Abstract
Vitamin D is a well-studied agent for cancer chemoprevention and treatment. Its chief circulating metabolite, 25-hydroxyvitamin D, is converted into the active hormone 1,25-dihydroxyvitamin D (1,25D) by the cytochrome P450 enzyme CYP27B1 in kidney and other tissues. 1,25D is then deactivated by CYP24A1 and ultimately catabolized. Colorectal carcinoma cells express CYP27B1 and CYP24A1 that locally regulate 1,25D with potential implications for its impact on carcinogenesis. While 1,25D inhibits cancer growth, the effects of polymorphic variations in genes encoding proteins involved in 1,25D homeostasis are poorly understood. Using an RXR-VDR mammalian-two-hybrid (M2H) biological assay system, we measured vitamin D metabolite uptake and activation of the vitamin D receptor (VDR) pathway in colon cancer cells that expressed one of five CYP27B1 single nucleotide polymorphisms (SNPs) or four CYP24A1 SNPs. Compared to the wild-type control, four of five CYP27B1 SNPs reduced enzymatic activity while one (V166L) increased activity. For CYP24A1, all tested SNPs reduced enzyme activity. Quantitative real-time PCR analyses supported the results of M2H experiments. The observed SNP-directed variation in CYP functionality indicated that vitamin D homeostasis is complex and may be influenced by genetic factors. A comprehensive understanding of 1,25D metabolism may allow for a more personalized approach toward treating vitamin D-related disorders and evaluating risk for carcinogenesis.
Keywords: Vitamin D, colon cancer, cytochrome P450, vitamin D receptor, vitamin D metabolism
Introduction
Vitamin D and two of its metabolites, 25-hydroxycalciferol [25D] and 1,25-dihydroxycalciferol [1,25D], have been studied intensively in relation to several disease outcomes, including cancer, using both population-based and basic science approaches. A reduction in risk for colorectal cancer has been consistently associated with higher circulating concentrations of 25D (1), which is the biomarker of vitamin D most commonly employed in epidemiological studies. Nonetheless, it is 1,25D that is considered to be the active molecule, as it binds to the vitamin D receptor (VDR) and exhibits regulatory effects on over 1000 genes (2, 3). The activity of 1,25D is particularly relevant with respect to potential chemopreventive or chemotherapeutic actions of vitamin D metabolites (3). Effects of 1,25D on cancer cell lines include inhibition of cell proliferation and promotion of differentiation (4–7).
Colon carcinoma (Caco-2) cells have been shown to display 25-hydroxyvitamin D3 1-alpha hydroxylase (CYP27B1) activity(8). This observation is of interest because CYP27B1 is responsible for the hydroxylation of 25D to form 1,25D, and as such, it indicates that colorectal cells can independently synthesize the active hormone. Increased functional activity of this enzyme in the colon has the potential to elevate 1,25D levels at the local level (3); therefore, a thorough understanding of the cellular-level regulation of 1,25D metabolism through CYP27B1 is crucial to understanding tissue-specific hormone effects.
Another key enzyme influencing circulating and cellular 1,25D concentrations is CYP24A1, which is the primary molecule involved in 1,25D catabolism (2, 3, 9). CYP24A1 hydroxylates 1,25D at the C24 position to form 1,24,25D (9), a metabolite that is less active than 1,25D in activating VDR, and which in turn is catabolized further to excretion products (9). A less active variant of CYP24A1 would likely result in greater concentrations of 1,25D at the tissue level.
Genetic polymorphisms in both CYP27B1 and CYP24A1 have been identified, and some work has shown these to be significantly related to circulating concentrations of vitamin D metabolites (10) or to risk for colorectal cancer (11) in population studies, though these associations are inconsistent (12, 13). Nonetheless, the functional effects of genetic variation in these enzymes is unclear (12), and could result in differences in the amount of 1,25D available in colon cells for anti-neoplastic activity. Therefore, the objective of the current work was to elucidate the functional effects of selected single nucleotide polymorphisms (SNPs) in CYP27B1 and CYP24A1 using site-directed mutagenesis in human colon cancer cells.
Materials and Methods
Cell culture
HCT-116 (human colorectal adenocarcinoma) cells were obtained from the American Type Culture Collection (ATCC) biological resource center (Manassas, VA) and grown at 37°C in a humidified atmosphere of 5% carbon dioxide. Subconfluent cells were transfected in Costar 24-well polystyrene plates from Corning Inc. (Corning, NY) using Express-In Transfection Reagent supplied by Thermo Scientific (Waltham, MA). Cells were plated at a density of 60,000 cells/well approximately 24 h prior to transfection in Dulbecco’s modified eagle medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin.
The UMR-106 (rat osteosarcoma) cell line was obtained from ATCC (Manassas, VA) and cultured as recommended. Culture media, fetal bovine serum, and penicillin–streptomycin stocks were obtained from Gibco (Invitrogen Corp., Carlsbad CA). Crystalline 1,25-dihydroxyvitamin D3 (1,25D) was obtained from Axxora (Farmingdale, NY). Cells were cultured at <80% confluence between passages 10–30 in 6-well plates (9.4 cm2), seeded at either 175 or 200 × 103/well, and treated with 1,25D for the indicated times, usually 4 or 24 hours.
Site-directed mutagenesis
Synthesis of CYP27B1 and CYP24A1 point mutants within full-length wild-type (WT) pSG5-CYP27B1 and pSG5-CYP24A1 vectors was accomplished using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, California) according to the protocol of the manufacturer. All mutants were confirmed by DNA sequencing.
Transient transfection and hormone treatment
For the mammalian two-hybrid assays, human RXRα was cloned into pCMV-BD and human VDR was cloned into pCMV-AD (14). The transfection procedure (Fig. 1) was adapted from the manufacturer’s protocol. Briefly, each well received 2 μl Express-In, along with 25 ng pCMV-BD-hRXRα (bait) and 25 ng pCMV-AD-hVDR (prey), plus 200 ng pFR-Luc (reporter gene) and 20 ng pRL-NULL (Renilla reniformis luciferase used to monitor transfection efficiency). All hormone treatments were consistently performed in 10% FBS-containing medium.
