Abstract
Purpose
We tested the hypothesis that low intensity vibration training in mice improves contractile function of hindlimb skeletal muscles and promotes exercise-related cellular adaptations.
Methods
We subjected C57BL/6J mice to 6 wk, 5 d·wk−1, 15 min·d−1 of sham or low intensity vibration (45 Hz, 1.0 g) while housed in traditional cages (Sham-Active, n=8; Vibrated-Active, n=10) or in small cages to restrict physical activity (Sham-Restricted, n=8; Vibrated-Restricted, n=8). Contractile function and resistance to fatigue were tested in vivo (anterior and posterior crural muscles) and ex vivo on the soleus muscle. Tibialis anterior and soleus muscles were evaluated histologically for alterations in oxidative metabolism, capillarity, and fiber types. Epididymal fat pad and hindlimb muscle masses were measured. Two-way ANOVAs were used to determine effects of vibration and physical inactivity.
Results
Vibration training resulted in a 10% increase in maximal isometric torque (P=0.038) and 16% faster maximal rate of relaxation (P=0.030) of the anterior crural muscles. Posterior crural muscles were unaffected by vibration, with the exception of greater rates of contraction in Vibrated-Restricted mice compared to Vibrated-Active and Sham-Restricted mice (P=0.022). Soleus muscle maximal isometric tetanic force tended to be greater (P=0.057) and maximal relaxation was 20% faster (P=0.005) in Vibrated compared to Sham mice. Restriction of physical activity induced muscle weakness but was not required for vibration to be effective in improving strength or relaxation. Vibration training did not impact muscle fatigability or any indicator of cellular adaptation investigated (P≥0.431). Fat pad but not hindlimb muscle masses were affected by vibration training.
Conclusion
Vibration training in mice improved muscle contractility, specifically strength and relaxation rates, with no indication of adverse effects to muscle function or cellular adaptations.
Keywords: Exercise, Fatigue, Mechanical oscillation, Strength, Whole body vibration
Vibration training has emerged as a therapeutic strategy to improve the musculoskeletal system in health and rehabilitation. In health, it is utilized as an exercise modality to better athletic performance (reviewed in (35)). In rehabilitation, bone has been the primary focus because it has been shown to be osteogenic (32). Vibration training is also being explored as an alternative exercise modality for conditions of muscle weakness, such as in geriatrics and with bedrest. The results of these studies have been largely mixed. For example, a meta-analysis showed that whole body vibration training was affective in improving leg muscle strength in older adults (14), while another analysis concluded that there is only weak support for the efficacy of whole body vibration for muscle strength (17). Similarly, some but not all studies on young healthy adults show that vibration training improves muscle function (reviewed in (26). Thus, the broader application of vibration training to musculoskeletal health is limited because its effects on skeletal muscles are not unanimous.
One aspect that likely contributes to the various results of vibration training on skeletal muscle function is the magnitude of acceleration, or intensity of the vibration. This parameter is typically expressed as a fraction of gravity (g) and it is a primary factor that dictates the mechanical signal delivered from the oscillating platform to the subject standing on the device. The majority of commercially-available, whole body vibration equipment delivers accelerations >1.0 g, referred to as “high intensity” vibration in this paper. Vibration platforms that deliver accelerations <1.0 g (“low intensity”) have also been investigated. In terms of effects on skeletal muscle, it has been shown that 2–12 months of low intensity vibration training results in increased muscle strength (19), balance (19), grip strength (25), and muscle mass (11, 24). Subjects in those studies were selected based on clinical conditions associated with poor bone health, and it is possible that muscle weakness was also a characteristic of those subjects. Thus, it is not clear if low intensity vibration training has the potential to enhance skeletal muscle of healthy individuals without muscle weakness.
Low intensity vibration training is utilized to investigate osteogenic effects in animal models including sheep, rats, and mice (e.g., (28, 29, 36)), but myogenic effects have been much less studied. In two notable studies, BALB/c mice were subjected to low intensity vibration training for a duration of 6 wk. Xie and coworkers reported that cross-sectional areas of soleus muscle and type I and II fibers within that muscle were greater in vibrated than control mice (37). However, Murfee and coworkers reported low intensity vibration reduced the number of arterioles and venules in the distal region of soleus muscle, an undesirable microvascular adaptation (20). Additionally, others have reported that high intensity vibration causes injury to rodent muscle as indicated by fiber swelling and centrally located nuclei (21, 22). However, none of these studies evaluated the extent to which muscle function was beneficially or detrimentally altered with vibration training. Thus, a more comprehensive analysis of skeletal muscle following vibration training, particularly low intensity vibration training, is needed to determine function adaptations. As such, the primary objective of this study was to utilize a low intensity vibration platform, designed specifically for mice, to test the hypothesis that contractility is improved in muscles of the hindlimb in response to vibration training. A subset of mice housed in small cages to evoke physical inactivity was also studied in order to determine if vibration training was more effective under conditions promoting muscle weakness.
Traditional exercise training can elicit changes in skeletal muscle strength, oxidative capacity, and fiber type distributions, which in turn can affect muscle’s resistance to fatigue. There are indications that vibration training may influence muscle fatigue (15, 27). Therefore, histological analyses reflecting oxidative capacity, capillarity, and fiber types, as well as functional analyses of muscle fatigability and recovery from fatigue, were assessed to determine if low intensity vibration training can provide a strong enough stimulus to evoke such cellular and parallel functional adaptations in muscles of mice.
Methods
Animals and Study Design
Male C57BL/6J mice aged 8 wk were housed at 20–23 °C on a 12:12 hour light:dark cycle in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Mice were provided food ad libitum and intake was recorded weekly. Mice were randomized to one of four conditions, either without or with vibration treatment (Sham and Vibrated, respectively) and either housed in traditional sized cages to permit normal cage activity (Sham-Active, n=8; Vibrated-Active, n=10) or small cages that restricted physical activity (Sham-Restricted, n=8; Vibrated-Restricted, n=8). Sample size was determined a priori from our past experience using C57BL/6 mice and physiological muscle outcome measurements in our lab. Specifically, the number of mice used was based on that needed to detect a 10% difference among groups in strength assuming a minimum power of 0.7 and an α-level of 0.05. Mice in restricted groups were placed individually in small cages as reported previously (23), except that in the current study a ceiling was also used to minimize vertical movement (length × width × height: 12 × 8.5 × 6.3 cm). This small-cage intervention reduces ambulation by ~ 85% (23). Mice in traditional cages were housed three per cage. All mice received vibration or sham treatment 5 d·wk−1 for a total of 6 wk.
