional sensitivities, however, do not translate to enhanced therapeutic responses at distant sites: For patients with HPV-positive oropharyngeal cancer, various therapeutic regimens including cisplatin induction chemotherapy do not differentially affect the elimination of occult distant metastases.[17] Accordingly, patients with HPV positive HNSCCs develop distant metastases at about the same incidence as patients with HPV negative HNSCCs.[20] HPV status may have more of an influence on the pattern rather than incidence of distant spread. HPV positive metastatic HNSCCs are now surfacing at distant sites rarely targeted by HPV negative cancers including the skin and brain.[20, 21, 24]
Of the patients who had undergone excisions of squamous cell carcinomas to the brain over a 27 year period at the Johns Hopkins Hospital, 7 (18%) were from the head and neck. These metastatic HNSCCs were overrepresented by HPV positive carcinomas from the oropharynx, with all of the HPV positive cases occurring over the last 15 years. Metastatic HPV-related HNSCC to the brain was not observed in the 17 years prior to 1998. This time trend mirrors the rising overall incidence of HPV-related HNSCC and the reversal in the ratio of HPV-positive to HPV-negative cases noted over the past decade.[25] Admittedly, metastatic-HPV related HNSCC to the brain is still uncommon, but the rising incidence of HPV-related HNSCC suggests the possibility of a parallel upsurge in the appearance of distant metastases including unexpected sites like the brain.
The interval from treatment of the primary carcinoma to clinical manifestation of distant spread may be influenced by HPV status. As a general rule, the clinical behavior of HNSCC is characterized by a relatively short distant metastasis free survival time. The vast majority (about 90%) of patients who develop distant metastases do so within 2 years following treatment of the primary tumor.[4, 26] In the 4 patients we report with HPV-related HNSCC, only 1 manifested with a brain metastasis within this 2 year window. Strikingly, 2 patients manifested with brain metastases more than 4 years after treatment of their primary tumors. This finding is in line with other observations regarding the influence of HPV status on time intervals to distant spread. Huang et al.[20] noted that distant metastasis free survival is extended for patients with HPV-related HNSCCs compared to patients with HPV-unrelated HNSCCs. Bishop et al.[27] reported patients with HPV-related oropharyngeal carcinomas who developed lung metastases 8 years after treatment of their primary cancers, well beyond the 2–5 year interval that is usually set as the threshold for distinguishing primary from metastatic HNSCC. The potential for these HPV-related carcinomas to metastasize long after curative therapy argues for extended patient follow-up and a measured assessment of patient cure.
The appearance of a brain metastasis remains an ominous finding for patients with previously treated HNSCCs, irrespective of HPV status. In our series, extensive treatment involving multiple invasive neurosurgical interventions, systemic chemotherapy, and radiation therapy was attempted to palliate symptoms in patients who presented with neurological decline. While stabilization of disease was noted for a short period of time, multimodal therapy was invariably followed by tumor recurrence and/or progression. Patients with HPV-positive brain metastases did experience a longer median survival compared to patients with HPV-negative brain metastases, but the study population was not large enough to demonstrate statistical significance. Although HPV-positivity may extend the overall time course for patients with HNSCC who develop distant metastases, we find no evidence to support an expectation that HPV-related HNSCC is curable once it has metastasized to the brain.
In summary, HPV status is a powerful prognostic indicator that signals improved clinical outcomes for patients with HNSCC, but HPV status does not eradicate the threat of distant metastases. In our experience, just over half of the HNSCCs that metastasized to the brain were HPV-related. The potential for HPV-related HNSCCs to metastasize long after curative therapy argues for extended patient follow-up, but therapeutic options may be limited to palliative care when the brain is targeted as a metastatic site.
Acknowledgments
Funding: This work has been partially funded by the National Institute of Dental and Craniofacial Research (R01 DE013152-11).
References
- 1.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annual review of pathology. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2006;135:451–457. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA. Management of advanced stage squamous cell carcinoma of the head and neck. The American journal of the medical sciences. 1986;291:405–415. doi: 10.1097/00000441-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Takes RP, Rinaldo A, Silver CE, Haigentz M, Jr, Woolgar JA, Triantafyllou A, Mondin V, Paccagnella D, de Bree R, Shaha AR, Hartl DM, Ferlito A. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012;48:775–779. doi: 10.1016/j.oraloncology.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Papac RJ. Distant metastases from head and neck cancer. Cancer. 1984;53:342–345. doi: 10.1002/1097-0142(19840115)53:2<342::aid-cncr2820530228>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, El Mofty S, Perez CA. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. The Laryngoscope. 2001;111:1079–1087. doi: 10.1097/00005537-200106000-00028. [DOI] [PubMed] [Google Scholar]
- 7.de Bree R, Mehta DM, Snow GB, Quak JJ. Intracranial metastases in patients with squamous cell carcinoma of the head and neck. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001;124:217–221. doi: 10.1067/mhn.2001.112478. [DOI] [PubMed] [Google Scholar]
- 8.Probert JC, Thompson RW, Bagshaw MA. Patterns of spread of distant metastases in head and neck cancer. Cancer. 1974;33:127–133. doi: 10.1002/1097-0142(197401)33:1<127::aid-cncr2820330119>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. The lancet oncology. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerging infectious diseases. 2010;16:1671–1677. doi: 10.3201/eid1611.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D’Souza G, Gravitt PE, Westra W, Psyrri A, Kast WM, Koutsky LA, Giuliano A, Krosnick S, Trotti A, Schuller DE, Forastiere A, Ullmann CD. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, D.C. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 12.Kim L, King T, Agulnik M. Head and neck cancer: changing epidemiology and public health implications. Oncology (Williston Park) 2010;24:915–919. 924. [PubMed] [Google Scholar]
- 13.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 14.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 15.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 16.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Seminars in radiation oncology. 2012;22:128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 20.Huang SH, Perez-Ordonez B, Liu FF, Waldron J, Ringash J, Irish J, Cummings B, Siu LL, Kim J, Weinreb I, Hope A, Gullane P, Brown D, Shi W, O’Sullivan B. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Muller S, Khuri FR, Kono SA, Beitler JJ, Shin DM, Saba NF. HPV Positive Squamous Cell Carcinoma of the Oropharynx. Are we Observing an Unusual Pattern of Metastases? Head and neck pathology. 2012;6:336–344. doi: 10.1007/s12105-012-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 23.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 24.Kraft S, Faquin WC, Krane JF. HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. The American journal of surgical pathology. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 25.Ernster JA, Sciotto CG, O’Brien MM, Finch JL, Robinson LJ, Willson T, Mathews M. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. The Laryngoscope. 2007;117:2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 26.Leon X, Quer M, Orus C, del Prado Venegas M, Lopez M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680–686. doi: 10.1002/1097-0347(200010)22:7<680::aid-hed7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Bishop JA, Ogawa T, Chang X, Illei PB, Gabrielson E, Pai SI, Westra WH. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. The American journal of surgical pathology. 2012;36:142–148. doi: 10.1097/PAS.0b013e3182395c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]