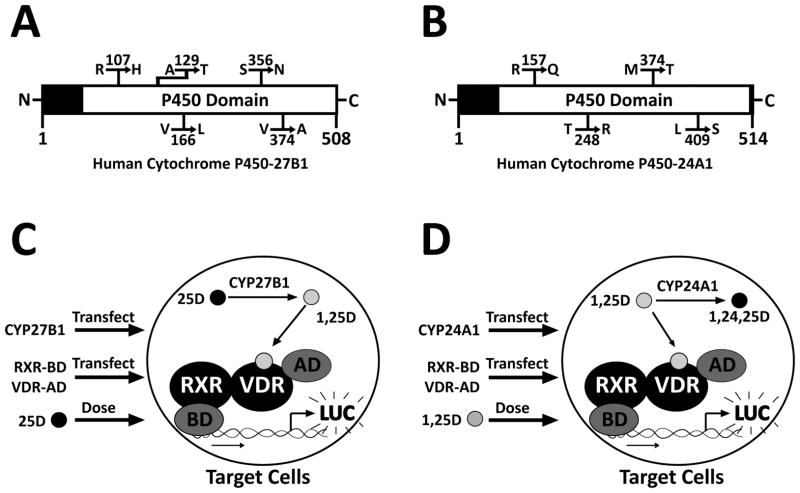
Figure 1. Schematic representation of CYP27B1 and CYP24A1 proteins, as well as the novel experimental systems employed in this study.
These diagrams show the locations of resulting amino acid mutations within the P450 domains of (A) the human cytochrome P450-27B1 protein and (B) the human cytochrome P450-24A1 protein. (C) A novel experimental assay was employed to study the CYP27B1 SNPs. SNP-containing plasmids were introduced into target cells along with a mammalian two-hybrid luciferase reporter gene via liposome transfection. This was followed by treatment with inactive 25D. As CYP27B1 catalyzes the addition of a hydroxyl group to 25D, forming 1,25D, the resulting active hormone binds to VDR, causing heterodimerization with DNA-bound RXR, and transcription of the luciferase reporter gene. Increased CYP27B1 enzymatic activity is reflected by increased luciferase transcription. (D) A similar experimental system was employed to study the CYP24A1 SNPs, however, as CYP24A1 causes the inactivation of 1,25D by the addition of a third hydroxyl group, increased CYP24A1 activity is reflected by decreased luciferase transcription.
For the experiments to evaluate assay sensitivity and substrate specificity (Fig. 2), cells were additionally transfected with either pSG5-empty or pSG5-CYP27B1 and incubated for approximately 24 h in minimal DMEM medium without FBS, penicillin, or streptomycin. The following day, cells were treated with either ethanol, 1 nM 1,25D, 100 nM 24D, or 10–200 nM 25D and incubated for approximately 15 hours prior to lysis and luciferase assay. For the CYP27B1 SNPs analysis experiments (Fig. 3), cells were transfected with either pSG5-empty, human wild type pSG5-CYP27B1, or one of the following CYP27B1 SNPs: R107H (rs28934604), A129T (rs58915677), V166L (rs8176344), S356N (rs13377933), or V374A (rs2229103; see Table 1). Following 24-hour incubation, cells were treated with either ethanol or varying concentrations of 25D for approximately 15 hours before lysis and luciferase assay.
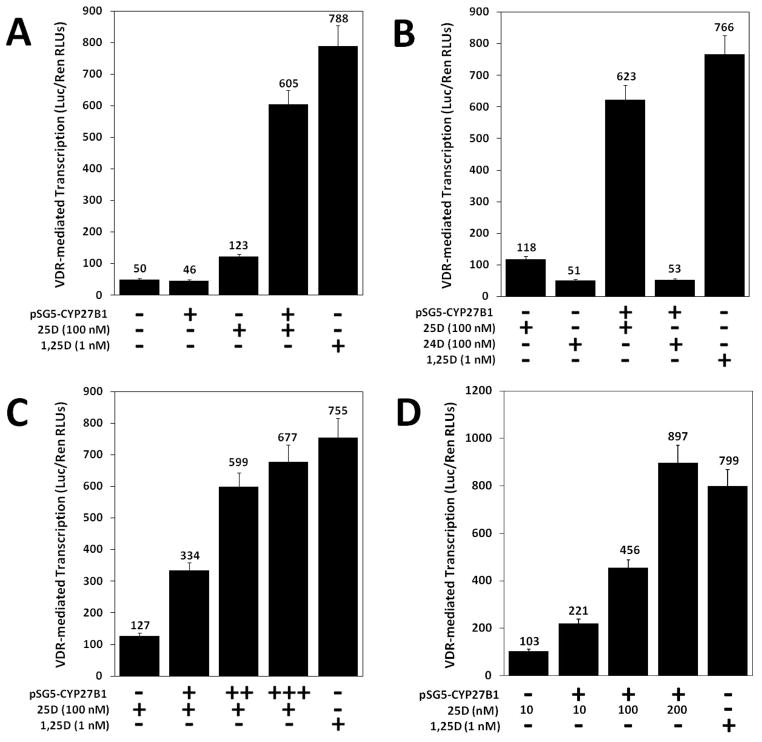
Figure 2. Expression and evaluation of WT human CYP27B1 in target cells.
A 1,25D-VDR luciferase transcription system was successfully developed to detect levels of 1,25D synthesized via the CYP27B1 enzyme. In a mammalian two-hybrid assay system, either pSG5-empty (designated by “−”) or pSG5-CYP27B1 (designated by “+”) was transfected into HCT-116 cells, followed by vitamin D treatment. (A) Cells with and without CYP27B1 were treated with either ethanol or 100 nM 25D to analyze the sensitivity of the assay system. (B) Cells with and without CYP27B1 were treated with either 100 nM 25D or 100 nM 24D to evaluate CYP27B1 substrate specificity. (C) Cells were transfected with increasing amounts of pSG5-CYP27B1 and treated with 100 nM 25D. (D) Cells were transfected with a constant concentration of pSG5-CYP27B1 and treated with increasing concentrations of 25D (10, 100, and 200 nM). Data are representative of three independent experiments with triplicate samples in each group.
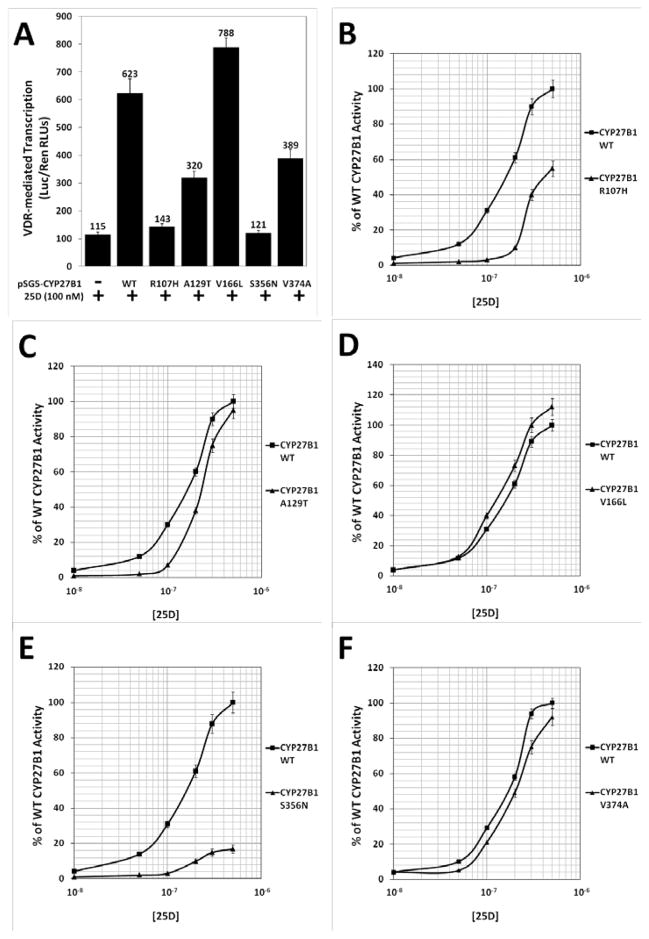
Figure 3. Analysis of CYP27B1 SNPs for 1-hydroxylase activity in target cells.