After 6 wk of treatment, when mice were 14 wk of age, contractile functions were analyzed for the anterior and posterior crural muscle groups of the left hindlimb and the soleus muscle of the right hindlimb. These muscles and muscle groups were selected because they are in close proximity to the vibrating platform and transmissibility of the vibration signal in not damped by soft tissue in the mouse hindlimb (9). For in vivo testing of the anterior and then posterior crural muscles, mice were anesthetized with fentanyl citrate (0.2 mg·kg−1 body mass [BM]), droperidol (10 mg·kg−1 BM) and diazepam (5 mg·kg−1 BM). Immediately following in vivo analyses, mice were injected i.p. with sodium pentobarbital (50 mg·kg−1 BM) and muscles were excised. First, the soleus muscle was dissected for ex vivo contractile analyses and then tibialis anterior (TA), extensor digitorum longus (EDL), gastrocnemius and contralateral soleus muscles were dissected, weighed, and either mounted in optimal cutting temperature compound or frozen in LN2. Epididymal fat pads were excised and weighed as well. Mice were then sacrificed with an overdose of sodium pentobarbital (200 mg·kg−1 BM). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and adhere to ACSM animal care and standards. All investigators were blinded to treatment groups while performing analyses of contractile function and dissections.
Vibration training
Mice in the vibration groups were exposed to low intensity vibration via a vertically oscillating platform. The vibration system was designed after the work of Fritton et al (10), with modifications including the use of a cylindrical platform driven by accelerometer feedback to ensure fidelity, minimize error and eliminate wave-transmission modes to create a uniform exposure across the entire platform. Briefly, a circular platform was driven by a linear actuator (BEI, Kimco; Magnetics, San Marcos, CA) using custom-built LabVIEW software (National Instruments; Austin, TX). The frequency and acceleration of vibration were continually monitored and fed back into the system using an accelerometer (Endevco, 50 g; San Juan Capistrano, CA) mounted to the underside of the aluminum platform. During vibration, mice were temporarily housed within one of four compartments of an acrylic cage fixed to the top of the platform. To maximize the exposure of the vibratory stimulus to the mouse hindlimb, a ceiling height of 6.3 cm was used to minimize vertical activities such as rearing and jumping and to be consistent with housing of the restricted mice. The vibration stimulus was applied for 15 min, 5 d·wk−1 for 6 wk using a vibration frequency of 45 Hz and an acceleration magnitude of 1.0 g. These parameters created a peak-to-peak displacement of 0.24 mm. Sham mice were placed in an identical acrylic cage for the same duration of time as Vibrated mice, but did not receive any vibration stimulus.
In vivo measurements of anterior and posterior crural muscle contractility
The anterior (TA, EDL and extensor hallucis longus muscles) and then posterior (soleus, plantaris, and gastrocnemius muscles) crural muscle groups were tested for contractile capacities in anesthetized mice using methods previously described (4, 13). Contraction of the anterior crural muscles was elicited via percutaneous stimulation by platinum-iridium needles (Model E2-12, Grass Technologies; West Warwick, RI) placed on either side of the left common peroneal nerve. Peak-isometric torque was defined as the greatest torque measured by a 300B-LR servomotor (Aurora Scientific; Aurora, Ontario, Canada) during a 200-ms stimulation using 1-ms square-wave pulses at 300 Hz and increasing voltage from 3.0 to 9.0 V (models S48 and SIU5, Grass Technologies). Fatigability of the anterior crural muscles was then assessed by subjecting the muscles to 120 submaximal isometric contractions over 2 min using 330 ms stimulations at 50 Hz. A rest period of 5 min followed and then recovery from fatigue was tested by re-measuring peak isometric torque. Immediately following testing of the anterior crural muscles, the left common peroneal nerve was severed and testing of the posterior crural muscles began. Contraction of this muscle group was elicited by stimulating the sciatic nerve (7). Peak-isometric torque was determined during a 200-ms stimulation with varying voltages (3.0 to 7.0 V) at 300 Hz using 1-ms square-wave pulses. Fatigability was assessed by 120 isometric contractions produced over a 2-min duration using 500 ms stimulations at 60 Hz. After 5 min of rest, recovery from fatigue was tested by re-measuring peak isometric torque of the posterior crural muscles. Isometric torque-time tracings from the peak pre-fatigue contractions were analyzed to determine maximal rates of contraction and relaxation for both anterior and posterior muscle groups.
Ex vivo soleus muscle contractility
Isolated soleus muscles were mounted to a dual-mode muscle lever system (300C-LR, Aurora Scientific) in a 1.5-ml bath containing oxygenated (95% O2) Krebs-Ringer bicarbonate buffer at 25 °C using 5-0 suture. Muscle lengths from proximal to distal myotendinous junctions were measured using digital calipers after muscles were set to their anatomic resting length (Lo). Contractile characteristics were measured as described previously (18) in the order of passive stiffness, maximal isometric tetanic force (Po), and active stiffness. Tetanic force-time tracings were analyzed to determine maximal rates of contraction and relaxation. Following these measurements, fatigability was tested by employing a protocol of 60 1-s tetanic contractions at a rate of 12 tetani per minute for 5 min and then re-measuring Po 5 and 10 min later to assess recovery (12). Soleus muscles were then removed from the bath assembly, trimmed, blotted, weighed, and frozen in liquid nitrogen. Soleus muscle mass and Lo were used to calculate physiological cross-sectional area, which was then used for calculating specific Po (5, 34).
