Summary
Chimeric antigen receptors re-direct T cells to surface antigens. Discovery and validation of appropriate target antigens expands the possible indications for CAR-T cells. BCMA is expressed only on mature B cells and plasma cells and promotes their survival. BCMA is a promising target for CAR-T cells in multiple myeloma.
In this issue of Clinical Cancer Research, Carpenter and colleagues1 explore the potential of targeting the B cell maturation antigen (BCMA) with chimeric-antigen receptor (CAR)-transduced T cells, with the goal of developing a clinical T cell therapy to treat multiple myeloma.
CAR-T cells are autologous or allogeneic T cells genetically engineered to express a chimeric antigen receptor (CAR) specific for a cell-surface structure, typically a protein or carbohydrate. The specificity of the CAR is based on the Fab region of an antibody, typically engineered into a single-chain variable fragment (scFv), while the CAR’s signaling domains are derived from native human T cell receptor signaling domains (CD3z) which may be fused in tandem to additional costimulatory proteins that promote T cell proliferation, cytokine release, and resistance to apoptosis. CAR-T cells are emerging as powerful therapy with the curative potential of allogeneic stem cell transplant but without the complications of allogeneic graft vs. host disease. In the last two years, pilot studies of CD19-directed CAR-T cells have been reported by several groups to induce prolonged remissions in chemotherapy resistant or refractory CD19+ malignancies2, 3. Clinical trials of CAR-T cells directed to CD204 and GD2 (in neuroblastoma)5 have been reported, and there are many ongoing studies of CAR-T cells in various tumors.
Although there is still an ongoing effort in the field to determine the optimal molecular and biomechanical aspects of CAR design, the biggest hurdle to widespread development of CAR-T cells for malignancies is finding suitable antigenic targets. The requirements for an appropriate target antigen for directing CAR-T are conceptually simple but strict:
The target must be expressed on the surface.
Off-tumor expression of the target, even at low levels, must not be present in an essential organ or cell type (i.e. hematopoietic stem cells).
To avoid antigen escape, all the tumor cells must express the target, or, alternatively, the target must be essential for maintenance of the tumorigenic phenotype.
The first requirement is a consequence of the nature of MHC-independent antibody binding. The second requirement is based on the toxicity profile of CAR-T cells, which has demonstrated that cells expressing low levels of the target antigen are rapidly lysed. In the case of the Her2/neu6 antigen, for example, low-level expression in the lungs resulted in rapid and fatal toxicity; similarly, CARs directed to carbonic anhydrase IX resulted in T-cell mediated cholangitis due to low-level expression of the target in the bile duct epithelium7. This type of toxicity reflects the sensitivity of the T cell to signaling from engagement of its target, and has also been seen in T cells that have been redirected to MHC-restricted tumor antigens. The third requirement has now been clinically demonstrated; in a recent trial of CD19-directed CAR-T for B cell acute lymphoblastic leukemia (ALL), a patient whose tumor expressed CD19 heterogeneously relapsed with CD19-negative disease after an initial complete response induced by the CAR-T8. Thus, choosing the most appropriate target antigen, in the context of the targeted disease, is arguably the most crucial component in developing CAR-T therapies.
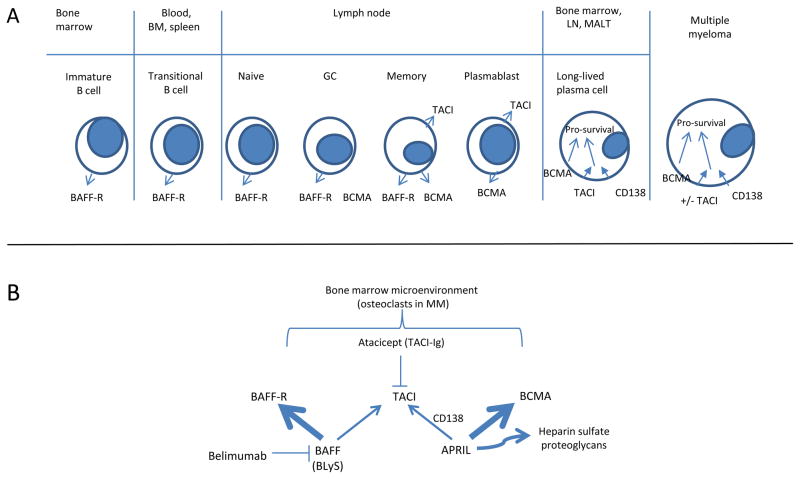
B cell maturation antigen (BCMA) is a tumor necrosis family receptor (TNFR) member that is expressed on terminally differentiated B cells; engagement of BCMA by its ligands delivers pro-survival signals to mature B cells, plasma cells, and multiple myeloma cells. The two ligands for BCMA are B cell activator of the TNF family (BAFF, also known as BLyS) and a proliferation inducing ligand (APRIL). Two other related TNFR family members, BAFF-R and transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), are expressed in earlier stages of B cell development. The primary ligand for BAFF-R is BAFF, whereas the primary ligand for BCMA is APRIL.9 TACI, which is co-expressed with both BCMA and BAFF-R in memory B cells, and only with BCMA in plasmablasts, long-lived plasma cells, and some multiple myeloma cells, binds to BAFF independently but requires CD138 to act as a co-receptor to bind APRIL10. In human multiple myeloma, BCMA is thought to play a critical role in protecting the myeloma cells from apoptosis; the tumor microenvironment, and osteoclasts in particular, secrete APRIL and BAFF. (Figure).
