Abstract
Purpose
Multiple myeloma (MM) is a usually incurable malignancy of plasma cells. New therapies are urgently needed for MM. Adoptive transfer of chimeric antigen receptor (CAR)-expressing T cells is a promising new therapy for hematologic malignancies, but an ideal target antigen for CAR-expressing T cell therapies of MM has not been identified. B-cell maturation antigen (BCMA) is a protein that has been reported to be selectively expressed by B-lineage cells including MM cells. Our goal was to determine if BCMA is a suitable target for CAR-expressing T cells.
Experimental Design
We conducted an assessment of BCMA expression in normal human tissues and MM cells by flow cytometry, quantitative PCR, and immunohistochemistry. We designed and tested novel anti-BCMA CARs.
Results
BCMA had a restricted RNA expression pattern. Except for expression on plasma cells, BCMA protein was not detected in normal human tissues. BCMA was not detected on primary human CD34+ hematopoietic cells. We detected uniform BCMA cell-surface expression on primary MM cells from 5 of 5 patients. We designed the first anti-BCMA CARs to be reported, and we transduced T cells with lentiviral vectors encoding these CARs. The CARs gave T cells the ability to specifically recognize BCMA. The anti-BCMA-CAR-transduced T cells exhibited BCMA-specific functions including cytokine production, proliferation, cytotoxicity, and in vivo tumor eradication. Importantly, anti-BCMA-CAR-transduced T cells recognized and killed primary MM cells.
Conclusions
BCMA is a suitable target for CAR-expressing T cells, and adoptive transfer of anti-BCMA-CAR-expressing T cells is a promising new strategy for treating MM.
Keywords: multiple myeloma, chimeric antigen receptor, adoptive T cell therapy, B-cell maturation antigen, immunotherapy
Introduction
Multiple myeloma (MM) is a malignancy characterized by an accumulation of clonal plasma cells (1–3). Current therapies for MM often cause remissions, but nearly all patients eventually relapse and die (1, 2). There is substantial evidence of an immune-mediated elimination of myeloma cells in the setting of allogeneic hematopoietic stem cell transplantation; however, the toxicity of this approach is high, and few patients are cured (1, 4). Although some monoclonal antibodies have shown promise for treating MM in preclinical studies and early clinical trials, consistent clinical efficacy of any monoclonal antibody therapy for MM has not been conclusively demonstrated (5–7). There is clearly a great need for new immunotherapies for MM, and developing an effective antigen-specific adoptive T-cell therapy for this disease would be a major advance.
Adoptive transfer of T cells genetically modified to recognize malignancy-associated antigens is a promising approach for cancer therapy (8, 9). T cells can be genetically modified to express chimeric antigen receptors (CARs), which are fusion proteins that include an antigen recognition moiety and T cell activation domains (9, 10). For B-lineage malignancies, substantial progress has been made recently in developing adoptive T cell approaches that utilize anti-CD19 CARs (11–18). Anti-CD19-CAR-transduced T cells have cured leukemia and lymphoma in mice (19, 20). Several patients obtained remissions in early clinical trials of adoptively transferred anti-CD19-CAR-transduced T cells, and T cells transduced with anti-CD19 CARs also eradicated normal B cells (12, 13, 17, 21). Unfortunately, CD19 is rarely expressed on the malignant plasma cells of MM, so treating MM with CAR-expressing T cells will require identifying other antigens to target (22, 23).
One candidate antigen for immunotherapies of MM is B-cell maturation antigen (BCMA, CD269) (24, 25). BCMA RNA was detected universally in MM cells, and BCMA protein was detected on the surface of plasma cells from multiple myeloma patients by several investigators (26–29). BCMA is a member of the tumor necrosis factor receptor (TNF) superfamily (30, 31). BCMA binds B-cell activating factor (BAFF) and a proliferation inducing ligand (APRIL) (31–33). Among nonmalignant cells, BCMA has been reported to be expressed mostly by plasma cells and subsets of mature B cells (24, 25, 32, 34, 35). Mice deficient in BCMA were healthy and had a normal physical appearance (36, 37). BCMA-deficient mice had normal numbers of B cells, but survival of long-lived plasma cells was impaired (34, 36).
We reasoned that BCMA would be an appropriate target antigen for treating MM with CAR-expressing T cells. Except for expression on plasma cells, we found that BCMA is not expressed on the cells of major human organs. We designed lentiviral vectors that encoded BCMA-specific CARs. T cells transduced with these vectors performed BCMA-specific functions including cytokine production, proliferation, and cytotoxicity.