Protein Analyses
To determine if greater rates of relaxation with vibration could be attributed to enhanced calcium handling, we quantified the expression of two calcium sequestering proteins (6). Briefly, frozen TA muscles were pulverized by mortar and pestle, solubilized in 1% sodium dodecyl sulfate with 5 mM EGTA and a cocktail of protease inhibitors, and measured for total protein concentration using a NanoDrop ND-100 spectrophotometer (Thermo Fisher Scientific; Wilmington, DE). Proteins were separated on 4–20% poly-arcylamide gels (120 V for 90 min), transferred to polyvinylidene difluoride membranes, and then probed using antibodies against: sarcoplasmic reticulum calcium ATPase (SERCA) 1a (1:1000 dilution of MA3-911; Pierce; Rockford, IL), parvalbumin (1:5000 dilution of PARV-19; Sigma; St. Louis, MO) and glyceraldehyde 3-phosphate dehydrogenase (1:5000 dilution of G9545; Sigma). Secondary antibodies were diluted (1:10,000) and detected and analyzed with the Odyssey Infrared Imaging System (Li-Cor Biosciences; Lincoln, NE) using the 700- and 800-nm channels.
Histological Analyses
Ten-micron thick transverse sections were cut from the mid-belly of TA and soleus muscles on a cryostat at −20 °C. Muscles used for histology were contralateral to those tested in vivo or ex vivo for contractility. Sections were stained for nicotinamide adenine dinucleotide (NADH)-tetrazolium reductase reactivity as an indicator of mitochondrial enzyme activity (1) and by periodic acid-Schiff reaction to determine the number of capillaries per fiber (2). For each stain, 3–5 muscles per group were evaluated, with ~300 fibers/muscle assessed for NADH reactivity and ~200 fibers/muscle for capillarity. Lastly, fiber type distribution based on myosin heavy chain isoforms were determined and classified as types I, 2a, or 2b using antibodies BA-F8, SC-71, and BF-F3, respectively (as originally developed by Schiaffino and obtained from the Developmental Studies Hybridoma Bank under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242) and immunofluorescent secondary antibodies (3). If a fiber did not react to antibodies against any of these myosin heavy chain isoforms, it was classified as a 2x fiber. Three to five muscles per group were assessed for fiber type, with all fibers of each muscle being evaluated (range 354 to 661 fibers for soleus muscle and 2363 to 3243 fibers for TA muscle). Data were calculated as the percentage of NADH-positive fibers, average number of capillaries per fiber, percentage of fiber type, and average fiber cross-sectional area per fiber type for each muscle analyzed. Those muscle means were then used for statistical analyses. Investigators were blinded to treatment groups while performing all histological analyses.
Statistical Analyses
Data were analyzed using two-way ANOVAs to determine effects of vibration (Sham vs. Vibrated) and physical inactivity (Active vs. Restricted). Holmes-Sidak post-hoc tests were done when significant interactions were detected. Significance was accepted at P≤0.05. Statistical analyses were carried out using SigmaStat version 3.5 (Systat Software Inc; Chicago, IL).
Results
Six weeks of low intensity vibration was well tolerated by mice. There were no detectable changes in behavior when vibration was initiated or during the 15-min exposure. Also, Vibrated and Sham mice behaved similarly in the vibration and sham chambers, respectively. Aggressive behavior of the Vibrated-Active mice was noted for ~5 min when the mice were first returned to their cages immediately following vibration. Food intake during the first week of the study was 13–26% greater for Vibrated-Active and Sham-Restricted mice compared to Sham-Active mice (Table 1). There was no lasting effect of vibration on food intake, but at week 6, Restricted mice consumed ~15% more food per day than Active mice (Table 1).
Table 1.
Effects of six weeks of low intensity vibration training on food intake, body and fat pad masses, and muscle size characteristics of mice that maintained normal physical activity and those that had restricted physical activity.
| Sham-Active | Vibrated-Active | Sham-Restricted | Vibrated-Restricted | P-values for Two-Way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Main effect of Vibration | Main effect of Activity | Interaction (Vibration × Activity) | |||||
| Food intake, week 1 (g/24 hr) | 3.1 (0.2) | 3.5* (0.1) | 3.9* (0.1) | 3.6 (0.2) | - | - | 0.034 |
| Food intake, week 6 (g/24 hr) | 3.0 (0.1) | 3.2 (0.1) | 3.6 (0.1) | 3.6 (0.1) | 0.454 | <0.001 | 0.518 |
| Body mass, week 1 (g) | 21.2 (0.3) | 21.2 (0.5) | 21.6 (0.5) | 21.7 (0.4) | 0.949 | 0.377 | 0.940 |
| Body mass, week 6 (g) | 24.2 (0.5) | 24.7 (0.7) | 22.9 (0.7) | 23.5 (0.5) | 0.376 | 0.073 | 0.924 |
| Epididymal fat pad mass (mg) | 430 (18) | 407 (22) | 439 (44) | 371 (22) | 0.642 | 0.117 | 0.415 |
| Fat pad mass: body mass (%) | 1.78 (0.07) | 1.66 (0.10) | 1.90 (0.15) | 1.57 (0.07) | 0.037 | 0.875 | 0.312 |
| Soleus muscle length (mm) | 14.2 (0.2) | 14.2 (0.2) | 14.1 (0.2) | 13.9 (0.2) | 0.557 | 0.303 | 0.752 |
| Soleus muscle CSA (mm2) | 0.85 (0.02) | 0.85 (0.02) | 0.79 (0.04) | 0.84 (0.05) | 0.486 | 0.236 | 0.502 |
| Tibialis anterior muscle protein content (mg) | 5.69 (0.30) | 5.81 (0.21) | 4.70 (0.62) | 5.59 (0.28) | 0.185 | 0.114 | 0.310 |
Values are means (SE). CSA, cross-sectional area.