Both single-concentration and dose-response experiments were used to assess activity among the CYP27B1 SNPs. (A) To evaluate CYP27B1 SNP activity, pSG5-CYP27B1 WT and the five SNPs were transfected into HCT-116 cells, in a mammalian two-hybrid assay system, followed by treatment with 100 nM 25D. (B–F) CYP27B1 WT and the five SNPs were also transfected into HCT-116 cells and treated with a range of 25D concentrations. Data are representative of three independent experiments with triplicate samples in each group.
Table 1.
Identification, classification and synthesis of human CYP27B1 and CYP24A1 SNPs via site-directed mutagenesis.
| CYP27B1 | Region | dbSNP rs# Cluster Id | dbSNP allele | Protein Residue (*=Ref) | Amino Acid |
|---|---|---|---|---|---|
| R107H | Exon 2 | rs28934604 | G | Arg* | 107 |
| A | His | 107 | |||
| A129T | Exon 2 | rs58915677 | G | Ala* | 129 |
| A | Thr | 129 | |||
| V166L | Exon 3 | rs8176344 | G | Val* | 166 |
| C | Leu | 166 | |||
| S356N | Exon 6 | rs13377933 | G | Ser* | 356 |
| A | Asn | 356 | |||
| V374A | Exon 6 | rs2229103 | T | Val* | 374 |
| C | Ala | 374 | |||
|
| |||||
| CYP24A1 | Region | dbSNP rs# Cluster Id | dbSNP allele | Protein Residue (*=Ref) | Amino Acid |
|
| |||||
| R157Q | Exon 3 | rs35051736 | G | Arg* | 157 |
| A | Gln | 157 | |||
| T248R | Exon 6 | rs16999131 | C | Thr* | 248 |
| G | Arg | 248 | |||
| M374T | Exon 8 | rs6022990 | T | Met* | 374 |
| C | Thr | 374 | |||
| L409S | Exon 9 | rs6068812 | T | Leu* | 409 |
| C | Ser | 409 | |||
For CYP24A1 preliminary experiments (Fig. 4), cells were transfected with either pSG5-empty or pSG5-CYP24A1 and incubated in minimal DMEM as above for approximately 24 hours. The following day, cells were treated with 100 nM 25D or 1 nM 1,25D and incubated for approximately 15 hours. For the CYP24A1 SNPs analysis experiments (Fig. 5), cells were transfected with either pSG5-empty, human wild type pSG5-CYP24A1, or one of the following CYP24A1 SNPs: R157Q (rs35051736), T248R (rs16999131), M374T (rs6022990), L409S (rs6068812; see Table 1). Following 24-hour incubation, cells were treated with either ethanol or 1,25D for approximately 15 hours before lysis and luciferase assay.
Figure 4. Expression and evaluation of WT human CYP24A1 in target cells.
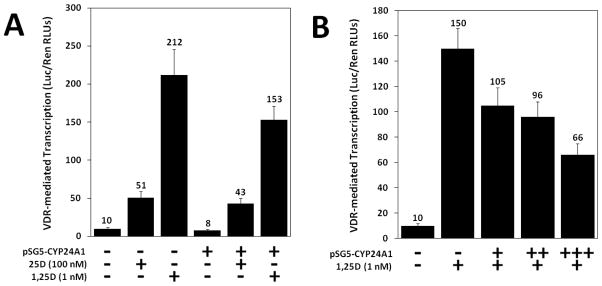
A 1,25D-VDR luciferase transcription system was successfully developed to detect levels of 1,25D inactivation via the CYP241 enzyme. In a mammalian two-hybrid assay system, HCT-116 cells were transfected with either pSG5-empty (designated by “−”) or pSG5-CYP24A1 (designated by “+”) followed by treatment with either 25D or 1,25D. (A) Cells were transfected as indicated and treated with either 100 nM 25D or 1 nM 1,25D. (B) Cells were transfected with increasing concentrations of pSG5-CYP24A1 plasmid and treated with 1 nM 1,25D. Data are representative of three independent experiments with triplicate samples in each group.
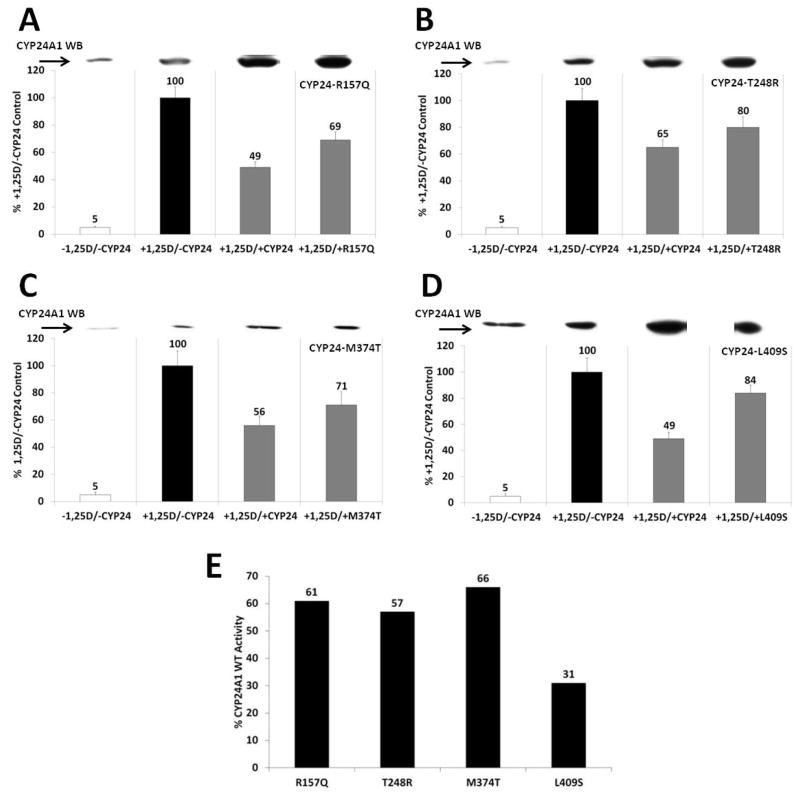
Figure 5. Expression and assessment of CYP24A1 SNPs.