Materials and Methods
Cell lines and primary cells
H929, U266, and RPMI8226 are all BCMA+ multiple myeloma cell lines that were obtained from ATCC. A549 is a BCMA-negative lung cancer cell line (ATCC). TC71 is a BCMA-negative sarcoma cell line. CCRF-CEM is a BCMA-negative T cell line (ATCC). BCMA-K562 are K562 cells (ATCC) transduced with the gene for full-length BCMA in our laboratory. NGFR-K562 are K562 cells transduced with the gene for low-affinity nerve growth factor in our laboratory (38). The same gammaretroviral vector and methods were used to transduce BCMA-K562 and NGFR-K562. We used tissue samples or peripheral blood mononuclear cells (PBMC) from 6 patients with MM designated Myeloma Patients 1–6. We used PBMC from 3 subjects with melanoma: Donor A, Donor B, and Donor C. We obtained primary CD34+ hematopoietic cells from 3 healthy normal donors. All of the human samples mentioned above were obtained from patients enrolled on IRB-approved clinical trials at the National Cancer Institute.
Real-time qPCR to quantitate BCMA transcript copies
We quantitated BCMA cDNA copies in samples of cDNA from human tissues included in the Human Major Tissue qPCR panel II (Origene) by performing qPCR with a BCMA-specific primer and probe set (Applied Biosystems, catalog number 4331182). As a positive control, we quantitated BCMA cDNA copies in cDNA of MM cells from a plasmacytoma of a patient with advanced multiple myeloma. RNA was extracted from the plasmacytoma cells with an RNeasy mini kit (Qiagen), and cDNA was synthesized with standard methods. A standard curve for the BCMA qPCR was created by amplifying dilutions of a plasmid that encoded the full-length cDNA of BCMA (Origene). The qPCR accurately detected copy numbers from 102 to 109 copies of BCMA per reaction. We also quantitated the number of β-actin cDNA copies in the same tissues with a Taqman β-actin primer and probe kit (Applied Biosystems). A β-actin standard curve was created by amplifying serial dilutions of a β-actin plasmid. All qPCR reactions were carried out on a Roche LightCycler480 machine.
Immunohistochemistry
BCMA stains were performed on formalin-fixed paraffin-embedded tissue sections and on a commerical normal human tissue array (Pantomics Inc. catalog number MN0661). NGFR-K562 cells (BCMA-negative), BCMA-K562 cells (BCMA-positive) and sections from reactive tonsils were used as controls. Sections were deparaffinized in xylene, rehydrated in graded-alcohol, placed in 1X low pH antigen retrieval solution (Dako, catalog number S1699), and steamed for 30 minutes. Sections were blocked with Dako protein-block-serum-free (catalog number X0909) for 30 minutes, and blocked with 5% bovine serum albumin (Sigma-Aldrich) for another 30 minutes. The sections were then incubated for 60 minutes at room temperature (RT) with either 1μg/mL anti-BCMA goat polyclonal antibody (R&D Systems, catalog number AF193) or with an isotype control of 1μg/mL normal purified goat IgG. Biotinylated rabbit anti-goat IgG was used as the secondary antibody (Vector Lab, catalog number BA-5000; 7.5 μg/mL, 30 minute incubation, RT). Vectastain ABC Kit (Vector Lab, catalog number PK4005; 30 minute incubation, RT) and DAB+Chromogen (Dako, catalog number K4011; 5 minute incubation, RT) were used for detection. For BCMA and MUM1 double staining, BCMA stained sections were re-retrieved in hot Dako 1X low pH antigen retrieval solution and microwaved for 6 min. Sections were blocked with Dako protein-block-serum-free for 30 min, incubated with MUM1 mouse monoclonal antibody (Dako, M7259; 1:100 dilution, 120 min incubation, RT) and detected with Ventana BenchMark XT with Ventana ultraView universal alkaline phosphatase red detection kit (AZ, 760-501). Images were taken with an Olympus Bx41 microscope: objective UPlanFI 100x/1.30 oil, adaptor U-TV0.5xC, digital camera Q-imaging Micropublisher 5.0 RTV. The images were captured with “Q-Capture Version 3.1” and imported to Adobe Photoshop 7.0.
Construction of anti-BCMA chimeric antigen receptors (CARs)
Sequences of 2 mouse-anti-human-BCMA antibodies were obtained from a patent (39). The specific antibodies used were designated in the patent as C12A3.2 and C11D5.3. The heavy chain and light chain variable-region sequences of these antibodies were used to design single chain variable fragments (scFvs) with the following pattern: light chain variable region-linker-heavy chain variable region. The linker had the following amino acid sequence: GSTSGSGKPGSGEGSTKG (15). CARs incorporating variable regions from C12A3.2 and C11D5.3 were designated anti-bcma1 and anti-bcma2 respectively. DNA sequences encoding these CARs were designed. The sequence of each CAR followed this pattern from the 5′ end to the 3′ end: the CD8α signal sequence, scFv, hinge and transmembrane regions of the human CD8α molecule, the cytoplasmic portion of the CD28 molecule, and the cytoplasmic portion of the CD3ζ molecule. The sequences used for CD8α, CD28, and CD3ζ were obtained from the National Center for Biotechnology Information website (www.ncbi.nlm.nih). Guidance regarding the portions of each molecule to include in the CARs was obtained from prior work (38). DNA encoding the CARs was codon optimized and synthesized by GeneArt AG with appropriate restriction sites. The CAR sequences were ligated into a lentiviral vector plasmid designated pRRLSIN.cPPT.MSCV.coDMF5.oPRE (40). The coDMF5 portion of this vector was replaced with the CAR sequences by using standard methods. Two anti-BCMA-encoding CAR vectors were constructed: pRRLSIN.cPPT.MSCV.anti-bcma1.oPRE and pRRLSIN.cPPT.MSCV.anti-bcma2.oPRE. A negative-control CAR that contained the SP6 scFv that recognized the hapten 2,4,6-trinitrophenyl was also constructed (10). This CAR was referred to as SP6. The SP6 CAR was cloned into the same lentiviral vector as the anti-BCMA CARs and contained the same signaling domains as the anti-BCMA CARs.