Significantly different from Sham-Active
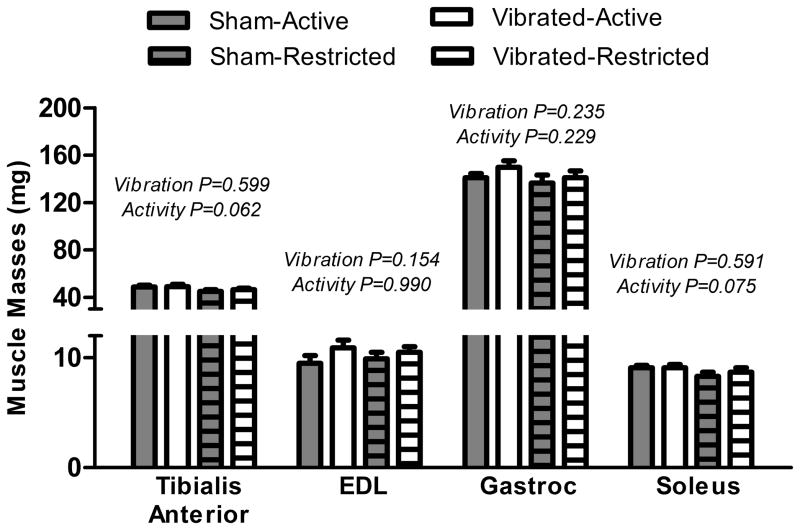
Body mass did not significantly differ among groups at the beginning or end of the study (Table 1). Mass of the epididymal fat pad was not affected by vibration or activity, however, fat pad mass relative to body mass was lower in Vibrated mice (Table 1). There were trends for Restricted mice to have smaller TA and soleus muscle masses compared to Active mice, but there was no effect of vibration on any hindlimb muscle mass measured (Figure 1). Furthermore, length and physiological cross-sectional area of soleus muscles and protein content of TA muscles were not were not significantly affected by vibration (Table 1). Collectively, there was no indication that vibration induced hypertrophy of hindlimb muscles.
Figure 1.
Low intensity vibration training did not affect mouse hindlimb muscle masses. Restricted activity trended toward tibialis anterior and soleus muscle atrophy. Main effect P-values from two-way ANOVA’s are indicated above each set of bars; no significant interactions between Vibration and Activity were detected (P≥0.549).
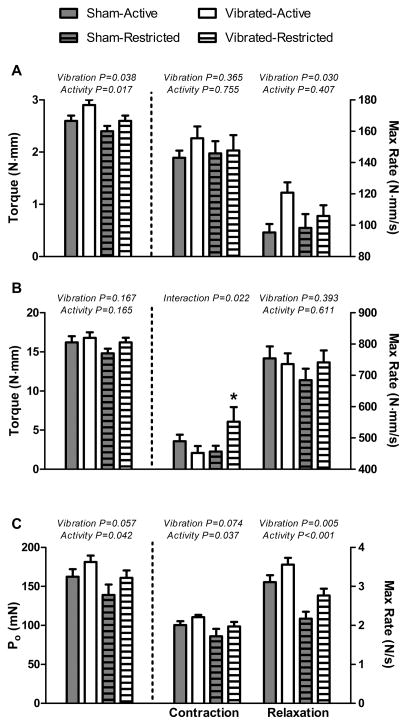
There were some indications that vibration training improved muscle contractility. Maximal isometric torque generated by anterior crural muscles was ~10% greater in Vibrated compared to Sham mice (Figure 2A), and occurred whether mice were active or restricted (interaction P= 0.642). As expected, there was also a main effect of activity with Restricted mice having ~10% lower maximal torque than Active mice (Figure 2A). When normalized by mass of the TA muscle, anterior crural muscle torque of Vibrated mice was ~8% greater than that of Sham mice (57.7 ± 1.2 vs. 53.3 ± 1.2 N·mm·g−1, respectively; P=0.021), with no effect of activity (P=0.138). The maximal rate of contraction by the dorsiflexors was unaffected by vibration training, but the maximal rate of relaxation following isometric contraction was ~16% faster in dorsiflexors of Vibrated than Sham mice (Figure 2A). To determine if the vibration-induced increase in muscle relaxation was due to greater levels of calcium-handling proteins, the expression of SERCA and parvalbumin was measured; neither protein were different between TA muscles from Vibrated and Sham mice (P≥0.361).
Figure 2.
Effects of low intensity vibration training on mouse hindlimb muscle contractility. A: In vivo testing of anterior crural muscle function showed that maximal isometric torque and rate of relaxation were greater in mice subjected to vibration (Vibrated) compared to those that were not (Sham), irrespective if mice maintained normal cage activity (Active) or were restricted in their physical activity by being housed in small cages (Restricted). B: In vivo testing of posterior crural muscle function revealed minimal effects of vibration training. C: Ex vivo testing of isolated soleus muscle contractility showed that maximal rate of relaxation was greater in Vibrated compared to Sham mice. Po, maximal isometric tetanic force. Data are mean, SE. Main effect P-values from two-way ANOVA’s are indicated above each set of bars when there was not a significant interaction between Vibration and Activity. *Significantly different from Vibrated-Active and Sham-Restricted, as determined from Holmes-Sidak post hoc testing
In contrast to the anterior crural muscles, contractility of the posterior crural muscles was largely unaffected by vibration and activity (Figure 2B). The exception was that Vibrated-Restricted mice had greater rates of contraction by the plantarflexors than Vibrated-Active and Sham-Restricted mice.
There was a trend for maximal isometric tetanic force (Po) by isolated soleus muscles to be greater in Vibrated than Sham mice (P=0.057; Figure 2C), regardless of activity (interaction P=0.887). As expected, Restricted mice had lower soleus muscle Po than did Active mice (P=0.042; Figure 2C). Specific Po was not affected by vibration training or activity (P≥0.244), and averaged 19.71 ± 0.79 N·cm−2 across all groups. Similarly, active stiffness was not different among groups (P≥0.245; 252 ± 9.7 N·m−1 for all groups). Maximal rate of contraction tended to be faster and maximal rate of relaxation was ~20% faster in soleus muscles of Vibrated than Sham mice (Figure 2C). There were main effects of activity on rates of contraction and relaxation with soleus muscles from Restricted mice being slower than those from Active mice. There was no effect of vibration or activity on soleus muscle passive stiffness (P≥0.689; 11.59 ± 0.4 N·m−1 across all groups).
