Abstract
Purpose
Malignant mesothelioma (MM) is a devastating disease with a need for new treatment strategies. In the present study we demonstrated the importance of ERK5 in MM tumor growth and treatment.
Experimental Design
ERK5 as a target for MM therapy was verified using mesothelial and mesothelioma cell lines as well as by xenograft SCID mouse models.
Results
We first showed that crocidolite asbestos activated ERK5 in LP9 cells and mesothelioma cell lines exhibit constitutive activation of ERK5. Addition of doxorubicin resulted in further activation of ERK5 in MM cells. ERK5 silencing increased DOX-induced cell death and DOX retention in MM cells. In addition, shERK5 MM lines exhibited both attenuated colony formation on soft agar and invasion of MM cells in vitro that could be related to modulation of gene expression linked to cell proliferation, apoptosis, migration/invasion and drug resistance as shown by microarray analysis. Most importantly, injection of shERK5 MM cell lines into SCID mice showed significant reduction in tumor growth using both subcutaneous and intraperitoneal models. Assessment of selected human cytokine profiles in peritoneal lavage fluid from IP shERK5 and control tumor-bearing mice showed that ERK5 was critical in regulation of various proinflammatory (RANTES/CCL5, MCP-1) and angiogenesis related (IL-8, VEGF) cytokines. Finally, use of doxorubicin and cisplatin in combination with ERK5 inhibition showed further reduction in tumor weight and volume in the IP model of tumor growth.
Conclusion
; ERK5 inhibition in combination with chemotherapeutic drugs is a beneficial strategy for combination therapy in MM patients.
Keywords: Malignant mesothelioma, asbestos, Mitogen activated protein kinases, Extracellular signal regulated kinase 5, Gene expression
Introduction
Malignant mesothelioma (MM) is an asbestos-associated cancer of the pleura, pericardium and peritoneum with an average time of survival less than 12 months after initial diagnosis (1, 2). Currently no successful single-modality therapy exists for MM patients. Doxorubicin (DOX) is one of the first DNA damaging chemotherapeutic drugs tested in MM and is still being used in combination with other modalities (3, 4). Both DOX and cisplatin in single agent-phase III studies increased survival in 7 to 23 % of patients (5).
Chemotherapeutic agents can activate signaling pathways responsible for drug resistance. For example, chemotherapeutic agents used in the treatment of MM can activate ERK1/2 signaling (6). Moreover, constitutive activation of extracellular signal regulated kinase 1 and 2 (ERK 1/2) occurs in many cancers including MMs (7) where they play important roles in MM development (8). Extracellular signal regulated kinase 5 (ERK5) is a mitogen activated protein kinase (MAPK) that is activated by dual phosphorylation via a unique MAPK/ERK kinase 5 (MEK5). The MEK5/ERK5 pathway is essential for blood vessel and cardiac development, and removal of MEK5 or ERK5 is embryonically lethal with defects in these tissues (9). Additionally, the MEK5/ERK5 pathway is important in regulating the proliferation and survival of endothelial cells (10, 11) and cells of the immune system (12). Recently it has been reported that high ERK5 expression in oral squamous cell carcinoma correlated with advanced tumor stage and the presence of lymph node metastasis (13). ERK5 immunoreactivity was significantly upregulated in high grade prostate cancer when compared to benign prostatic hyperplasia, and ERK5 overexpressing PC3 cells had enhanced proliferative, migrative, and invasive capabilities in vitro and were dramatically more efficient in tumor formation (14, 15). A link between overexpression of ERK5 and decreased disease-free survival in breast cancer patients has recently been reported (16), and inhibition of ERK5 decreased both proliferation and sensitization of cells to anti-HER2 therapies. In addition, ERK5 is a target for gene amplification at 17p11 in hepatocellular carcinoma (HCC) and is detected in approximately 50% of primary HCC tumors (17).
Although there is an existing link between ERK5 and various cancers, nothing is known about the role of ERK5 in MM tumorigenesis. Previous studies from our laboratory have shown that asbestos-induced proliferation of murine epithelial cells requires ERK5 activation (18). In addition, hepatocyte growth factor (HGF) mediated cell proliferation in selected MM cell lines is ERK5 dependent (19). In this preclinical study we show mechanistically that ERK5 plays a critical role in parameters of MM tumor development and demonstrate that inhibition of ERK5 alone or in combination with DOX or cisplatin is a potential therapeutic strategy for MMs.
Materials and Methods
Cell culture and exposure to agents
Human peritoneal mesothelial LP9/TERT-1 (LP9) cells (20) were obtained from Dr. James Rheinwald (Brigham and Women’s Hospital, Harvard University, Boston, MA). Human MM cell lines, H2373, H2595, H2461 and HP-1 were contributed by Dr. Harvey Pass (New York University, New York, NY) (21). HMESO cells, originally designated H-MESO-1, were isolated by Reale et al (22). All cells were cultured as reported previously (6). Cell lines were validated by STR DNA fingerprinting using the Promega CELL ID System (Promega, Madison, WI). The STR profiles are of human origin, and did not match known DNA fingerprints in the Cell Line Integrated Molecular Authentication database (http://bioinformatics.istge.it/clima/), but will serve as a reference for future work. The characterization of the NIEHS reference sample of crocidolite asbestos has been reported previously (23). Following sterilization under ultraviolet light overnight, particulates were prepared as described before (24) and a volume of this suspension was added to cells in medium to achieve the desired final concentration of 5 μg/cm2 area dish, a concentration causing apoptosis and compensatory proliferation of surrounding pleural mesothelial cells (25). The non-pathogenic control particle glass beads (GB), was used at an equal surface area (Polysciences Inc., Warrington, PA). The EGFR inhibitor, AG1478 (20 μM) and c-Met (HGFR) kinase inhibitor II (10 μM) were obtained from Calbiochem (La Jolla, CA). All inhibitors were added at effective concentrations reported previously in the literature for 24 h. Control cultures received medium without inhibitors but with vehicle (≤0.1% DMSO) instead and were treated identically. DOX and cisplatin were purchased from Sigma (St. Louis, MO) and epidermal growth factor (EGF, 5 ng/ml) was purchased from Calbiochem (La Jolla, CA). All experiments were performed in duplicate or more.
Western blot analysis
Western blot analysis were performed as described previously (26), using antibodies specific to total and phosphorylated ERK 1/2 and ERK5 (rabbit polyclonal anti-phospho-ERK5, 1:500, rabbit polyclonal anti-ERK5, 1:1000, rabbit polyclonal anti-ERK1/2, 1:1000 (Cell Signaling Technology, Danvers, MA), and total β-actin 1:2000 (Abcam, Cambridge, MA). QuantityOne was used to quantify band density, and phosphorylated protein levels were normalized to respective total protein levels, i.e. pERK5/ERK5). Blots are representative of at least 2–3 different experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNA was prepared using an RNeasy plus mini kit according to the manufacturer’s protocol (Qiagen, Valencia, CA) as described previously (26). Total RNA (1μg) was reverse-transcribed with random primers using the Promega AMV Reverse Transcriptase kit (Promega, Madison, WI) according to the recommendations of the manufacturer. Gene expression was quantified by TaqMan Real Time Q-PCR using the 7700 Sequence Prism Detector (Perkin Elmer Applied Biosystems, Foster City, CA) as described previously (8). Triplicate assays were performed with RNA samples isolated from at least 2 independent experiments.