(A–D) HCT-116 cells were transfected with either CYP24A1 wild type or a polymorphism and treated with 1 nM of 1,25D. A mammalian two-hybrid assay system was used to analyze enzymatic activity. Western blot analysis using a monoclonal antibody against hCYP24A1 was also used to assess CYP24A1 expression of both endogenous and transfected CYP24A1. (E) The percent reduction in CYP24A1 activity of each SNP relative to the WT CYP24A1 control (both in the presence of 1,25D) was determined by calculating the % reduction of the WT versus the control (second bar minus third bar in each panel), and the % reduction of the SNP versus the control (second bar minus fourth bar in each panel) for the SNPs, and then taking the ratio of %SNP reduction:%WT reduction, in each panel. All CYP24A1 SNPs show reduced activity, with L409S displaying markedly lower activity. Data are representative of three independent experiments with triplicate samples in each group.
For the CYP27B1 and CYP24A1 qPCR analyses (Fig. 6), cells were transfected with either an empty pSG5 vector, or a pSG5 plasmid containing the cDNA for WT human CYP27B1, WT human CYP24A1, or the CYP27B1 and CYP24A1 SNPs indicated in Table 1. Cells were transfected with 1.0 μg expression vector/well 24 hours post-seeding, using Xtreme-GENE 9 DNA Transfection Reagent (Roche, Palo Alto, CA) at a ratio of 3 μl/μg DNA according to manufacturer’s recommendations. Twenty-four hours after transfection, fresh total media was applied, containing either ethanol vehicle, 5 nM 1,25D for 4 hours in the CYP24A1 group, or 100 nM 25D for 24 hours in the CYP27B1 group.
Figure 6. UMR-106 cells transiently cotransfected with cDNA expression vectors containing either wild type or SNPs of human CYP27B1 or CYP24A1.
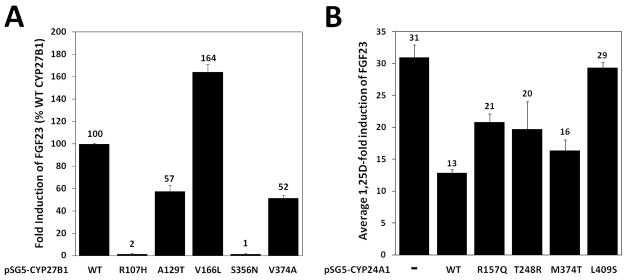
(A) Quantitative RT-PCR demonstrates fold-induction of rFGF23 expression in response to treatment with 100 nM 25D for 24 hours. Results are shown as a percentage relative to the average fold induction observed in the hCYP27B1 wild-type group and did not occur without hCYP27B1 transfection. Actual fold effect of each group was calculated (2−ΔΔCT) with the average wild type induction of rFGF23 (114-fold) set to 100%. Error bars are SEM of the ratios with all groups showing statistical significant reductions (P < 0.01; n=3) compared to wild type (first column) using a 2-sided student’s t-test. (B) UMR-106 cells transiently cotransfected with cDNA expression vectors containing either wild type or SNPs of human CYP24A1. Quantitative RT-PCR demonstrates fold-induction of rFGF23 expression in response to treatment with 5 nM 1,25D for 4 hours. 1,25D fold-induction of rFGF23 for each group was calculated (2−ΔΔCT) with the average induction of the mock-transfected cells set as the control group (31-fold). Each hCYP24A1-transfected SNPs displays an attenuated rFGF23 average fold induction. Two-sided student’s t-tests showed significant activity of each construct (P < 0.01; n=3) compared to mock-transfected groups (first column), with R157Q and L409S being significantly different from the wild type (p < 0.01; n=3) and showing reduced attenuation of the 1,25D-induced rFGF23.
Mammalian two-hybrid assay
After transient cell transfection and incubation with ligands, cells were collected and the amount of reporter gene product (luciferase) produced in the cells was measured using the Dual-Luciferase Reporter Assay System according to the manufacturer’s protocol (Promega, Madison, WI). To control for transfection efficiency, the RLUs produced by the inducible firefly luciferase reporter were divided by the RLUs produced by the constitutively expressed Renilla luciferase. The mean ratio of firefly/Renilla luciferase was determined for each treatment group and the standard deviation was calculated (expressed as error bars). All data are reported as either the average of three or more experiments, or are representative of two or more trials. Each experimental treatment group was replicated in at least three, and often as many as six, wells.
Western blotting
Cell lysates were heated at 95°C in SDS sample buffer in the presence of 2-mercaptoethanol and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred by electrophoresis to Amersham Hybond-P polyvinylidene difluoride transfer membranes (GE Healthcare, Wauwatosa, WI) and then treated with diluted antibodies as follows: CYP24A1 rabbit polyclonal Ab (H-87), CYP24A1 mouse monoclonal Ab (E-7), or CYP27B1 rabbit polyclonal Ab (H-90) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG was utilized as the secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and signals were detected using SuperSignal (Pierce Biotechnology, Rockford, IL).
Total RNA isolation and cDNA synthesis
Total RNA was isolated from approximately 2 million cells using an Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. First strand cDNA was synthesized from 2 μg of RNA using an iScript kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions in 40 μl total volume.
Real-time PCR
Quantitative real time PCR (qrtPCR) was performed with Applied Biosystems SYBR Green 2X PCR Master Mix (Life Technologies, Carlsbad CA) in a System 7500 Fast thermal cycler using 1.5 μl of first strand DNA and 0.5 μl of 18 μM primer mixture in 10 μl total volume per well in triplicate. For detection of rat FGF23 transcripts, the forward primer was 5′-ACGGAACACCCCATCAGACTATC-3′ and the reverse primer was 5′-TATCACTACGGAGCCAGCATCC-3′, yielding a 71 nt product, while for the reference gene rat GAPDH the forward prime was 5′-AGGTCGGTGTGAACGGATTTG-3′ and the reverse primer was 5′-CATTCTCAGCCTTGACTGTGCC-3′ yielding a 180 nt product. Rat CYP24A1 was detected using forward primer 5′-GATCACCTTTCCAAGAAGGAACT3′ and reverse primer 5′ AGAGAATCCACATCAAGCTGTTC3′ yielding a 94 nt product as validation of 1,25D activity (data not shown). The transfected human CYP24A1 was detected using forward primer 5′-CAGCGAACTGAACAAATGGTCG-3′ and reverse primer 5′-TCTCTTCTCATACAACACGAGGCAG-3′, while the transfected human CYP27B1was detected using forward primer 5′-GAATTGCAAATGGCTTTGGCCCAG-3′ and reverse primer 5′-CTGTAGGTTGATGCTCCTTTCAGG-3′.