Figure 1.
Figure A. Expression of the three related TNF-R family members BAFF-R, TACI and BCMA during B cell development. BM, bone marrow. GC, germinal center. LN, lymph node. MALT, mucosa-associated lymphoid tissue. B. Relationships of the ligands BAFF and APRIL to their respective receptors. Both ligands are expressed and/or secreted by multiple bone marrow cell types, particularly osteoclasts in multiple myeloma. Belimumab, antibody to BAFF, is FDA-approved for systemic lupus erythematosus; Atacicept, a fusion protein of TACI-Ig, is designed to block APRIL and BAFF and is in clinical development for both rheumatoid arthritis and multiple myeloma.
The BAFF pathways are highly active in some autoimmune diseases. BCMA is mostly known for its functional activity in mediating the survival of plasma cells that maintain long-term humoral immunity11, but it may not be absolutely required for this function, as mature B cells from BCMA-deficient lupus-prone mice could still differentiate into plasma cells and have a worsened autoimmunity12; this is thought to occur via TACI signaling. Whether compensatory signaling through TACI could or will occur in humans is unknown, since BCMA has not yet been directly targeted with an antibody or CAR-T in humans. It is unknown BCMA-TACI+ antigen escape variants of myeloma will be selected by CAR-T cells, and this question is best answered by clinical testing.
In this paper, Carpenter et al have focused on defining BCMA as a suitable antigen for CAR-T cell therapies. They begin by demonstrating bright surface expression on primary myelomas, which confirms previous studies of surface expression of BCMA on mature B cells. What is not clear is whether the expression of BCMA is homogeneous in primary myeloma cells, though it is encouraging that BCMA signals are functionally involved in the maintenance of the tumor phenotype. They also perform an in depth analysis of protein expression of BCMA in a many normal organs, with particular attention to gut tissues that were thought to express BCMA on the basis of mRNA expression; reassuringly, a beautiful immunohistochemical analysis demonstrated that BCMA expression in the gut is a result of resident B cells and plasma cells that form part of the gut-associated lymphoid tissues. Ultimately, the disappointing experience in the field is that clinical toxicities may not be predicted from in vitro and xenogeneic models.
Given the early successes with CD19-CARs for B cell malignancies, it is logical to target multiple myeloma as a malignancy because of the extensive prior characterization of plasma cells. One caveat is that myeloma tends to be a heterogeneous disease; it is possible that some myeloma cells will express TACI over BCMA, though the potential for antigen escape should be relatively straightforward to asses by flow cytometry from bone marrow aspirates. Finally, the predisposition of CAR-T cells to home to the bone marrow weighs the odds in favor of developing CAR-T cells for myeloma and other hematologic malignancies.
Footnotes
Conflicts of interest:
MVM: no conflicts; MVM is supported a Young Investigator Award from the Conquer Cancer Foundation and NCI K08CA166039. CHJ: sponsored research and royalty income from Novartis
Bibliography
- 1.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell Maturation Antigen is a Promising Target for Adoptive T-cell Therapy of Multiple Myeloma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold S, et al. Complete remissions of ALL by chimeric antigen receptor-expressing T cells. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1215134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coquery CM, Erickson LD. Regulatory Roles of the Tumor Necrosis Factor Receptor BCMA. Crit Rev Immunol. 2012;32:287–305. doi: 10.1615/critrevimmunol.v32.i4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreaux J, Sprynski AC, Dillon SR, Mahtouk K, Jourdan M, Ythier A, et al. APRIL and TACI interact with syndecan-1 on the surface of multiple myeloma cells to form an essential survival loop. Eur J Haematol. 2009;83:119–29. doi: 10.1111/j.1600-0609.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. The Journal of experimental medicine. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang C, Loo WM, Greenley EJ, Tung KS, Erickson LD. B cell maturation antigen deficiency exacerbates lymphoproliferation and autoimmunity in murine lupus. Journal of immunology. 2011;186:6136–47. doi: 10.4049/jimmunol.1001931. [DOI] [PMC free article] [PubMed] [Google Scholar]