Creation of shERK5 stable MM lines
Confluent HMESO or H2373 cells were transfected with either ERK5 or scrambled control Sure Silencing Plasmids (4 shConstructs per gene per cell line were used) from SA Biosciences (Frederick, MD), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After selection for 14 days in G418 (400 μg/ml)-containing medium, clones were screened by qRT-PCR for inhibition of ERK5 mRNA levels as compared to scrambled control (shCon) transfected clones. Two maximally inhibited clones from shERK5 transfected MM cells were processed by limited dilution to obtain clones in which individual ERK5 were inhibited by more than 70% in comparison to shCon clones. Following this procedure, shERK5 clones exhibiting inhibition of >70–80 % ERK5 expression were obtained. Inhibition of ERK5 in transfected MM cell lines was determined by qRT-PCR as well as by Western blot analysis.
Affymetrix gene profiling
Microarrays were performed on samples from 3 independent experiments as described previously (27). Each of the samples was analyzed on a separate array, i.e., N=3 arrays per MM cell line (3 independent biological replicates) (shCon and shERK5). A Human U133A 2.0 array (Affymetrix, Santa Clara, CA) was scanned twice (Hewlett-Packard GeneArray Scanner), the images overlaid, and the average intensities of each probe cell compiled. Microarray data were analyzed using GeneSifter software (VizX Labs, Seattle, WA). This program used a “t test” for pairwise comparison and a Benjamini-Hochberg test for false discovery rate (FDR 5%) to adjust for multiple comparisons. A 2-fold cut-off limit was used for significance.
Cell viability in response to Doxorubicin (DOX)
Transfected H2373 cells (shCon and shERK5) were grown to near confluence in 12 well plates and reduced to 0.5% FBS containing media 1 day before treatment. They were then treated with DOX for 24 h (N=3). Cells were trypsinized and counted with a haemocytometer.
Invasion assay
Modified Boyden chamber Transwell polycarbonate filters (6.5 mm in diameter, 8 μm pore size, Costar) were coated with 100 μl of Matrigel (BD Biosciences, Bedford, MA) at a 1:20 dilution in serum-free DMEM/F12 medium and were air dried for 24 h. Cells (1 X 105 cells) were then plated on coated inserts in serum-free medium. Medium containing 10% FBS was placed in the bottom wells. After 48–72 h, invading cells adherent to the undersurface of the inserts were determined as previously described (8).
Growth in soft agar
Anchorage-independent growth of MM cells was assessed by a colony formation assay in soft agar as previously published (8). The CytoSelect™ Cell Transformation Assay (Cell Biolabs, Inc. San Diego, CA) was used following the manufacturer’s instructions.
Flow cytometry
shCon and shERK5 MM cells were grown to confluence and then treated with DOX (0.5 or 5.0 μM) for 1 or 5 h. Negative controls had no drug added. Cells were washed 3X with PBS, trypsinized, counted, suspended in PBS, and DOX fluorescence was examined by flow cytometry as previously described (6).
Flourescence microscopy
shCon and shERK5 cells were grown to confluence in 4-chambered culture slides (BD Falcon, Bedford, MA) in media containing 10% FBS. Cells were either untreated or treated with 0.5 or 5 μM DOX for 1h or 5h at 37°C. Slides with attached cells were then washed in PBS and fixed in 100% MeOH for 20 min at −20°C. They were then washed in PBS and water, allowed to dry, coverslipped with Aqua Poly/Mount (Polysciences Inc., Warrington, PA) and were stored at 4°C until fluorescent images were acquired using an Olympus BX50 Light Microscope with attached mercury epi-fluorescence illumination.
Assessment of human MM tumor development in a subcutaneous (SC) SCID mouse model
HMESO or H2373 cells stably transfected with either shERK5 or shCon were injected into 4 subcutaneous sites (5 X 106 cells per injection site) on the dorsa of 6 wk-old Fox Chase SCID mice (Charles River Laboratories, Wilmington, MA). All mice (N=5–6/group) were weighed weekly and examined every other day for morbidity and for tumor growth (measured using a digital caliper). When the largest axis of the tumors in the shCon mice reached 1 cm, all mice were weighed and euthanized with IP injections of sodium pentobarbital, necropsied to determine possible gross metastases. Tumor volumes were calculated using the formula (π x long axis x short axis x short axis)/6 (28). Tumors were characterized by using previously described histochemical criteria (29). All experiments using mice were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Vermont, College of Medicine.
Assessment of human MM tumor development in an intraperitoneal (IP) SCID mouse model
ERK5 inhibited (shERK5) or control (shCon) HMESO cells (5X106 cells in 50 μl 0.9% NaCl, pH 7.4) were injected into the lower left quadrant of the peritoneal cavity of 6 week-old male Fox Chase SCID mice (n=5–6 mice/group). Two weeks post cell injection, two groups (shCon-DOX and shERK5-DOX) started receiving DOX (0.5 mg/kg, IP), 3 X per week for 2 weeks. The other two groups (shCon-saline and shERK5-saline) received equal volumes of saline for the same period of time. Four weeks post-MM cell injections, mice were euthanized as described above. Peritoneal lavage fluid (PLF) was collected and animals were closely examined for the presence of tumors. Weights and volumes of tumors were determined as reported previously (30). In a separate group of mice, cisplatin treatment was given as a single IP injection (2 mg/kg in 500 μl saline) two weeks after MM cell injection. Animals of this group were also euthanized 4 weeks post-MM cell injections.
Determination of inflammatory cell profiles in peritoneal lavage fluid (PLF) in the IP SCID mouse model
Following euthanization of mice PLF was collected as described previously (30). PLF was centrifuged at 1,000 rpm for 5 min at 4°C, the supernatant removed and stored at −80°C for cytokine analysis, and the cell pellet resuspended in 500 μl of 5% bovine serum albumin (BSA) in PBS. Cytospins were prepared and differential cell counts were assessed as described previously (30).
Analysis of cytokine concentrations in PLF
A custom made ELISA plate from Signosis BioSignal Capture (Sunnyvale, CA) containing 8 cytokines and growth factors (IL-6, IL-8, bFGF, G-CSF, VEGF, MCP-1, RANTES, PDGF-BB) was used as per manufacturer’s instructions. The cytokine selection was made based on our previously published report that these cytokines are significantly elevated in the PLF of an IP MM model after injection of 2 individual human MM lines (30). Amounts of different cytokines or growth factors in PLF were calculated using the standard for each cytokine run simultaneously on the same plate.
Statistical analyses
In all in vitro assays, at least 2–3 independent samples were examined at each time point per group in duplicate or triplicate experiments. Data were evaluated by either ANOVA using the Student Neuman-Keul’s procedure for adjustment of multiple pairwise comparisons between treatment groups, the non-parametric Kruskal-Wallis and Mann-Whitney tests or a two-tailed Student’s t-test. Differences with p values ≤ 0.05 were considered statistically significant. The difference in tumor growth rates in the subcutaneous MM model was assessed using a hierarchical regression model to take into account the correlation between repeated measurements on the same tumor and multiple tumors in the same animal. In this analysis, the regression coefficient describing tumor growth is modeled as a function of treatment group as well as random variation due to differences between animals and tumors on the same animal.