Reactions were performed in 96-well PCR plates and read on an ABI 7500 Fast instrument. Data were analyzed using the comparative cycle threshold (Ct) method as a means of relative quantitation, normalized to an endogenous reference (GAPDH) and relative to a calibrator (normalized Ct value obtained from vehicle-treated cells) and expressed as 2−ΔΔCt according to the Applied Biosystems manufacturer’s protocol.
Results
Selection of CYP27B1 and CYP24A1 SNPs and development of assays to evaluate colonic intracellular vitamin D metabolism
The five CYP27B1 and four CYP24A1 human polymorphisms were chosen for analysis based on population diversity, and, using data available in the dbSNP database at the time of selection, including only nonsynonymous SNPs. Each SNP results in a missense mutation where a single amino acid is replaced by another with different physicochemical properties, potentially changing the functionality of the resulting protein. For example, the CYP24A1 R157Q mutant substitutes a polar, uncharged glutamine in place of the original positively charged arginine. These nine SNPs include few conservatively replaced amino acids, and therefore are generally the most common polymorphisms likely to result in a functionally altered protein product.
Figures 1A and 1B depict linear schematics of the CYP27B1 and CYP24A1 proteins, respectively. Most notable about the SNP loci selected for analysis is that they are all positioned within the active P450 domain that contains an iron(III) protoporphyrin-IX ring covalently linked to a nearby cysteine ligand (15). This cysteine heme prosthetic group is enzymatically responsible for the oxidative reaction carried out by the proteins; thus, mutations in this domain may have an effect on overall enzyme function.
In order to evaluate the relative activity of the SNPs, an assay system was developed to measure intracellular 1,25D levels. As described above, active 1,25D binds to VDR, which heterodimerizes with RXR and then binds to DNA to stimulate transcription. For this system, RXR was cloned into a plasmid containing a heterologous DNA binding domain (BD), and VDR was cloned into a plasmid containing a transcriptional activation domain (AD). In the presence of 1,25D, AD-VDR (prey) binds the hormonal ligand and dimerizes with BD-RXR (bait). When bait and prey are brought together the transcriptional activation domain is tethered to the correct DNA sequence by the binding domain, through the RXR-VDR dimer. This assembled complex stimulates transcriptional initiation of the firefly luciferase reporter gene which can be quantified via luminescence. In this system, a linear relationship exists between the levels of intracellular 1,25D and the amount of luciferase production, allowing for a quantitative assessment of 1,25D levels inside the cell.
Figures 1C and 1D illustrate the mammalian two-hybrid assay system utilized for the evaluation of both SNPs. In the first system (Fig. 1C), exogenous CYP27B1, RXR-BD, VDR-AD, and inactive 25D are introduced to target cells. As exogenous WT or a CYP27B1 SNP catalyzes the formation of 1,25D, RXR-VDR dimerization occurs, and the firefly luciferase (LUC) gene is transcribed. More active CYP27B1 variants would synthesize more 1,25D, leading to more luciferase production, while less active SNPs would produce less luciferase.
The CYP24A1 system (Fig. 1D) utilized a similar design, except CYP24A1 was introduced and target cells were treated with 1,25D. The 1,25D treatment results in luciferase production, while exogenous CYP24A1 expression leads to 1,25D degradation and lowers the overall production of the luciferase protein. The outcome of this system is essentially the reverse of that with CYP27B1, with a more active CYP24A1 enzyme yielding lower firefly luciferase output, due to higher rates of 1,25D inactivation.
Assessment of the 1,25D-luciferase transcription system for evaluating CYP27B1 SNPs
Due to the expression of CYP27B1 in colon carcinoma cells reported previously (8), a luciferase-based transcription system was developed to detect enzymatic activity beyond CYP27B1 endogenous levels. The leftmost column (column one) of Fig. 2A represents target cells transfected with an insertless expression vector, pSG5-empty (indicated by “−”) and treated with ethanol only. These cells are effectively untreated, “natural” cells that represent the basal CYP27B1 activity. As indicated by columns one and two, without 25D substrate, synthesis of 1,25D (as measured by the amount of luciferase activity) remains unchanged with or without transfection of exogenous CYP27B1 (indicated by “+”). A lack of exogenous CYP27B1 and treatment with 25D (Fig. 2A middle column) showed a slight increase in 1,25D-induced firefly luciferase transcription, indicating modest endogenous CYP27B1-mediated production of 1,25D. Transfection of exogenous CYP27B1, followed by treatment with 25D, yielded a nearly 5-fold increase in 1,25D-induced firefly luciferase transcription (Fig. 2A, column 4), and an increase in CYP27B1 protein as assessed by western blotting (data not shown). The positive control (far right column), in which transfected cells are treated with 1,25D, indicates a potent induction of luciferase by the active 1,25D-VDR complex. Taken together, these results illustrate that by overexpressing exogenous CYP27B1, fluctuations due to endogenous activity are negligible and the system allows the evaluation of CYP27B1 activity in the various CYP27B1 SNPs expression vector constructs.
In addition to negating the influence of endogenous enzymatic activity, this assay system does not detect CYP27B1 metabolites other than 1,25D. Cells transfected both with and without exogenous CYP27B1 that have been treated with 24D (Fig. 2B, columns 2 and 4) showed firefly transcription levels only slightly above those of cells treated with ethanol only (Fig. 2A, columns 1 and 2), while treatment with 25D (to generate 1,25D) again reveals a vigorous induction of the reporter gene (Fig. 2B, column 3). Increased amounts of transfected CYP27B1, with 25D hormone treatment held constant, lead to elevated detectable 1,25D synthesis (Fig. 2C), and increased CYP27B1 protein expression (data not shown). Similarly, higher concentrations of 25D substrate (Fig. 2D) also resulted in increasingly elevated levels of detectable 1,25D synthesis, indicating that the assay system can be employed to test dose-response associations.