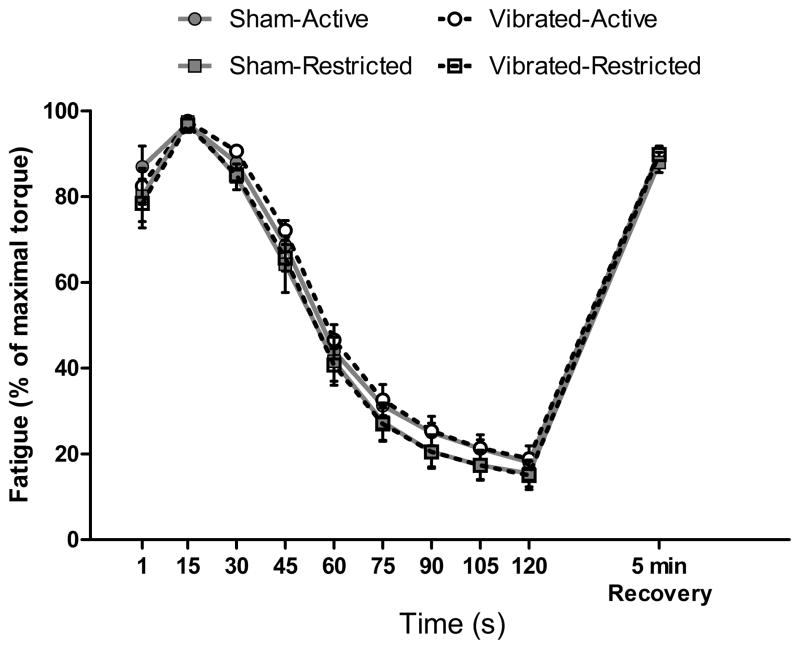
Muscle fatigability, as measured by percent loss of torque or force over the course of repeated contractions, and recovery from fatigue were not affected by vibration training in any of the three muscle or muscle groups studied. Torque of the anterior crural muscles was reduced by ~80% at the end of the 2-min bout of fatiguing contractions with no effect of vibration or activity (P≥0.431; Figure 3). Five minutes later, torque generation was equally recovered, irrespective of vibration or activity (P≥0.403; Figure 3). Similarly, posterior crural muscles lost ~70% of torque during the bout of fatiguing contractions with no effect of vibration or activity on torque loss (P≥0.126) or recovery of torque 5 min later (P≥0.388). The 60 isometric contractions performed in 5 min by isolated soleus muscles caused force to go down ~55% regardless if mice had vibration trained or not, or if mice were active or restricted (P≥0.464). By 10 min after the fatiguing protocol, soleus muscles recovered to ~95% of pre-fatigue Po with no difference among groups (P≥0.228).
Figure 3.
Torque loss and recovery from a fatiguing bout of isometric contractions by the anterior crural muscles from Vibration and Sham mice that maintained normal cage activity (Active) or were restricted in their physical activities (Restricted). Fatigue was calculated as the percentage of torque relative to peak torque during the 120-contraction protocol. Plotted are the relative torques of every fifteenth contraction during the protocol. Peak isometric torque generated 5 min later is also plotted. For each group, this recovery torque was not different than peak isometric torque before the protocol began, indicating that the torque loss represented transient fatigue as opposed to a more lasting muscle injury. There were no differences among groups in torque during or after the fatigue protocol. Data are means, SE. Error bars not seen are contained within symbols.
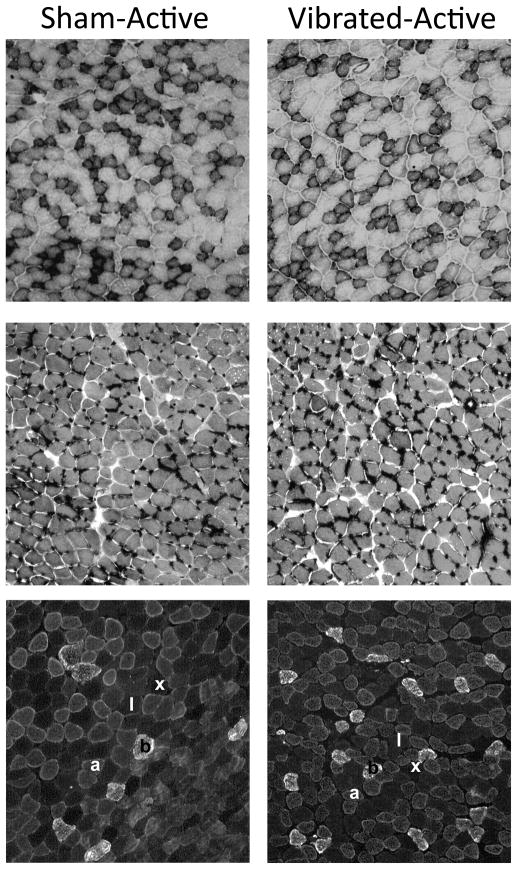
Vibration training had no effect of TA muscle fiber NADH reactivity, capillarity, or fiber type composition (Table 2). Mice that were restricted in their activity had TA muscles with relatively fewer NADH-positive fibers, fewer Type 2x fibers, and more Type 2b fibers, compared to those of normally active mice. Soleus muscles from Vibrated and Sham mice were not different in NADH reactivity, fiber type composition, or fiber cross-sectional areas (Table 2 and Figure 4). Capillarity, as determined by number of capillaries per fiber, was 24% greater in vibrated soleus muscles, but only within the Restricted mice (Table 2).
Table 2.