Results
Asbestos causes activation of ERK5 in human mesothelial cells (LP9/hTERT)
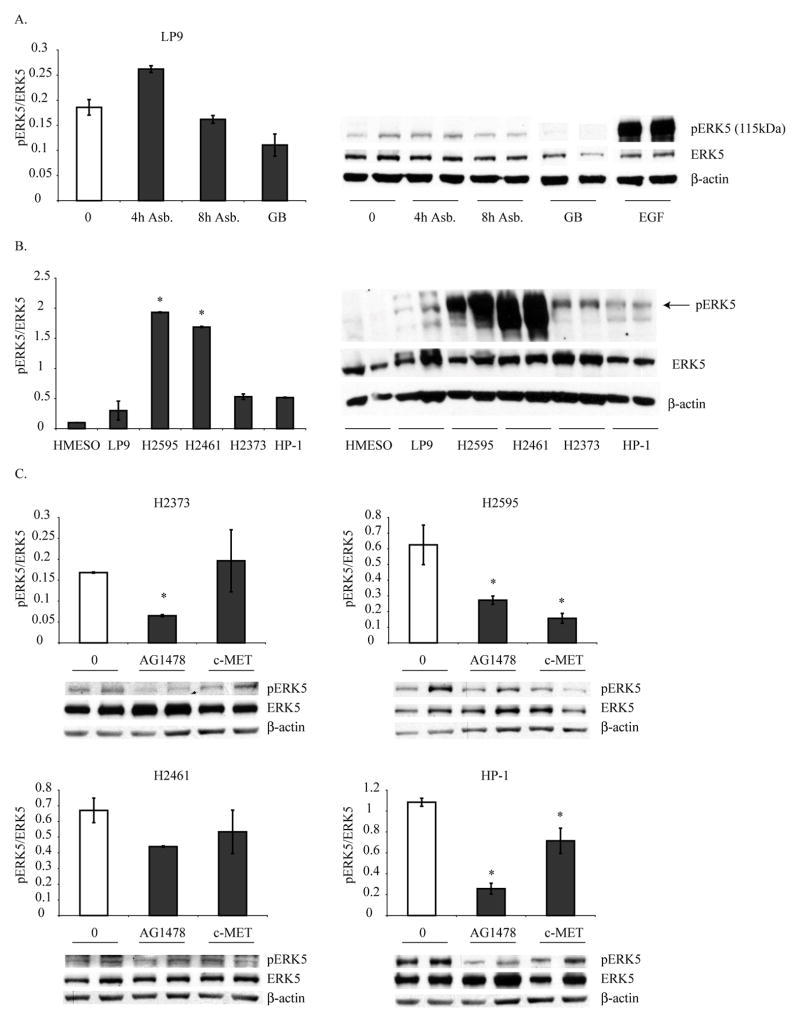
Human immortalized mesothelial cells (LP9) were exposed to crocidolite asbestos (1 and 5 μg/cm2) for 4 and 8 h, and ERK5 activation was assessed by Western blot analysis. Asbestos exposure caused increases in pERK5 levels (Fig 1A). Asbestos at a low concentration (1 μg/cm2) was not effective in activating ERK5 in these cells (data not shown). Glass beads (GB) a negative control at the same surface area concentration was ineffective. EGF was a striking positive control, suggesting a possible mechanism of asbestos-induced ERK5 activation through the EGFR.
Figure 1. Asbestos activates ERK5 which is constitutively activated in MMs.
A) Crocidolite asbestos (5 μg/cm2) increased ERK5 phosphorylation at 4 h after addition to human mesothelial cells (LP9). Glass beads (GB, a non-fibrous negative control) at the same surface area concentration had no effect on ERK5 activation, and epidermal growth factor (EGF) (5 ng/ml for 1h) significantly activated ERK5 several fold. B) Different human MM lines showed constitutive activation of ERK5 as compared to the LP9 cells. *p≤0.05 as compared to LP9. C) Effect of EGFR (AG1478, 20μM, 24 h) or HGFR/c-MET inhibitor (10 μM, 24 h) on constitutive pERK5 levels in 4 MM cell lines. *p≤0.05 as compared to DMSO (0) treated solvent controls. Each graph represents an independent experiment with its own control group.
Constitutive activation of ERK5 in human MMs
We then assessed 5 human MM cell lines (HMESO, H2373, H2595, H2461 and HP-1) for constitutive activation (phosphorylation) of ERK5. Four out of 5 MM lines showed increases in pERK5 levels as compared to LP9 (Fig 1B). Total ERK5 levels were not different in MM cell lines from LP9 cells. EGFR was also constitutively activated in MM cell lines as assessed by Western blot analysis (data not shown). To show that constitutive activation of ERK5 in MM cell lines is driven by EGFR or c-Met, we incubated 4 MM cell lines with either EGFR (AG1478, 20 μM) or a c-Met inhibitor (c-Met kinase inhibitor II, 10 μM) for 24 h before assessment of ERK5 activation. As shown in Fig 1C, both inhibitors had significant effects on diminishing elevated ERK5 activation in MM cell lines, suggesting the involvement of these 2 pathways in ERK5 activation in MMs.
Doxorubicin (DOX) activates ERK5 in MM cells
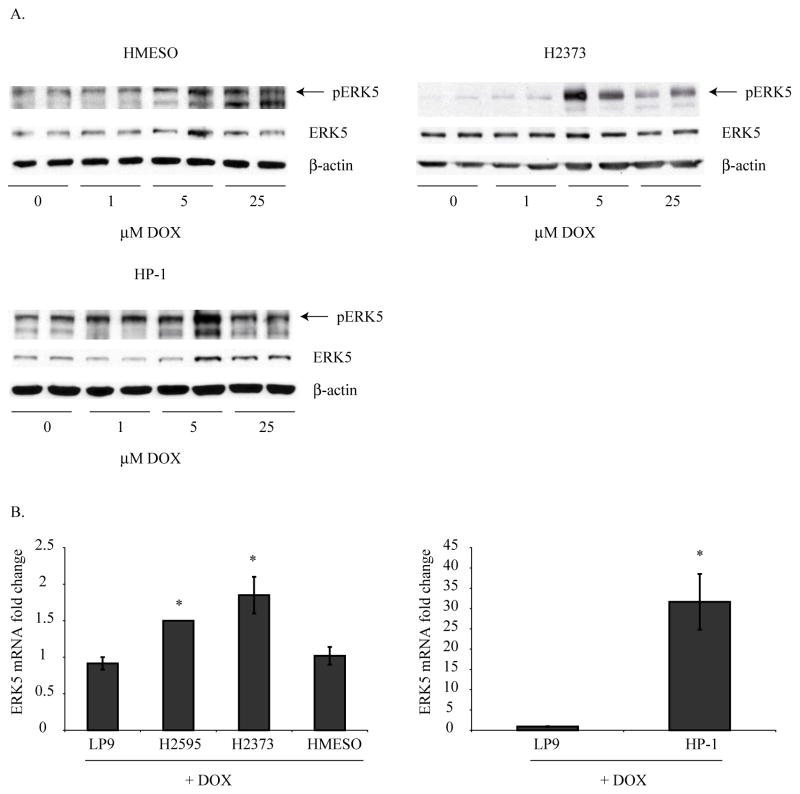
To assess the role of the chemotherapeutic drug, DOX, on ERK5 activation in MM cells, 5 different MM cell lines were exposed to DOX (1, 5 or 25 μM) for 24 h, and ERK5 activation was determined by Western blot analysis. As shown in Figure 2, 3 out of 5 MM cell lines showed significant activation of ERK5 by DOX. The 2 MM cell lines which showed very high constitutive levels of activated ERK5 (Fig 1B) did not show any further activation of ERK5 after DOX treatment (data not shown).
Figure 2. Doxorubicin (DOX) activates ERK5 in various human MM cell lines.
A) DOX treatment (1, 5, 25 μM, for 24 h) of various human MM cell lines resulted in activation of ERK5 as assessed by Western blot analysis. B) DOX treatment of LP9 and various MM cell lines resulted in increased steady-state mRNA levels of ERK5. Increases in ERK5 levels were significantly higher in MM cells as compared to DOX-treated LP9 cells. *p≤0.05 as compared to LP9.