CYP27B1 SNPs show both modest and dramatic differences in 1α-hydroxylase activity
Having designed an assay system that is able to effectively quantify CYP27B1-mediated production of intracellular 1,25D in colonic cells, the next step was to compare the CYP27B1 SNPs (Table 1) to the WT enzyme. To do this, HCT-116 were transfected with an empty pSG5 vector, pSG5-WT-CYP27B1, or a pSG5-CYP27B1 SNP expression plasmid. Cells were then treated with either a constant concentration of 25D or a range from 10−8 to 0.5×10−6 M. All CYP27B1 variants showed statistically significantly (p < 0.05) decreased activity with the exception of V166L, a conservative replacement, when CYP27B1-transfected cells were treated with 100 nM 25D (Fig. 3A). R107H (Fig. 3B) displayed nearly undetectable activity at low- to mid- concentrations of 25D, with at most approximately 55% of WT activity (p < 0.05) at high concentrations of hormone. A129T (Fig. 3C) also showed little activity at low- to mid-concentrations of hormone but with higher 25D concentrations, this polymorphism had moderate but statistically significant reductions in activity when compared to WT CYP27B1 (p < 0.05). Interestingly, the SNP V166L (Fig. 3D) revealed normal activity at low levels of hormone, with activity higher than WT activity at mid- and high- concentrations of 25D (p < 0.05; similar to Fig. 3A). CYP27B1 S356N (Fig. 3E) was the least active of the SNPs, showing negligible activity at low to mid concentrations of hormone and a small increase in activity at higher levels of 25D. However, its overall activity remained markedly lower than the WT enzyme (p < 0.05). CYP27B1 V374A (Fig. 3F) constitutes a conservative replacement and demonstrated slight but statistically significant reductions in activity compared to WT at all concentrations of 25D (p < 0.05).
Assessment of a 1,25D-luciferase transcription system for CYP24A1
A similar mammalian-two hybrid system as employed for the CYP27B1 experiments was used for evaluating the CYP24A1 SNPs (Figure 1). Over-expression of exogenous transfected CYP24A1 in target cells (Fig. 4A, three far-right columns) reduced levels of intracellular 1,25D, as shown by the reductions in basal (column 1), 25D-induced (column 2), or 1,25D-induced (column 3) luciferase transcription. Analogous to the CYP27B1 system, elevated amounts of transfected CYP24A1 (Fig. 4B) increase enzymatic deactivation of 1,25D, resulting in a dose-dependent reduction in luciferase activity (compare columns 2–5).
CYP24A1 SNPs all exhibit reduced 24-hydroxylase activity
Western blot analysis reveals the induction of endogenous CYP24A1 in colon carcinoma cells by the active 1,25D metabolite (upper inset, Fig. 5A–D). As expected, further transfection of CYP24A1 vectors results in additional and pronounced expression of exogenous WT or SNP CYP24A1 protein, indicating that a significant proportion of CYP24A1 expression was derived from cellular transfection. For all CYP24A1 SNP experiments, endogenous CYP24A1 activity in colonic cells transfected with pSG5-empty was set to 100% (Fig. 5A–D, second column, black bar). Transfection of exogenous CYP24A1 WT and SNPs, followed by treatment with 1,25D, resulted in increased expression of the CYP24A1 protein and in reduction of firefly luciferase activity, indicating increased 1,25D catabolism.
Figure 5A demonstrates that transfection of exogenous WT CYP24A1 results in a 51% drop in firefly activity, while the R157Q SNP showed only a 31% reduction. In contrast, exogenous WT CYP24A1 yielded a 35% reduction in 1,25D-induced firefly luciferase transcription while the T248R SNP (Fig. 5B) resulted in only a 20% reduction. Similarly, CYP24A1 M374T results (Fig. 5C) showed a 44% drop in firefly activity for the WT protein and a 29% drop for the M374T SNP. Finally, the CYP24A1 L409S variant (Fig. 5D) exhibited a 51% drop in 1,25D-induced firefly activity by exogenous WT CYP24A1 and only a small 16% drop in firefly activity by the L409S SNP. Taken together, and summarized in Fig. 5E, the percent reduction in catabolic CYP24A1 activity (as measured by luciferase output) of each SNP, expressed as a ratio of the percent reduction in luciferase in the presence of the WT enzyme, revealed that of the CYP24A1 SNPs tested, the L409S was the most affected in terms of its ability to catabolize 1,25D, exhibiting only 31% of wild type activity, followed by T248R at 57%, R157Q at 61%, and M374T at 66% of the wild-type protein. The reduction in activity for all SNPs was statistically significant at p < 0.05.
Real time PCR data results
To evaluate the effects of the human CYP27B1 and CYP24A1 SNPs in conjunction with an endogenous 1,25D-VDR target gene, we selected the rat osteosarcoma bone cell line UMR106, and the FGF23 gene as the reporter of 1,25D activity. Prior studies have shown robust 1,25D-induced FGF23 expression that is both dose- and time-dependent in this cell line, with maximum mRNA levels observed at 24 h when evaluated by qRT-PCR (16). However, FGF23 levels gradually decline in UMR106 cells with advanced (>30) passages. We avoided this issue and observed internal consistency in FGF23 expression by evaluating multiple SNPs in parallel experiments in UMR106 cells grown between passages 15–30 only. The induction of rFGF23 depicted in Fig. 6A represents the relative amount of 1,25D-mediated induction via production of intracellular 1,25D from the 25D prohormone by the transfected CYP27B1, following treatment of cells with 100 nM 25D for 24 hours. The WT CYP27B1-directed expression of FGF23 observed under these conditions is 114-fold (set to 100% in Fig. 6A, column 1). This level of FGF23 induction is almost entirely abrogated under similar conditions for the R107H and S356N groups (Fig. 6A, columns 2 and 5; p < 0.05), although when the dose was increased to 200 nM 25D, we did observe a modest but significant increase in activity (data not shown). Interestingly the V166L demonstrated a rare statistically significant hyperactivity (p < 0.05), while the A129T and V374A SNPs induced about 50% FGF23 compared to WT (p < 0.05).
The assay to assess CYP24A1 SNP activity applied the same cell line and reporter gene as above, although the experimental scheme is modified for the CYP24A1 catabolic enzyme. In this experiment, the cultures were transfected with CYP24A1 WT or SNP variants, then treated with 1,25D instead of the 25D prohormone. In this assay, the attenuation of 1,25D-directed FGF23 induction is monitored at a shorter time point of 4 h. The pSG5-empty group (−) treated with 5 nM 1,25D reveals an average 31-fold induction of FGF23 (Fig. 6B, column 1). Using this value as a baseline, the transfection of WT pSG5-CYP24A1 results in the greatest attenuation of 1,25D-mediated FGF23 expression (13-fold, column 2), while the pSG5-L409S SNP displays a severely impaired ability to catabolize 1,25D, thus resulting in a 29-fold induction of FGF23 (column 6) which is almost identical to that observed with the pSG5-empty control (p < 0.01). The other CYP24A1 SNPs analyzed, namely R157Q, T248R, and M374T each displayed a statistically significant (p < 0.01) reduction in activity compared to WT CYP24A1, as evidenced by a blunting of the attenuation of FGF23 induction (Fig. 6B, 21-, 20- and 16-fold, respectively, versus 13-fold for WT). Importantly, these qPCR data with an endogenous 1,25D target gene reporter effectively mimic the results obtained previously with CYP27B1 (Fig. 3) and CYP24A1 (Fig. 5) employing the M2H assay.