Effects of six weeks of low intensity vibration training on histological assessments of oxidative capacity, as indicated by NADH reactivity and capillarity, and fiber type distribution of tibialis anterior and soleus muscles of mice that maintained normal physical activity and those that had restricted physical activity.
| Sham-Active | Vibrated-Active | Sham-Restricted | Vibrated-Restricted | P-values for Two-Way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Main effect of Vibration | Main effect of Activity | Interaction (Vibration × Activity) | |||||
| Tibialis anterior muscle | |||||||
| NADH-positive fibers (%) | 64.4 (4.7) | 69.1 (3.7) | 57.6 (2.6) | 60.2 (2.6) | 0.292 | 0.033 | 0.760 |
| Capillaries per fiber | 3.73 (0.05) | 3.98 (0.22) | 3.46 (0.43) | 3.53 (0.21) | 0.641 | 0.309 | 0.805 |
| Type 2a fibers (%) | 6.8 (0.2) | 7.5 (1.4) | 4.4 (1.4) | 5.1 (0.2) | 0.649 | 0.168 | 0.996 |
| Type 2x fibers (%) | 43.6 (0.8) | 33.3 (5.0) | 12.3 (2.9) | 18.2 (2.9) | 0.716 | 0.003 | 0.197 |
| Type 2b fibers (%) | 49.6 (0.9) | 59.2 (6.0) | 83.3 (1.5) | 76.6 (2.7) | 0.836 | 0.005 | 0.264 |
| Soleus muscle | |||||||
| NADH-positive fibers (%) | 59.9 (2.0) | 59.6 (1.3) | 57.9 (3.7) | 64.5 (2.7) | 0.217 | 0.559 | 0.185 |
| Capillaries per fiber | 3.53 (0.10) | 3.26 (0.21) | 3.16 (0.22) | 3.91* (0.34) | - | - | 0.043 |
| Type 1 fibers (%) | 36.0 (0.7) | 32.2 (2.2) | 34.8 (2.2) | 34.6 (1.2) | 0.269 | 0.724 | 0.329 |
| Type 2a fibers (%) | 53.9 (0.4) | 56.5 (1.6) | 57.7 (1.8) | 56.8 (0.8) | 0.518 | 0.141 | 0.198 |
| Type 2x fibers (%) | 8.5 (1.3) | 9.2 (1.2) | 5.3 (3.0) | 6.0 (0.7) | 0.664 | 0.058 | 0.984 |
| Type 2b fibers (%) | 1.6 (1.0) | 2.2 (1.0) | 2.2 (0.5) | 2.7 (1.1) | 0.644 | 0.618 | 0.970 |
| Type 1 fiber CSA (μm2) | 1938.4 (151.2) | 1872.6 (98.7) | 1692.2 (136.5) | 2026.9 (119.9) | 0.312 | 0.725 | 0.142 |
| Type 2a fiber CSA (μm2) | 2302.8 (205.1) | 2430.9 (155.0) | 2370.0 (154.1) | 2440.0 (150.8) | 0.579 | 0.830 | 0.870 |
| Type 2x fiber CSA (μm2) | 2102.0 (138.2) | 1910.9 (109.7) | 2175.6 (28.2) | 2124.8 (232.2) | 0.451 | 0.373 | 0.660 |
| Type 2b fiber CSA (μm2) | 1799.7 (666.2) | 1606.7 (330.0) | 2154.4 (191.6) | 1933.0 (653.0) | 0.699 | 0.528 | 0.979 |
Values are means (SE). NADH, nicotinamide adenine dinucleotide. CSA, cross-sectional area.
Significantly different from Sham-Restricted
Figure 4.
Representative cross-sections of soleus muscles from mice that were caged in normal sized cages (Active) and were subjected to low intensity vibration training (Vibrated) or not (Sham). Top panel shows muscles stained for nicotinamide adenine dinucleotide (NADH)-tetrazolium reductase reactivity as an indicator of mitochondrial enzyme activity. Dark fibers were counted at positive. Middle panel shows muscles stained by periodic acid-Schiff reaction that labels capillaries. Bottom panel shows muscles triple-stained with antibodies against type 1, 2a, and 2b myosin heavy chain. Fibers denoted with “1” were classified as type 1 fibers, “a” as type 2a, and “b” as type 2b. These fibers were distinguished based on secondary antibodies that fluoresced fibers red, green, or blue. Fibers denoted by “x” were classified as type 2x because they did not react with any myosin heavy chain antibody and thus did not fluoresce.
Discussion
The results of this study partially support the hypothesis that low intensity vibration improves muscle contractility. We show that standing on a platform which vibrates at 1.0 g and 45 Hz for 15 min per day, 5 times per week for 6 weeks, increased strength and rate of relaxation of some but not all of the mouse hindlimb muscles tested (Figure 2). These vibration-induced improvements in muscle contractility were independent of mouse activity. That is, restriction of physical activity in subsets of mice successfully induced muscle weakness of the anterior crural muscles and soleus muscle, but that was not required for vibration to be effective in improving strength or relaxation.
The mechanism by which vibration improves strength is not clear. Acute vibration may facilitate contractility by enhancement of stretch- and H-reflexes (26) or increased muscle activation (8). Neuromuscular adaptations in response to weeks or months of vibration training are less clear. One way that traditional resistance exercise results in strength gains is by muscle hypertrophy, but our results do not give any indication that hypertrophy was induced by 6 weeks of low intensity vibration training in C57BL/6 mice. Above all, hindlimb muscle masses and protein contents were not greater in Vibrated compared with Sham mice (Figure 1, Table 1). Physiological cross-sectional area and length of soleus muscles (Table 1), as well cross-sectional areas of fibers from that muscle (Table 2), were not affected by vibration training providing further support that hypertrophy was not induced. Two previous studies on BALB/c mice subjected to 15 min per day of low intensity vibration for 6 weeks report data on soleus muscle and fiber size. Murfee et al found that soleus muscle area and number of fibers per area were not altered by vibration training (20) and Xie et al reported that the number of type I and type II muscle fibers and cross-sectional area of those fibers were not different between soleus muscles of sham and vibrated mice (36). However in the latter study, total cross-sectional area of soleus muscle was calculated to be 27% greater in mice that were subjected to vibration training (36). The differing soleus muscle cross-sectional area results between that study and ours may have to do with the method by which cross-sectional area was determined (anatomical versus physiological) or is possibly due to differential responses to vibration. That is, the designs of the two studies were the same except that we used an acceleration of 1.0 g and they used of 0.3 g. Overall, there is not convincing evidence that low intensity vibration training causes hypertrophy in hindlimb muscles of mice.