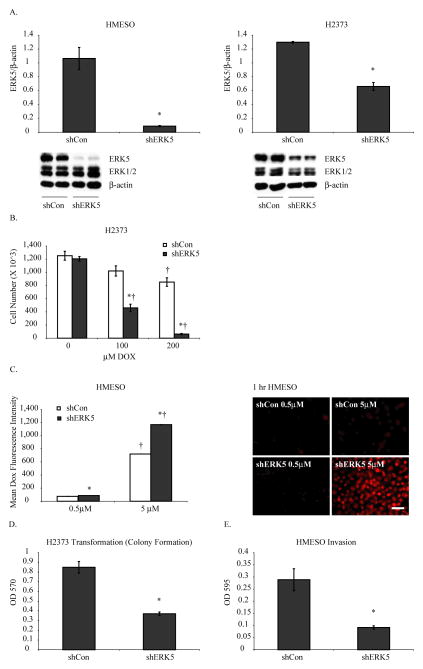
Figure 3. Human MM lines with stable ERK5 inhibition show reduced tumorigenic properties.
A) Stable transfection of human MM cells, HMESO and H2373, with shERK5 shows significant decreases in ERK5 protein as assessed by Western blot analysis as compared to scrambled control transfected cells (shCon). Transfection of human MM cell lines with shERK5 had no significant effects on protein levels of ERK1/2. *p≤0.05 as compared to shCon. B) ERK5 inhibited MM cells (H2373) show increased cell death in response to DOX (100, 200 μM, 24 h) as compared to shCon MM cells. *p≤0.05 as compared to shCon; †p≤0.05 as compared to respective control (0) of the same sh group. C) Stably inhibited ERK5 MM cells retain more DOX than shCon MM cells as assessed by flow cytometer and fluorescence microscopy (scale bar=50μm). *p≤0.05 as compared to shCon; †p≤0.05 as compared to 0.5 μM DOX of the same sh group. D) ERK5 silenced MM lines show fewer numbers of colonies than control MM cells. *p≤0.05 as compared to shCon. E) ERK5 silenced MM lines show significantly reduced migration in response to serum. *p≤0.05 as compared to shCon.
Steady-state mRNA levels of total ERK5 were also measured after DOX treatment (25 μM, 24 h), and an increasing trend was observed in all MM cell lines over DOX-treated LP9 cells. However, the HP-1 cell line showed a remarkable and highly significant increase as compared to LP9 treated with DOX (Fig 2B). These results suggest the role of DOX in activation of ERK5 and thereby in promotion of subsequent survival response.
ERK5 silencing attenuates tumorigenic properties of MM cells
We next created stably inhibited ERK5 (shERK5) MM lines (HMESO and H2373), which specifically showed downregulation of ERK5 (70–80%) without affecting ERK1 and ERK2 (Fig 3A). These cell lines were used to assess the role of ERK5 in various tumorigenic properties of MM cells under in vitro conditions. First, shERK5 and shCon MM cell lines were treated with different concentrations of DOX for 24 h, and viable cell numbers were determined by cell counting. A high concentration of DOX was used in the H2373 cell line because this line was very resistant to DOX as previously demonstrated (31). As shown in Figure 3B, ERK5 silencing rendered MM cells more sensitive to DOX-induced killing, suggesting a probable role of ERK5 in drug resistance. ERK5 silencing also caused significantly more DOX retention in cells as compared to shCon MM cells when assessed by flow cytometry and immunofluorescence (Fig 3C).To determine possible ERK5 involvement in parameters of tumorigenesis, invasion, migration and transformation assays were also performed. Although no effect of ERK5 inhibition on migration was observed in either MM cell line, colony formation on soft agar (Fig 3D) and invasion (Fig 3E) of MM cells was significantly reduced by ERK5 inhibition. Overall, the above data indicate a strong role of ERK5 in chemoresistance and promotion of MM tumorigenesis. We did not observe any significant effect of ERK5 inhibition on MM cell proliferation (data not shown).
ERK5 regulates MM tumor growth by modulating gene expressions
To understand the role of ERK5 in regulation of gene expression in MMs that are related to tumorigenesis, microarray analysis was performed on control and ERK5 inhibited stable MM cell lines (HMESO). Pairwise analysis with 2 fold threshold cutoff (p<0.05, FDR 5%) produced a list of 812 genes that were up or down regulated. Table 1 summarizes the important tumorigenesis related genes significantly modified by ERK5 inhibition. For example, ERK5 inhibition resulted in decreased proliferation/survival related genes and transcription factors like p21 activated kinase 7 (PAK7), chemokine ligand 5 (CCL5/RANTES), and AKT 3 as well as decreased invasion/migration related genes such as matrix metalloproteinases 1 and 9 (MMP1 and 9) and ATP binding Cassettes (ABC) (ABCC2, ABCA8, ABCC5, ABCB) involved in drug resistance. In addition, various apoptosis promoting genes were upregulated by ERK5 inhibition. These gene expression results show that ERK5 inhibition can attenuate tumor growth by regulating expression of many critical genes related to tumorigenesis.
Table 1.
Microarray analysis showing altered expression of important tumorigenesis related genes by ERK5 inhibition (shERK5) in HMESO cells as compared to control (shCon)
| Gene name | Increase/Decrease (fold) | Function | qRT-PCR Validation (fold decrease) |
|---|---|---|---|
| Matrix metalloproteinase 1 (MMP1) | Decreased (83) | Migration/invasion | 111 |
| Bone morphogenic protein 5 (BMP5) | Decreased (15) | Invasion | 108 |
| P21 (CDKN1A)-activated kinase7 (PAK7) | Decreased (11) | Proliferation/survival signaling | 67 |
| Chemokine (c-c) ligand 5 (CCL5) | Decreased (9) | Proinflammatory | |
| ABCC2 | Decreased (4) | Drug resistance | 7 |
| MEKK5 | Increased (4) | Apoptosis | |
| Interleukin-6 (IL-6) | Decreased (3) | Proinflammatory cytokine | 8 |
| Matrix metalloprotease 9 (MMP9) | Decreased (3) | Migration/invasion | |
| MAP2K6/MEK6/MKK6 | Increased (3) | Apoptosis | |
| ABCA8 (ABC1) | Decreased (3) | Drug resistance | 7 |
| MAPK10 | Increased (3) | Apoptosis | |
| ABCC5 | Decreased (2) | Drug resistance | |
| ABCB (MDR/TAP) | Decreased (2) | Drug resistance | |
| AKT3 | Decreased (2) | Proliferation/survival |
ERK5 inhibition attenuates subcutaneous (SC) MM tumor growth
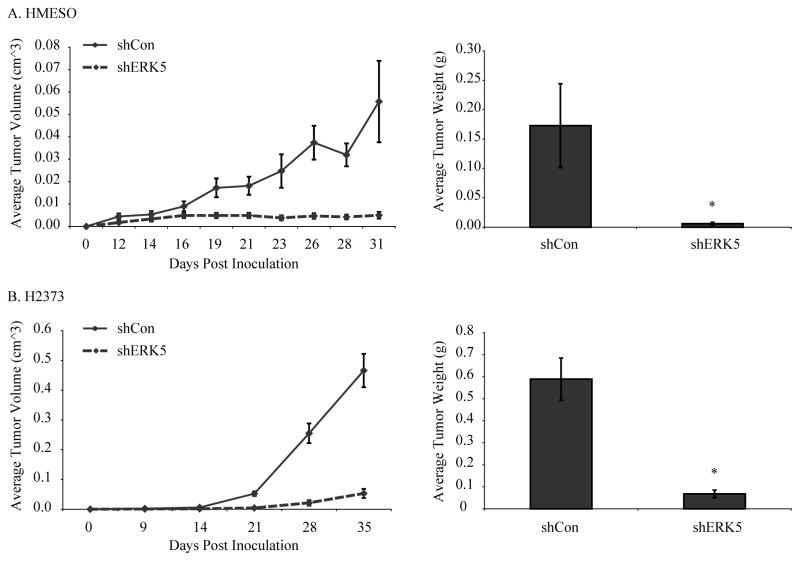
To assess the role of ERK5 in SC human MM cell growth, ERK5 inhibited (shERK5) and control (shCon) MM lines were injected SC into SCID mice as described previously (8) and tumor growth was followed over time (n=4–5 mice/group/time point or total of 16–20 observations/time point). ERK5 silencing resulted in a significantly slower growth rate of SC MMs as compared to controls. ERK5 silencing caused a significant inhibition in tumor growth using both MM cell lines as measured by both tumor volume over time and final tumor weights at autopsy (Fig 4). Negative control (shCon) data in Fig 4 have been published before as this also was the control group for a bigger experiment using shERK1 and shERK2 MMs (8).