Discussion
The objective of the present work was to examine whether genetic variation in CYP27B1 and CYP24A1 might affect enzymatic function in the 1,25D metabolic pathway in colon cancer cell lines, and therefore potentially alter tissue-level exposure to the hormone. In these experiments, we observed differential activity of CYP27B1 and CYP24A1 in the presence of SNPs that result in missense mutations with both a mammalian-two hybrid system, and quantitative real-time PCR analysis of an endogenous 1,25D target gene. These findings have potentially important implications for cancer prevention and control given the significance of these enzymes in regulating the metabolism of 1,25D, which has been shown to possess potent anti-carcinogenic effects in the colon (6, 7, 17).
CYP27B1 and CYP24A1 are both of critical importance in governing 1,25D concentrations (2, 3, 9), and it has been proposed that these two enzymes work in concert to regulate the concentrations of this hormone at the tissue level (9). The hydroxylation that converts 25D to 1,25D is performed by CYP27B1 (3); while CYP24A1 begins the catabolic processing of 1,25D by incorporating a hydroxyl group at the C24-position to form 1,24,25D, and continues to catalyze the further hydroxylation of this metabolite until degradation (9). CYP24A1 may also hydroxylate 25(OH)D to begin catabolism of this molecule, a process that has the potential to limit the availability of active prohormone (9).
Several factors may influence the degree to which these enzymes can alter cellular exposure to the anti-carcinogenic effects of 1,25D. First, compared to normal cells, the colonic expression of CYP27B1 is increased in early-stage colon tumors, but is reduced or abrogated as colon cells become increasingly undifferentiated (18). Therefore, the production of 1,25D may be stimulated in response to carcinogenic initiation but repressed in progression to undifferentiated tumors, thus excluding later-stage tumors from the anti-proliferating effects of this hormone and allowing for further uncontrolled growth (17). In contrast, expression of CYP24A1 remains high during tumorigenesis, allowing for sustained catabolism of 1,25D, and therefore again reducing tumor exposure to this hormone (17). Genetic variation in CYP24A1 and CYP27B1 is another factor that may contribute to availability of 1,25D during carcinogenesis.
Polymorphisms in CYP27B1 and CYP24A1 have been studied in relation to both vitamin D metabolite concentrations and risk of cancer. Genetic variation in both CYP27B1 and CYP24A1 has been linked to colorectal cancer risk in a human population study; however, the mechanism of action for this association is unknown (11). It may be the result of alterations in circulating or tissue-level availability of vitamin D metabolites. Significant associations between CYP27B1 and circulating 25D concentrations have been observed in some studies (10, 19), though a large genome-wide association study (GWAS) did not identify a significant relationship (20). However, the same GWAS investigation demonstrated that variation in CYP24A1 was significantly related to blood 25D levels (20). While no CYP27B1 or CYP24A1 polymorphisms are known to affect concentrations of 1,25D in circulation, both enzymes are expressed in the colon (8, 21). The activity of these enzymes is likely critically important in tissue-level exposure to 1,25D (9), and as such could have major implications for any inhibition of the carcinogenic pathway by this hormone.
In the current work, the majority of the CYP27B1 variants resulted in attenuation of enzymatic activity to different degrees; these SNPs included R107H, A129T, S356N, and V374A. Although the R107H variant appears relatively rarely in human populations, it is perhaps the most well-studied of these SNPs due to adverse outcomes when it is present (22, 23). Kitanaka et al. performed DNA sequencing of CYP27B1 among four patients with pseudovitamin D-deficiency rickets (PDDR), and discovered four genes with missense mutations, including R107H, which abrogated CYP27B1 activity (22). Sawada et al. (23) confirmed these findings by expressing cDNAs from normal and PDDR patients in Escherichia coli JM109 cells. Among the cells expressing the R107H mutant CYP27B1, activity was reported as less than 1% than that of WT (23). Our results support and extend this observation, as R107H was one of two SNPs that resulted in striking reductions in CYP27B1 activity in both the M2H and qRT-PCR experiments. The results also revealed another SNP that exhibited such drastic attenuation of enzymatic activity, namely S356N; however, to date there are no allele frequency or functional data available for this polymorphism. It is possible that its potent effects on CYP27B1 function render it a rare variant in human populations.
Two other SNPs, A129T and V374A, caused a reduction in activity compared to WT CYP27B1. Very little information is currently available for A129T, which has a minor allele frequency (MAF) in human populations of approximately 0.013% (24). Regarding V374A, a SNP for which the MAF is 0.02 (24), it was one of 791 SNPs in human cytochrome P450 enzymes recently evaluated by Wang et al. using sorting intolerant from tolerant (SIFT) calculations and polymorphism phenotyping (25). Both methods predicted that V374A would be a deleterious SNP (25), which is supported by our results. It is unknown whether there are differences in MAF for these two SNPs by race/ethnicity, and whether the impact of such SNPs varies between populations with differing genetic backgrounds, though these are strong possibilities given prior findings for genetic variation in genes within the vitamin D pathway (19, 26). In fact, variation in MAF by race/ethnicity has been reported for V166L, the only SNP that exhibited increased enzymatic activity over CYP27B1 WT at high doses of 25D (24). The overall MAF frequency for V166L has been reported as 0.03; while in a small sample of those reporting Pacific Rim heritage, the MAF is 0.13 (24). Very little is known about circulating concentrations of vitamin D metabolites associated with this SNP. However, given the functional differences in the presence of V166L and the variation in MAF between racial and ethnic groups, further study is warranted to evaluate whether this SNP may affect the availability of 1,25D at the cellular level, as this may affect susceptibility to cancer (27). A more efficient enzyme for conversion of prohormone 25D to 1,25D would be expected to produce higher concentrations of the hormone at the cellular level and possibly confer protective or chemopreventive effects. Also of interest was the finding that the activity of V166L remained at normal levels when exposed to low levels of hormone, but increased to higher than WT at mid-and high concentrations of 25D. These results suggest the possibility that higher concentrations of circulating 25D may overcome genetic background to induce rapid production of 1,25D at the target cell. It is therefore possible that the intake of vitamin D needed to achieve and maintain optimal circulating concentrations of vitamin D metabolites may vary by genotype.