Improvement in the intrinsic ability of muscle to generate torque or force is another mechanism by which strength gains can be realized. Maximal torque generation by the anterior crural muscles of mice that were subjected to vibration training was ~10% greater than that of non-vibrated mice (Figure 2A). Anterior crural muscle torque normalized by TA muscle mass was ~8% greater in Vibrated than Sham mice suggesting that some intrinsic ability of the TA muscle to generate torque was affected by vibration training. Specific force and active stiffness measurements of soleus muscle do not support this supposition, however, as these indicators of muscle quality were not different between Vibrated and Sham mice, even though Po trended toward being greater in Vibrated mice (P=0.057; Figure 2C). Collectively, these data leave some ambiguity as how muscle strength is improved by vibration training. Given that the anterior as opposed to the posterior crural muscles were affected by vibration, it would have been interesting to analyze contractility of the extensor digitorum longus muscle and should be done in future studies. No other studies that we are aware of have measured muscle function following vibration training in mice. We suggest that more functional types of studies need to be conducted to firmly establish the extent to which low intensity vibration training improves muscle strength and furthermore to determine underlying mechanism of strength gains when they occur. Furthermore, investigation is needed into the response of different muscles and muscle groups. The anterior and posterior muscle groups analyzed in this study, while theoretically receiving similar mechanical stimuli from the vibrating platform, responded very differently in terms of contractility adaptations with those measured in the posterior muscle group being minimally affected (Figure 2B).
The maximal rate of muscle relaxation was improved by vibration in 2 of 3 muscle or muscle groups studied. Specifically, the maximal rates of relaxation following maximal isometric contraction by anterior crural and soleus muscles were 16–20% greater in Vibrated than Sham mice (Figure 2A). Muscle proteins related to calcium handling were investigated in attempt to determine possible mechanisms underlying the faster muscle relaxation, but expression levels of parvalbumin and SERCA were not different in TA muscles of Vibrated and Sham mice. Analyses of the content of these two proteins does not exclude the possibility of vibration-induced influences of calcium handling because structure or function of these or other calcium handling proteins could have occurred. To our knowledge, the influence of vibration training on calcium handling proteins and muscle relaxation has not been previously studied. An example indicating that vibration training has the potential to influence particular muscle proteins is a study showing that ryanodine receptor type 1 expression and function in soleus muscles of bed-rested humans is significantly affected by vibration training (31). Again, more investigations are required to determine the extent to which vibration-training affects muscle contractility parameters such as rates of contraction and relaxation, and mechanisms underlying any such adaptations. Using mouse models and strategies that bypass activation of muscle through the nerve and in response to reflexes may help to identify neural versus muscular adaptation to vibration training.
Fatigability of hindlimb muscles and recovery from fatigue were not affected by low intensity vibration training (e.g., Figure 3). Corresponding with these functional outcomes, there was no indication that vibration affected mitochondrial oxidative capacity in TA or soleus muscle as measured by the percentage of fibers stained for NADH reactivity (Table 2 and Figure 4). Similarly, fiber type compositions of TA and soleus muscles were not altered in response to vibration training (Table 2). Previous studies of low intensity vibration on mouse soleus muscle also found no fiber type transition (20, 37). Finally, capillarity was investigated because blood supply to contracting muscle is an important factor in fatigability and a previous study reported suppression of blood vessels in mouse soleus muscle in response to low intensity vibration (20). In the TA muscle there was no difference between Vibrated and Sham mice in terms of number of capillaries per muscle fiber (Table 2). In normally-active mice there was also no difference in soleus muscle capillarity, however, among the physically-restricted mice those that were subjected to vibration had 24% more capillaries per fiber than those from Sham mice (Table 2). These data indicate a beneficial vascular remodeling in response to low intensity vibration. Collectively, functional measurements of muscle fatigue and histological analyses related to muscles’ ability to resist and recover from fatigue, apart from soleus muscle capillarity, were not improved by low intensity vibration training.
An interesting finding of the present study was that the epididymal fat pad mass, when expressed relative to body mass, was ~12% lower in Vibrated than Sham mice (Table 1), agreeing with previous reports of reduced fat masses in mice in response to vibration training (16, 30). There is evidence from mouse and cell culture studies that low and high intensity vibration can influence adipogenesis and perhaps shift bone marrow cell fate away from adipocytes toward osteoblasts, which could contribute to vibration-induced loss of fat and gain of bone, respectively (16, 30, 33).
In conclusion, we show some evidence supporting the hypothesis that low intensity vibration training improves muscle contractility, specifically strength and relaxation rates. The cellular and molecular adaptations that occurred to cause these physiological improvements were not elucidated. It is important to recognize that mechanisms underlying the remodeling of muscle in response to low intensity vibration training as used in this study may or may not be the same as those that underlie responses to high intensity vibration. We specifically chose to focus on low intensity vibration because our goal is to investigate the therapeutic potential of vibration training for neuromuscular disease and low intensity is less likely to be deleterious to fragile, diseased muscle. Thus, an important point in regard to the present study is that no adverse effects of 6 weeks of low intensity vibration training were observed.
Acknowledgments
The authors thank Greg Cochrane and Angela Greising for technical assistance. This research was supported by grants from the Muscular Dystrophy Association (DAL and DJN), University of Minnesota Undergraduate Research Opportunity (JNM), and the NIH; K02-AG036827 (DAL), P30-AR0507220 (University of Minnesota Muscular Dystrophy Center), and T32-AR07612 provided support to SAN, KAB, and JAC.
Footnotes
Authors disclose no conflict of interest. Furthermore, publication of these results of the present study do not constitute an endorsement by the ACSM.