Figure 4. Inhibition of ERK5 attenuates human MM tumor growth rate in a subcutaneous SCID mouse model.
A) ERK5-silenced (shERK5) HMESO MM cells or control (shCon) cells were injected at 4 subcutaneous sites on the dorsa of SCID mice (n= 4 mice per group), and tumor growth was followed over time (31 days). Statistical analysis revealed that shCon and shERK5 growth curves differed significantly (left, p<0.01). Comparison on each day showed shCon and shERK5 differed on days 19 through 31. B) ERK5 silenced (shERK5) H2373 MM cells or control (shCon) cells were injected at 4 subcutaneous sites on the dorsa of the SCID mice (n=5 mice per group) and tumor growth was monitored over time (for 35 days). Statistical analysis showed that growth rates for shCon differed significantly from shERK5 (left). The comparisons at each time point were based on the non-parametric Mann-Whitney tests. The differences in tumor growth rates between different groups (in both A and B left graphs) were assessed using a hierarchical regression model to take into account the correlation between repeated measurements on the same tumor and multiple tumors in the same animal. In this analysis, the regression coefficient describing tumor growth is modeled as a function of treatment group as well as random variation due to differences between animals and tumors on the same animal. The average final tumor weights measured at autopsy were also reduced in shERK5 MM cells injected into SCID mice (A,B right). *p≤0.05 as compared to shCon.
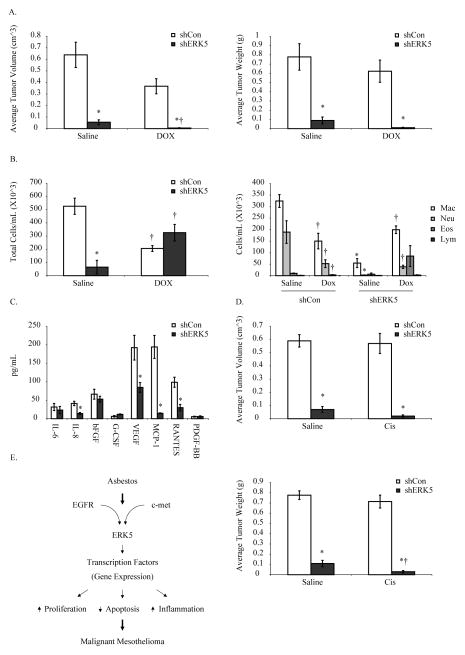
ERK5 silencing attenuates MM tumor burden in an intraperitoneal (IP) MM model and acts synergistically with DOX and cisplatin
shERK5 and shCon (HMESO) cells were injected IP into SCID mice as described previously (30). Tumor burden (volume and weight) (Fig 5A), total and differential cell counts in PLF (Fig 5B) and selected human cytokine levels in PLF (30) (Fig 5C) were assessed 4 wks post cell injections. A DOX alone treatment group was included and (0.5 mg/kg) did not produce any significant decreases in tumor burden by itself (Fig 5A). In contrast ERK5 inhibition significantly decreased tumor burden and further reduced tumor burden when combined with DOX (Fig 5A). ERK5 silencing also attenuated accumulation of inflammatory cells in PLF (Fig 5B), suggesting a possible role of ERK5 in inflammation. No significant effects of ERK5 inhibition on DOX-induced total or differential cell numbers in PLF were observed (Fig 5B, 5C). As depicted in Figure 5C, ERK5 silencing also significantly decreased the levels of many important cytokines and growth factors in PLF such as IL-8, VEGF, MCP-1 and RANTES. Taken together, data suggest that ERK5 regulates tumor growth in part by modulating inflammation.
Figure 5. ERK5 silencing inhibits MM tumor growth in an intraperitoneal (IP) MM model and synergizes with DOX and cisplatin.
A) ERK5-inhibited (shERK5) or control (shCon) HMESO MM cells were injected intraperitoneally into SCID mice (n=5 mice per group). DOX treatment (0.5 mg/kg in 500 μl saline, 3X per week for 2 weeks) was started 2 weeks post cell injections. Mice were sacrificed 4 weeks post cell injections, and tumor volumes and weights were calculated. *p≤0.05 as compared to shCon; †p≤0.05 as compared to respective saline-treated group. B) Total and differential cell counts performed on the peritoneal lavage fluid (PLF) collected from tumor bearing mice, showing the inflammatory cell profiles of control and shERK5 tumor bearing mice. *p≤0.05 as compared to shCon; †p≤0.05 as compared to respective saline controls. C) PLF supernatant from tumor bearing mice was analyzed for 8 different human cytokines using a custom kit as described in Methods. *p≤0.05 as compared to shCon. D) ERK5 inhibited (shERK5) and control (shCon) HMESO MM cells were injected IP in SCID mice (n=5 mice per group) and 2 weeks later a single dose of cisplatin (2 mg/kg, in 500 μl saline) was injected IP. Four weeks post cell injection, mice were sacrificed, and tumor volumes (top) and weights (bottom) were recorded. *p≤0.05 as compared to shCon; †p≤0.05 as compared to respective saline group. E) Schema showing how ERK5 can lead to MM development.
Cisplatin is part of the first-line FDA-approved treatment regimen for MM treatment, thus we chose to evaluate the effect of cisplatin in combination with ERK5 silencing using the same model. We selected a one-time dose of cisplatin at 2 mg/kg that does not result in tumor growth inhibition (32). The findings of this experiment further confirmed that ERK5 inhibition in combination with cisplatin, produces synergistic effects on diminishing MM tumor volume and weight (Fig 5D).
Discussion
Our group has shown that ERK1/2 contributes to the proliferation of human MM cells in vitro as well as in vivo. Moreover, we have demonstrated that ERK1 and ERK2 are needed for growth of a sarcomatoid MM cell line in SCID mice, whereas ERK2 alone is necessary for the development of epithelioid MM tumors. Here, using various in vitro and in vivo models, we demonstrate for the first time that ERK5 plays crucial roles in MM development and can be targeted in combination with chemotherapeutic drugs to attenuate MM tumor growth.