As observed with CYP27B1, CYP24A1 SNPs exhibited a broad range of effects on activity; however, in this case each of the four tested polymorphisms resulted in lower activity as compared to WT enzyme. Little is known about the effects of these SNPs in human populations, as they tend to be rare variants. However, one SNP examined in the current work, R157Q, is located two amino acids from R159Q, a SNP that has been associated with idiopathic infantile hypercalcemia (28). There is also marked variation in MAF between different racial/ethnic groups for some CYP24A1 polymorphisms. The MAF for M374T, for example, appears to be absent in those reporting white race; but is 0.15 among African-Americans (24). Because the reduced catabolism of 1,25D at the cellular level associated with this SNP would likely result in higher concentrations of this metabolite, variation such as this may partially account for the observation that while African-Americans tend to have lower concentrations of the prohormone 25D, this population has also been reported to have higher circulating levels of 1,25D than whites (27, 29, 30).
Vitamin D metabolism, predominantly controlled by CYP27B1 and CYP24A1, is a vital mediator of mineral homeostasis(31). Moreover, CYP expression in a variety of cancer cells likely affects tissue level 1,25D bioavailability and thus could potentially limit vitamin D concentrations in the tumor microenvironment(32). In summary, the present study demonstrates that CYP27B1 and CYP24A1 polymorphisms result in marked changes in enzymatic activity in colon cancer cells. These alterations would likely result in differential 1,25D exposure in colonic cells, which could render individuals more susceptible to the development of colon cancer.
Acknowledgments
Grant Support
This work was supported by grants from the National Cancer Institute to ETJ (R01CA140285, CA-41108, CA-23074, and CA-77145) and R01CA140285 to PWJ.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Zhang X, Giovannucci E. Calcium, vitamin D and colorectal cancer chemoprevention. Best Pract Res Clin Gastroenterol. 2011;25(4–5):485–94. doi: 10.1016/j.bpg.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Haussler MR, Haussler CA, Whitfield GK, Hsieh J-C, Thompson PD, Barthel TK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Bio. 2010;121(1–2):88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441(1):61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54(3):805–10. [PubMed] [Google Scholar]
- 5.Frampton RJ, Omond SA, Eisman JA. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. 1983;43(9):4443–7. [PubMed] [Google Scholar]
- 6.Giuliano AR, Franceschi RT, Wood RJ. Characterization of the vitamin D receptor from the Caco-2 human colon carcinoma cell line: effect of cellular differentiation. Arch Biochem Biophys. 1991;285(2):261–9. doi: 10.1016/0003-9861(91)90358-p. [DOI] [PubMed] [Google Scholar]
- 7.Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993;53(16):3712–8. [PubMed] [Google Scholar]
- 8.Cross HS, Bareis P, Hofer H, Bischof MG, Bajna E, Kriwanek S, et al. 25-Hydroxyvitamin D(3)-1alpha-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66(3–5):287–92. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 9.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Bio. 2010;121 (1–2):471–7. doi: 10.1016/j.jsbmb.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 11.Dong LM, Ulrich CM, Hsu L, Duggan DJ, Benitez DS, White E, et al. Vitamin D related genes, CYP24A1 and CYP27B1, and colon cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2540–8. doi: 10.1158/1055-9965.EPI-09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Ann Rev Nutr. 2009;29:111–32. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 13.Ahn J, Albanes D, Berndt SI, Peters U, Chatterjee N, Freedman ND, et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis. 2009;30(5):769–76. doi: 10.1093/carcin/bgp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartik L, Whitfield GK, Kaczmarska M, Lowmiller CL, Moffet EW, Furmick JK, et al. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21:1153–61. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev. 2004;104(9):3947–80. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 16.Kolek OI, Hines ER, Jones MD, LeSeuer LK, Lipko MA, Kiela PR, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G1036–42. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 17.Cross HS, Nittke T, Kallay E. Colonic vitamin D metabolism: implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol Cell Endocrinol. 2011;347(1–2):70–9. doi: 10.1016/j.mce.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Bises G, Kallay E, Weiland T, Wrba F, Wenzl E, Bonner E, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem. 2004;52(7):985–9. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- 19.Signorello LB, Shi J, Cai Q, Zheng W, Williams SM, Long J, et al. Common variation in vitamin D pathway genes predicts circulating 25-hydroxyvitamin D Levels among African Americans. PloS One. 2011;6(12):e28623. doi: 10.1371/journal.pone.0028623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner D, Kallay E, Cross HS. 1alpha,25-dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol Cell Endocrinol. 2007;263(1–2):55–64. doi: 10.1016/j.mce.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. New Eng J Med. 1998;338(10):653–61. doi: 10.1056/NEJM199803053381004. [DOI] [PubMed] [Google Scholar]
- 23.Sawada N, Sakaki T, Kitanaka S, Takeyama K, Kato S, Inouye K. Enzymatic properties of human 25-hydroxyvitamin D3 1alpha-hydroxylase coexpression with adrenodoxin and NADPH-adrenodoxin reductase in Escherichia coli. Eur J Biochem/ FEBS. 1999;265(3):950–6. doi: 10.1046/j.1432-1327.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- 24.Database of Single Nucleotide Polymorphisms (dbSNP) dbSNP accession, dbSNP Build 137 for human. 2012 [cited 2012 08/31/12]; Available from: Available from: http://www.ncbi.nlm.nih.gov/SNP/
- 25.Wang LL, Li Y, Zhou SF. A bioinformatics approach for the phenotype prediction of nonsynonymous single nucleotide polymorphisms in human cytochromes P450. Drug MetabDispos. 2009;37(5):977–91. doi: 10.1124/dmd.108.026047. [DOI] [PubMed] [Google Scholar]
- 26.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93(9):3381–8. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs ET, Martinez ME, Jurutka PW. Vitamin D: marker or mechanism of action? Cancer Epidemiol Biomarkers Prev. 2011;20(4):585–90. doi: 10.1158/1055-9965.EPI-10-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. New Eng J Med. 2011;365(5):410–21. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 29.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–6. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 30.Doherty TM, Tang W, Dascalos S, Watson KE, Demer LL, Shavelle RM, et al. Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96(5):1477–81. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- 31.St-Arnaud R, Naja RP. Vitamin D metabolism, cartilage and bone fracture repair. Mol Cell Endocrinol. 2011;347(1–2):48–54. doi: 10.1016/j.mce.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Luo W, Hershberger PA, Trump DL, Johnson CS. 24-Hydroxylase in cancer: Impact on vitamin D-based anticancer therapeutics. J Steroid Biochem Mol Bio. 2012 doi: 10.1016/j.jsbmb.2012.09.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]