Abstract
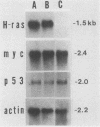
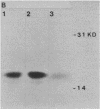
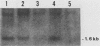
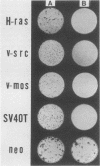
A flat revertant, R1, was isolated from human activated c-Ha-ras-1 (hu-ac-Ha-ras) gene-transformed NIH 3T3 cells (EJ-NIH 3T3) treated with mutagens. R1 contained unchanged transfected hu-ac-Ha-ras DNA and expressed high levels of hu-ac-Ha-ras-specific mRNA and p21 protein. Transfection experiments revealed that NIH 3T3 cells could be transformed by DNA from R1 cells but R1 cells could not be retransformed by Kirsten sarcoma virus, DNA from EJ-NIH 3T3 cells, hu-ac-Ha-ras, v-src, v-mos, simian virus 40 large T antigen, or polyomavirus middle T antigen. Somatic cell hybridization studies showed that R1 was not retransformed by fusion with NIH 3T3 cells and suppressed anchorage independence of EJ-NIH 3T3 and hu-ac-Ha-ras gene-transformed rat W31 cells in soft agar. These results suggest that the reversion and resistance to several oncogenes in R1 is due not to cellular defects in the production of the transformed phenotype but rather to enhancement of cellular mechanisms that suppress oncogenic transformation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Direct identification of palmitic acid as the lipid attached to p21ras. Mol Cell Biol. 1986 Jan;6(1):116–122. doi: 10.1128/mcb.6.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Oren M. Overproduction of p53 antigen makes established cells highly tumorigenic. Nature. 1985 Jul 11;316(6024):158–160. doi: 10.1038/316158a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Kedes L., Hickey R. J., Skoultchi A. I. Expression of human cardiac actin in mouse L cells: a sarcomeric actin associates with a nonmuscle cytoskeleton. Cell. 1984 Mar;36(3):709–715. doi: 10.1016/0092-8674(84)90351-9. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yutsudo M., Hakura A. Rat mutant cells showing temperature sensitivity for transformation by wild-type Moloney murine sarcoma virus. Virology. 1983 Feb;125(1):242–245. doi: 10.1016/0042-6822(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Katz E., Carter B. J. A mutant cell line derived from NIH/3T3 cells: two oncogenes required for in vitro transformation. J Natl Cancer Inst. 1986 Oct;77(4):909–914. [PubMed] [Google Scholar]
- Kuzumaki N., Minakawa H., Miyazaki T., Haraguchi S., Matsuo T., Yoshida T. O. Individually distinct tumor-specific cell surface antigen identified by monoclonal antibody on a Rous sarcoma virus-induced mouse tumor. J Natl Cancer Inst. 1982 Aug;69(2):527–530. [PubMed] [Google Scholar]
- Kuzumaki N., Oda A., Yamagiwa S., Taniguchi N., Kobayashi H., Oikawa T. Establishment of four mouse hybridoma cell lines producing monoclonal antibodies reactive with ras oncogene product p21. J Natl Cancer Inst. 1986 Dec;77(6):1273–1279. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Avery R. J. Integration of proviral DNA in Kirsten murine sarcoma virus-infected mouse fibroblasts. J Gen Virol. 1984 Feb;65(Pt 2):309–316. doi: 10.1099/0022-1317-65-2-309. [DOI] [PubMed] [Google Scholar]
- Norton J. D., Cook F., Roberts P. C., Clewley J. P., Avery R. J. Expression of Kirsten murine sarcoma virus in transformed nonproducer and revertant NIH/3T3 cells: evidence for cell-mediated resistance to a viral oncogene in phenotypic reversion. J Virol. 1984 May;50(2):439–444. doi: 10.1128/jvi.50.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Kuzumaki N., Yamada T., Chiba I., Yamagiwa S. Suppression of transformed phenotypes in intraspecific somatic cell hybrid clones between the c-myc activating mouse plasmacytoma line and normal cells. Int J Cancer. 1987 May 15;39(5):604–610. doi: 10.1002/ijc.2910390511. [DOI] [PubMed] [Google Scholar]
- Oikawa T., Yuhki Y., Kondoh N., Abe K., Yuhki N., Ogiso Y., Kuzumaki N. c-myc expression and transformed phenotypes in hybrid clones between mouse plasmacytoma S194 cells and normal spleen cells or fibroblasts. Int J Cancer. 1988 Sep 15;42(3):435–440. doi: 10.1002/ijc.2910420321. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Sacks T. L., Hershey E. J., Stephenson J. R. Abelson murine leukemia virus-infected cell lines defective in transformation. Virology. 1979 Sep;97(2):231–240. doi: 10.1016/0042-6822(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Samid D., Flessate D. M., Friedman R. M. Interferon-induced revertants of ras-transformed cells: resistance to transformation by specific oncogenes and retransformation by 5-azacytidine. Mol Cell Biol. 1987 Jun;7(6):2196–2200. doi: 10.1128/mcb.7.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., McGrath J. P., Levinson A. D. A mutation at the ATP-binding site of pp60v-src abolishes kinase activity, transformation, and tumorigenicity. Mol Cell Biol. 1985 Jul;5(7):1772–1779. doi: 10.1128/mcb.5.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stenman G., Delorme E. O., Lau C. C., Sager R. Transfection with plasmid pSV2gptEJ induces chromosome rearrangements in CHEF cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):184–188. doi: 10.1073/pnas.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Characterization of morphologic revertants of murine and avian sarcoma virus-transformed cells. J Virol. 1973 Feb;11(2):218–222. doi: 10.1128/jvi.11.2.218-222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Shih M., Gilden R. V., Hatanaka M. Mutants of nonproducer cell lines transformed by murine sarcoma virus. 3. Detection and characterization of RNA specific for helper and sarcoma viruses. J Exp Med. 1974 Jul 1;140(1):218–224. doi: 10.1084/jem.140.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsh L. S., Shih T. Y. Metabolic turnover of human c-rasH p21 protein of EJ bladder carcinoma and its normal cellular and viral homologs. Mol Cell Biol. 1984 Aug;4(8):1647–1652. doi: 10.1128/mcb.4.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Yagihashi A., Sato N., Torigoe T., Okubo M., Konno A., Takahashi N., Yamashita T., Fujinaga K., Kuzumaki N., Kikuchi K. Identification of the transformation-associated cell surface antigen expressed on the rat fetus-derived fibroblast. Cancer Res. 1988 May 15;48(10):2798–2804. [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]