In cancers, there is limited clinical evidence that an increase in MEK5/ERK5 signaling may be important for disease progression. For example, it has been reported in breast cancer that MEK5 expression is up-regulated by constitutive activation of STAT3 (33) and increased ERK5 protein levels are associated with decreased disease-free survival (16). In addition, MEK5 is overexpressed in prostate cancer and presents a poor prognosis (15). Studies also have shown the regulation of ERK5 by EGF in different cells (34). Here we demonstrate activation of ERK5 by either asbestos or EGF in human mesothelial cells. We also make the link that constitutively activated ERK5 in various MM cell lines could be a consequence of activated EGFR or hepatocyte growth factor receptor (HGFR or c-MET), as inhibitors of these receptors significantly blocked ERK5 activation. These results support a previous study from our group showing that HGF promotes proliferation of different MM cells via ERK5 activation (19). Both EGFR (35) and HGFR (36) are overexpressed in mesothelioma and recently, the combined inhibition of both c-MET and EGFR has shown a greater anti-proliferative effect than that obtained by a single inhibitor (37). Our data also indicate that combined inhibition of both receptors may have a more striking effect on ERK5 inhibition in MM cells. In the present study we could not find a link between constitutive activation of ERK5 and MM aggressiveness. For example, HMESO cells with no significant constitutive ERK5 activation showed significant decreases in tumorigenesis by ERK5 inhibition both in vitro and in vivo. This suggests that even small endogenous levels of ERK5 play an important role in MM tumorigenesis and could be targets for therapy.
In our studies various MM cell lines exposed to DOX concentrations much lower than the LD50 concentration in these lines caused increases in ERK5 activation. These and previous work (6) suggests that chemotherapeutic drugs activate survival pathways in MM cells. These findings and our results in two MM SCID mice models show that inhibition of ERK5 in combination with chemotherapeutic drugs is a better approach to suppress tumorigenesis. Two of the 5 MM cell lines we studied that had large amounts of constitutive pERK5 (Fig 1) did not show any further ERK5 activation after DOX treatment (data not shown), signifying that a threshold activation occurs. Although a study by Yan et al. (38) shows that ERK5 gene and protein levels are upregulated in DOX-resistant hepatocellular carcinoma cell lines, we did not find a correlation between increased constitutive ERK5 activation and DOX-induced cell death (31) in any of the 5 MM cell lines we evaluated. Activation of ERK1/2 by chemotherapeutic drugs has been demonstrated previously from our group as well as others in various tumors, including MMs (6, 39).
We created two ERK5 silenced stable MM cell lines to study the effect of ERK5 on MM tumorigenesis. A significant inhibition of ERK5 protein levels were observed without any effects on ERK1 and ERK2 levels (Fig 3), suggesting the specificity of ERK5 inhibition. Although a role of ERK5 in cell survival, antiapoptotic signaling, angiogenesis, cell migration and cell proliferation has been reported previously in various cells and tumors, we are the first to report the effect of ERK5 inhibition on MM tumorigenesis. ERK5 inhibition decreased DOX-induced cell viability of MM cells and increased DOX retention as measured by flow cytometry and fluorescence microscopy. These findings indicate a role of ERK5 in chemoresistance of MMs and are consistent with reports describing ERK5-induced chemoresistance in breast cancer cell lines (16, 40). We further confirmed our in vitro data with in vivo MM tumor development models in SCID mice. In these experiments, ERK5 silenced MM cells showed a significant decrease in MM tumor growth rate (SC model) and tumor volume (IP and SC models) as compared to tumors from transfected control MM cells. Furthermore, ERK5 inhibition produced significantly better effects on MM tumor reduction using DOX or cisplatin than either drug alone. Acquired resistance is a significant problem with all current MM therapeutic approaches and therefore, inhibition of the ERK5 pathway in combination with chemotherapeutic drugs could be a more effective strategy for MM treatment.
To investigate the mechanisms by which ERK5 inhibition contributes to the process of MM chemoresistance, we performed microarray analysis on control and ERK5-silenced MM cell lines. As listed in Table 1, ATP-binding cassette (ABC) transporters [ABCB (MDR/TAP), ABCC2, ABCA8, ABCC5] which are known transporters of various molecules, including chemotherapeutic drugs, across extra- and intracellular membranes were down regulated 2–4 fold by ERK5 attenuation. Increased expression of one or more ABC proteins is observed in almost all chemoresistant cancers and this is likely responsible for observed drug resistance. Inhibition of certain ABC transporters by ERK5 could be one of many mechanisms of how ERK5 regulates chemoresistance as we have previously reported that MM cells overexpress many of these ABC transporters, some of which could be attenuated by ERK1/2 inhibition (6).
To further understand the mechanisms of reduced MM tumorigenesis by ERK5 silencing, we assessed inflammatory profiles in PLF samples from tumor-bearing SCID mice. ERK5 inhibition significantly attenuated infiltration of macrophages and neutrophils, known perpetrators of inflammation in PLF. In addition to affecting infiltration of inflammatory cells, ERK5 inhibition also significantly decreased levels of various human cytokines (IL-8, VEGF, MCP-1, CCL5/RANTES) in PLF. Human cytokine levels were assessed exclusively in PLF to ensure that only cytokines secreted by human MMs were evaluated. IL-8 and VEGF are two cytokines involved in the process of angiogenesis and are known to be highly elevated in MM. MM patients have the highest VEGF levels of any solid tumor patients (41) and elevated VEGF levels in pleural effusion are associated with diminished survival in MM patients. ERK5 has been reported to play important roles in endothelial cell proliferation, survival and angiogenesis (10, 42) and in an in vitro angiogenesis system, ERK5 is required for VEGF-induced tubular morphogenesis (11). In our study, ERK5 silencing attenuated levels of both IL-8 and VEGF protein in PLF as measured by ELISA. As VEGF and IL-6 (as shown in Table 1) are prototypical STAT3-indicible factors and STAT3 activation was inhibited in ERK5-attenuated MM cells (data not shown), the ERK5-mediated effects could in part be STAT3-dependent. Thus our data strongly support the role of ERK5 in the regulation of angiogenesis in MMs.
The chemokine CCL5 (RANTES) is produced by many cell types including mesothelial cells (43) and attracts eosinophils, monocytes and lymphocytes. CCL5 is known to be overexpressed in many cancers and plays important roles in proliferation, invasion and angiogenesis of cancer cells. We report for the first time that ERK5 silencing plays an important role in reducing the expression of this gene in MM cells in vitro as well as secretion of this protein in PLF of MM tumor bearing mice. Another multifunctional cytokine, IL-6, which is linked to the promotion of tumorigenesis in various malignancies including MM (44), is known to be regulated by ERK5 (45) and was also downregulated by ERK5 silencing in MM cells. Levels of macrophage chemoattractant protein-1 (MCP-1) were also decreased significantly in PLF of ERK5-inhibited groups. Decreased CCL5/RANTES and MCP-1 may be responsible for the decreased number of macrophages and neutrophils seen in PLF of these mice, as both of these cytokines are involved in chemoattraction of different inflammatory cells. A review of the current literature provides only limited information about role of ERK5 in inflammation (46), and offers no connection relating ERK5 to inflammation in cancer. As per this knowledge, ours is the first report showing that ERK5 inhibition can attenuate MM tumor growth primarily by inhibiting inflammation.
A novel contribution of our studies is the revelation of various ERK5-linked genes that may be related to MM tumorigenesis. The major genes which may be involved in MM tumorigenesis are described in Table 1. For example, the proliferation and survival signal related genes, PAK7 and AKT3, were significantly inhibited by ERK5 silencing. ERK5 has been implicated in both cell proliferation and survival of normal and cancer cells, and AKT has been shown to play a key role in these processes in many cancers (11, 47, 48). In addition, silencing of ERK5 decreased invasion of MM cells which may correlate with significantly diminished levels of MMPs 1, 9 and BMP5 genes actively involved in migration and invasion. In an earlier study, overexpression of MEK5/ERK5 was strongly associated with the bony metastases and reduced disease-specific survival of prostate cancer patients (15). In this study, MEK5/ERK5 expression was linked to increased proliferation, invasion and increased expression of MMP9.