References
- 1.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–8. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975;95:203–5. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- 3.Baltgalvis KA, Call JA, Cochrane GD, Laker RC, Yan Z, Lowe DA. Exercise Training Improves Plantarflexor Muscle Function in mdx Mice. Med Sci Sports Exerc. 2012;44:1671–79. doi: 10.1249/MSS.0b013e31825703f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–54. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Call JA, Eckhoff MD, Baltgalvis KA, Warren GL, Lowe DA. Adaptive strength gains in dystrophic muscle exposed to repeated bouts of eccentric contraction. J Appl Physiol. 2011;111:1768–77. doi: 10.1152/japplphysiol.00942.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Call JA, Ervasti JM, Lowe DA. TAT-μUtrophin mitigates the pathophysiology of dystrophin and utrophin double-knockout mice. J Appl Physiol. 2011;111:200–5. doi: 10.1152/japplphysiol.00248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res. 2003;17:621–4. doi: 10.1519/1533-4287(2003)017<0621:eaovlm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen BA, Silva MJ. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng. 2006;34:1149–56. doi: 10.1007/s10439-006-9133-5. [DOI] [PubMed] [Google Scholar]
- 10.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann Biomed Eng. 1997;25:831–9. doi: 10.1007/BF02684167. [DOI] [PubMed] [Google Scholar]
- 11.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin CT. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 12.Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol’s beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110:109–15. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingalls CP, Warren GL, Lowe DA, Boorstein DB, Armstrong RB. Differential effects of anesthetics on in vivo skeletal muscle contractile function in the mouse. J Appl Physiol. 1996;80:332–40. doi: 10.1152/jappl.1996.80.1.332. [DOI] [PubMed] [Google Scholar]
- 14.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25:975–88. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Zhang M, Chen G, Luo S, Liu F, Li J. Wavelet analysis of lumbar muscle oxygenation signals during whole-body vibration: implications for the development of localized muscle fatigue. Eur J Appl Physiol. 2012;112:3109–17. doi: 10.1007/s00421-011-2298-0. [DOI] [PubMed] [Google Scholar]
- 16.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikhael M, Orr R, Fiatarone Singh MA. The effect of whole body vibration exposure on muscle or bone morphology and function in older adults: a systematic review of the literature. Maturitas. 2010;66:150–7. doi: 10.1016/j.maturitas.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 2005;40:966–75. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Muir J, Judex S, Qin YX, Rubin CT. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture. 2011;33:429–35. doi: 10.1016/j.gaitpost.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murfee WL, Hammett LA, Evans C, Xie L, Squire M, Rubin C, Judex S, Skalak TC. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J Appl Physiol. 2005;98:2376–80. doi: 10.1152/japplphysiol.01135.2004. [DOI] [PubMed] [Google Scholar]
- 21.Necking LE, Dahlin LB, Friden J, Lundborg G, Lundstrom R, Thornell LE. Vibration-induced muscle injury. An experimental model and preliminary findings. J Hand Surg Br. 1992;17:270–74. doi: 10.1016/0266-7681(92)90113-g. [DOI] [PubMed] [Google Scholar]
- 22.Necking LE, Lundstrom R, Lundborg G, Thornell LE, Friden J. Skeletal muscle changes after short term vibration. Scand J Plast Reconstr Surg Hand Surg. 1996;30:99–103. doi: 10.3109/02844319609056390. [DOI] [PubMed] [Google Scholar]
- 23.Novotny SA, Warren GL, Lin AS, Guldberg RE, Baltgalvis KA, Lowe DA. Prednisolone treatment and restricted physical activity further compromise bone of mdx mice. J Musculoskelet Neuronal Interact. 2012;12:16–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Pitukcheewanont P, Safani d. Extremely low-level, short-term mechanical stimulation increases cancellous and cortical bone density and muscle mass of children with low bone density. The Endocrinologist. 2006;16:128–32. [Google Scholar]
- 25.Reyes ML, Hernandez m, Holmgren JL, Sanhueza E, Escobar RG. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving anutonomy in disabled children. J Bone Miner Res. 2011;26:1759–66. doi: 10.1002/jbmr.402. [DOI] [PubMed] [Google Scholar]
- 26.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108:877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 27.Rittweger J, Beller G, Felsenberg D. Acute physiological effects of exhaustive whole-body vibration exercise in man. Clin Physiol. 2000;20:134–42. doi: 10.1046/j.1365-2281.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 28.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Low mechanical signals strengthen long bones. Nature. 2001;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 29.Rubin C, Xu g, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. Faseb J. 2001;15:2225–9. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 30.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal B, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief,daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104:17879–84. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salanova M, Schiffl G, Rittweger J, Felsenberg D, Blottner D. Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem Cell Biol. 2008;130:105–18. doi: 10.1007/s00418-008-0399-6. [DOI] [PubMed] [Google Scholar]
- 32.Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int. 2010;21:1969–80. doi: 10.1007/s00198-010-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirkkonen L, Halonen H, Hyttinen J, Kuokkanen H, Sievanen H, Koivisto AM, Mannerstrom B, Sandor GK, Suuronen R, Miettinen S, Haimi S. The effects of vibration loading on adipose stem cell number, viability and differentiation towards bone-forming cells. J R Soc Interface. 2011;8:1736–47. doi: 10.1098/rsif.2011.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren GL, Hayes DA, Lowe DA, Williams JH, Armstrong RB. Eccentric contraction-induced injury in normal and hindlimb-suspended mouse soleus and EDL muscles. J Appl Physiol. 1994;77:1421–30. doi: 10.1152/jappl.1994.77.3.1421. [DOI] [PubMed] [Google Scholar]
- 35.Wilcock IM, Whatman C, Harris N, Keogh JW. Vibration training: could it enhance the strength, power, or speed of athletes? J Strength Cond Res. 2009;23:593–03. doi: 10.1519/JSC.0b013e318196b81f. [DOI] [PubMed] [Google Scholar]
- 36.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Xie L, Rubin c, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–62. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]