Overexpression of constitutively activated MEK5 restricts caspase-3 activity and inhibits apoptosis in endothelial cells (49). Our findings also demonstrate that ERK5 inhibition may inhibit MM tumor development by elevating the expression of apoptosis-related genes and thereby apoptosis. As shown in Table 1, MEKK5/ASK1/MAPKKK5 (activator of proapoptotic pathways, JNK), MKK6/MAPKK6 (activator of proapoptotic pathway p38 MAPK) and MAPK10 (JNK activator) were upregulated by ERK5 inhibition. Our gene expression studies suggest that the enhancing effect of ERK5 on survival, proliferation, migration/invasion, drug resistance and angiogenesis-related genes as well as its inhibitory effect on apoptotic genes, contribute to its multifunctional roles in tumorigenesis.
Based upon data, we conclude that ERK5 is constitutively activated in MM cells and plays a crucial role in MM tumorigenesis. Furthermore, ERK5 inhibition can significantly reduce tumor growth, and when used in combination with chemotherapeutic drugs, can have a far better effect on MM tumor reduction when compared to either drug alone. Overall, our findings suggest that the combined effects of anti-inflammatory, anti-chemoresistant, antiangiogenic, and proapoptotic properties associated with ERK5 inhibition play significant roles in curtailing MM tumor development and drug resistance. Since ERK-5 induced effects are multifaceted, redundancy in the pathways involved could lead to paradoxical results. The strength of this work lies in the fact that two different in vivo MM models in conjunction with two chemotherapeutic drugs resulted in the same outcomes. Future studies will entail examination of specific ERK5 inhibitors alone and in combination with chemotherapeutic drugs on MM tumor development.
Translational Relevance.
Malignant mesothelioma (MM) is a devastating cancer with poor therapeutic options. The current standard of care therapy with cisplatin plus pemetrexed only improves overall survival in MM patients from 9 to 12 months demonstrating the critical need for novel therapeutic agents. Here we are the first to show that inhibition of ERK5 in MM tumors in vivo resulted in significantly decreased tumor burden and produced a synergistic effect with chemotherapeutic drugs. These findings strongly implicate ERK5 as a novel target for MM treatment. ERK5 inhibitors such as XMD8-92 are now commercially available and could be evaluated along with standard of care chemotherapy regimens in future MM clinical trials.
Acknowledgments
We acknowledge the help from the Vermont Cancer Center (VCC) DNA Analysis Facility for qRTPCR and the INBRE/VGN Micorarray Facility for microarrays. We also acknowledge the help of Colette Charland for help with Flow Cytometer.
Grant Support: Mesothelioma Applied Research Foundation grant (A Shukla); NIEHS RO1 ES021110-02 grant (A Shukla); Vermont Cancer Center/Lake Champlain Cancer Research Organization grant (A Shukla) and NIEHS T32ESO77122 grant (BT Mossman)
Footnotes
Conflict of interests:
none
Microarray data submitted to GEO repository- Accession number GSE 21750
References
- 1.Mossman BT, Gee JB. Asbestos-related diseases. The New England journal of medicine. 1989;320:1721–30. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- 2.Mossman BT, Kamp DW, Weitzman SA. Mechanisms of carcinogenesis and clinical features of asbestos-associated cancers. Cancer investigation. 1996;14:466–80. doi: 10.3109/07357909609018904. [DOI] [PubMed] [Google Scholar]
- 3.Scherpereel A, Berghmans T, Lafitte JJ, Colinet B, Richez M, Bonduelle Y, et al. Valproate-doxorubicin: promising therapy for progressing mesothelioma. A phase II study. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2011;37:129–35. doi: 10.1183/09031936.00037310. [DOI] [PubMed] [Google Scholar]
- 4.Saxena A, Chua TC. Results of systemic pemetrexed-based combination chemotherapy versus cytoreductive surgery and hyperthermic intraperitoneal cisplatin and doxorubicin on survival in malignant peritoneal mesothelioma. Lung Cancer. 2009;66:269–70. doi: 10.1016/j.lungcan.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Janne PA. First line chemotherapy for malignant pleural mesothelioma. In: Pass NV HI, Carbone M, editors. Malignant Mesothelioma: Advances in pathogenesis, and translational therapies. New York, NY: Springer Science+Business Media, Inc; 2005. pp. 21–33. [Google Scholar]
- 6.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, et al. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Molecular cancer. 2010;9:314. doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Melo M, Gerbase MW, Curran J, Pache JC. Phosphorylated extracellular signal-regulated kinases are significantly increased in malignant mesothelioma. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2006;54:855–61. doi: 10.1369/jhc.5A6807.2006. [DOI] [PubMed] [Google Scholar]
- 8.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Butnor KJ, et al. ERK2 is essential for the growth of human epithelioid malignant mesotheliomas. International journal of cancer Journal international du cancer. 2011;129:1075–86. doi: 10.1002/ijc.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9248–53. doi: 10.1073/pnas.142293999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, et al. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. The Journal of clinical investigation. 2004;113:1138–48. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts OL, Holmes K, Muller J, Cross DA, Cross MJ. ERK5 is required for VEGF-mediated survival and tubular morphogenesis of primary human microvascular endothelial cells. Journal of cell science. 2010;123:3189–200. doi: 10.1242/jcs.072801. [DOI] [PubMed] [Google Scholar]
- 12.Rovida E, Spinelli E, Sdelci S, Barbetti V, Morandi A, Giuntoli S, et al. ERK5/BMK1 is indispensable for optimal colony-stimulating factor 1 (CSF-1)-induced proliferation in macrophages in a Src-dependent fashion. J Immunol. 2008;180:4166–72. doi: 10.4049/jimmunol.180.6.4166. [DOI] [PubMed] [Google Scholar]
- 13.Sticht C, Freier K, Knopfle K, Flechtenmacher C, Pungs S, Hofele C, et al. Activation of MAP kinase signaling through ERK5 but not ERK1 expression is associated with lymph node metastases in oral squamous cell carcinoma (OSCC) Neoplasia. 2008;10:462–70. doi: 10.1593/neo.08164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCracken SR, Ramsay A, Heer R, Mathers ME, Jenkins BL, Edwards J, et al. Aberrant expression of extracellular signal-regulated kinase 5 in human prostate cancer. Oncogene. 2008;27:2978–88. doi: 10.1038/sj.onc.1210963. [DOI] [PubMed] [Google Scholar]
- 15.Mehta PB, Jenkins BL, McCarthy L, Thilak L, Robson CN, Neal DE, et al. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene. 2003;22:1381–9. doi: 10.1038/sj.onc.1206154. [DOI] [PubMed] [Google Scholar]
- 16.Montero JC, Ocana A, Abad M, Ortiz-Ruiz MJ, Pandiella A, Esparis-Ogando A. Expression of Erk5 in early stage breast cancer and association with disease free survival identifies this kinase as a potential therapeutic target. PloS one. 2009;4:e5565. doi: 10.1371/journal.pone.0005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zen K, Yasui K, Nakajima T, Zen Y, Gen Y, Mitsuyoshi H, et al. ERK5 is a target for gene amplification at 17p11 and promotes cell growth in hepatocellular carcinoma by regulating mitotic entry. Genes, chromosomes & cancer. 2009;48:109–20. doi: 10.1002/gcc.20624. [DOI] [PubMed] [Google Scholar]
- 18.Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23:805–13. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Nino ME, Blumen SR, Sabo-Attwood T, Pass H, Carbone M, Testa JR, et al. HGF mediates cell proliferation of human mesothelioma cells through a PI3K/MEK5/Fra-1 pathway. American journal of respiratory cell and molecular biology. 2008;38:209–17. doi: 10.1165/rcmb.2007-0206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Molecular and cellular biology. 2000;20:1436–47. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pass HI, Stevens EJ, Oie H, Tsokos MG, Abati AD, Fetsch PA, et al. Characteristics of nine newly derived mesothelioma cell lines. The Annals of thoracic surgery. 1995;59:835–44. doi: 10.1016/0003-4975(95)00045-m. [DOI] [PubMed] [Google Scholar]
- 22.Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A. Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer research. 1987;47:3199–205. [PubMed] [Google Scholar]
- 23.Campbell WJ, Huggins CW, Wylie AG. Chemical and physical characterization of amosite, chrysotile, crocidolite, and nonfibrous tremolite for oral ingestion studies. Washington, DC: National Institute of Environmental Health Sciences; 1980. Report No.: 8542. [Google Scholar]
- 24.Shukla A, MacPherson MB, Hillegass J, Ramos-Nino ME, Alexeeva V, Vacek PM, et al. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. American journal of respiratory cell and molecular biology. 2009;41:114–23. doi: 10.1165/rcmb.2008-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg JL, Zanella CL, Janssen YM, Timblin CR, Jimenez LA, Vacek P, et al. Novel cell imaging techniques show induction of apoptosis and proliferation in mesothelial cells by asbestos. Am J Respir Cell Mol Biol. 1997;17:265–71. doi: 10.1165/ajrcmb.17.3.2991. [DOI] [PubMed] [Google Scholar]
- 26.Shukla A, Barrett TF, Nakayama KI, Nakayama K, Mossman BT, Lounsbury KM. Transcriptional up-regulation of MMP12 and MMP13 by asbestos occurs via a PKCdelta-dependent pathway in murine lung. Faseb J. 2006;20:997–9. doi: 10.1096/fj.05-4554fje. [DOI] [PubMed] [Google Scholar]
- 27.Shukla A, Macpherson MB, Hillegass J, Ramos-Nino ME, Alexeeva V, Vacek PM, et al. American journal of respiratory cell and molecular biology. 2008. Dec 18, Alterations in Gene Expression in Human Mesothelial Cells Correlate with Mineral Pathogenicity. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki E, Kim S, Cheung HK, Corbley MJ, Zhang X, Sun L, et al. A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67:2351–9. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- 29.King JE, Galateau-Salle F, Haselton PS. Histopathology of malignant pleural mesothelioma. In: O’Byrne K, Rusch V, editors. Malignant Pleural Mesothelioma. New York: Oxford University Press; 2006. pp. 61–103. [Google Scholar]
- 30.Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Beuschel SL, Butnor KJ, et al. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Annals of the New York Academy of Sciences. 2010;1203:7–14. doi: 10.1111/j.1749-6632.2010.05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillegass JM, Blumen SR, Cheng K, MacPherson MB, Alexeeva V, Lathrop SA, et al. Increased efficacy of doxorubicin delivered in multifunctional microparticles for mesothelioma therapy. International journal of cancer Journal international du cancer. 2011;129:233–44. doi: 10.1002/ijc.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandermeers F, Hubert P, Delvenne P, Mascaux C, Grigoriu B, Burny A, et al. Valproate, in combination with pemetrexed and cisplatin, provides additional efficacy to the treatment of malignant mesothelioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2818–28. doi: 10.1158/1078-0432.CCR-08-1579. [DOI] [PubMed] [Google Scholar]
- 33.Song H, Jin X, Lin J. Stat3 upregulates MEK5 expression in human breast cancer cells. Oncogene. 2004;23:8301–9. doi: 10.1038/sj.onc.1208026. [DOI] [PubMed] [Google Scholar]
- 34.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–6. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 35.Destro A, Ceresoli GL, Falleni M, Zucali PA, Morenghi E, Bianchi P, et al. EGFR overexpression in malignant pleural mesothelioma. An immunohistochemical and molecular study with clinico-pathological correlations. Lung Cancer. 2006;51:207–15. doi: 10.1016/j.lungcan.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Klominek J, Baskin B, Liu Z, Hauzenberger D. Hepatocyte growth factor/scatter factor stimulates chemotaxis and growth of malignant mesothelioma cells through c-met receptor. International journal of cancer Journal international du cancer. 1998;76:240–9. doi: 10.1002/(sici)1097-0215(19980413)76:2<240::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi K, Murakami H, Taniguchi T, Fujii M, Kawata S, Fukui T, et al. Combined inhibition of MET and EGFR suppresses proliferation of malignant mesothelioma cells. Carcinogenesis. 2009;30:1097–105. doi: 10.1093/carcin/bgp097. [DOI] [PubMed] [Google Scholar]
- 38.Yan F, Wang XM, Pan C. Expression of ERK5 in multidrug-resistant hepatocellular carcinoma cell line. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2009;29:483–6. [PubMed] [Google Scholar]
- 39.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. The Journal of biological chemistry. 2000;275:39435–43. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Nitschke AM, Xiong W, Zhang Q, Tang Y, Bloch M, et al. Proteomic analysis of tumor necrosis factor-alpha resistant human breast cancer cells reveals a MEK5/Erk5-mediated epithelial-mesenchymal transition phenotype. Breast cancer research : BCR. 2008;10:R105. doi: 10.1186/bcr2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linder C, Linder S, Munck-Wikland E, Strander H. Independent expression of serum vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in patients with carcinoma and sarcoma. Anticancer research. 1998;18:2063–8. [PubMed] [Google Scholar]
- 42.Hayashi M, Fearns C, Eliceiri B, Yang Y, Lee JD. Big mitogen-activated protein kinase 1/extracellular signal-regulated kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer research. 2005;65:7699–706. doi: 10.1158/0008-5472.CAN-04-4540. [DOI] [PubMed] [Google Scholar]
- 43.Katayama H, Yokoyama A, Kohno N, Sakai K, Hiwada K, Yamada H, et al. Production of eosinophilic chemokines by normal pleural mesothelial cells. American journal of respiratory cell and molecular biology. 2002;26:398–403. doi: 10.1165/ajrcmb.26.4.4613. [DOI] [PubMed] [Google Scholar]
- 44.Monti G, Jaurand MC, Monnet I, Chretien P, Saint-Etienne L, Zeng L, et al. Intrapleural production of interleukin 6 during mesothelioma and its modulation by gamma-interferon treatment. Cancer research. 1994;54:4419–23. [PubMed] [Google Scholar]
- 45.Carvajal-Vergara X, Tabera S, Montero JC, Esparis-Ogando A, Lopez-Perez R, Mateo G, et al. Multifunctional role of Erk5 in multiple myeloma. Blood. 2005;105:4492–9. doi: 10.1182/blood-2004-08-2985. [DOI] [PubMed] [Google Scholar]
- 46.Xiao C, Zhang L, Cheng QP, Zhang LC. The activation of extracellular signal-regulated protein kinase 5 in spinal cord and dorsal root ganglia contributes to inflammatory pain. Brain research. 2008;1215:76–86. doi: 10.1016/j.brainres.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Finegan KG, Robinson AC, Knowles L, Khosravi-Far R, Hinchliffe KA, et al. Activation of extracellular signal-regulated protein kinase 5 downregulates FasL upon osmotic stress. Cell death and differentiation. 2006;13:2099–108. doi: 10.1038/sj.cdd.4401969. [DOI] [PubMed] [Google Scholar]
- 48.Razumovskaya E, Sun J, Ronnstrand L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochemical and biophysical research communications. 2011;412:307–12. doi: 10.1016/j.bbrc.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 49.Pi X, Yan C, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circulation research. 2004;94:362–9. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]