Lentiviral Supernatant Production
Supernatant that contained lentiviruses encoding each CAR was produced by following a previously published protocol (40). To produce the supernatant, 293T-17 cells (ATCC) were transfected with the following plasmids as detailed previously: pMDG (encoding the vesicular stomatitis virus envelope), pMDLg/pRRE (encoding gag and pol), pRSV-Rev (encoding Rev), and the appropriate CAR-encoding plasmid (40).
T cell transductions
T cells were cultured as described previously (38). In brief, PBMC were stimulated with the anti-CD3 monoclonal antibody OKT3 (Ortho) in AIM V medium (Invitrogen) containing 5% human AB serum (Valley Biomedical) and 300 international units (IU)/mL of interleukin-2 (IL-2, Chiron). Thirty-six hours after the cultures were started, the activated PBMC were suspended in lentiviral supernatant with protamine sulfate and 300 IU/mL IL-2. The cells were centrifuged for 1 hour at 1200xg. The cells were then cultured for 3 hours at 37 °C. Next, the supernatant was diluted 1:1 with RPMI (Mediatech)+10% fetal bovine serum (Invitrogen) and IL-2. The cells were cultured in the diluted supernatant overnight and then they were returned to culture in AIM V medium plus 5% human AB serum with IL-2.
CAR detection on transduced T cells by anti-Fab antibody staining
T cells were stained with biotin-labeled polyclonal goat anti-mouse-F(ab)2 antibodies (anti-Fab, Jackson Immunoresearch) to detect the anti-BCMA CARs as described previously (38).
Flow cytometry
For anti-BCMA staining, cells were stained with polyclonal biotin-labeled goat-anti-human BCMA antibodies (R&D, catalog number BAF 193) followed by streptavidin (BD). Bone marrow cells were also stained with anti-CD38 (eBioscience) and anti-CD56 (BD). Flow cytometry analysis for all experiments was performed by using FlowJo (Tree Star, Inc.).
ELISA, CD107a, intracellular cytokine staining, and proliferation assays
These assays were performed by using standard methods as previously published (17, 38). Details of the methods for these techniques are in the Supplemental Methods.
Cytotoxicity Assay
Cytotoxicity assays were conducted as previously described (38). Cytotoxicity was measured by comparing survival of BCMA+ target cells relative to the survival of negative-control CCRF-CEM cells. Both of these cell types were combined in the same tubes with CAR-transduced T cells. CCRF-CEM negative control cells were labeled with the fluorescent dye 5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (CMTMR) (Invitrogen), and BCMA+ target cells were labeled with CFSE. Cocultures were set up in sterile 5 mL test tubes (BD) in duplicate at multiple T cell to target cell ratios. The target cells contained in the tubes were 50,000 BCMA+ target cells along with 50,000 CCRF-CEM negative-control cells. The cultures were incubated for 4 hours at 37°C. Immediately after the incubation, 7AAD (7-amino-actinomycin D) (BD) was added, and flow cytometry acquisition was performed. For each T cell plus target-cell culture, the percent survival of BCMA+ target cells was determined by dividing the percent live BCMA+ cells by the percent live CCRF-CEM negative control cells. The corrected percent survival of BCMA+ target cells was calculated by dividing the percent survival of BCMA+ target cells in each T cell plus target cell culture by the ratio of the percent live BCMA+ target cells to percent live CCRF-CEM negative-control cells in tubes containing only BCMA+ target cells and CCRF-CEM cells without effector T cells. This correction was necessary to account for variation in the starting cell numbers and for spontaneous target cell death. Cytotoxicity was calculated as follows: the percent cytotoxicity of BCMA+ target cells=100−corrected percent survival of BCMA+ target cells.
In vivo treatment model Murine Experiments
NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) from The Jackson Laboratory were used. Mice received intradermal injections of RPMI8226 cells. Tumors were allowed to grow for 17 to 19 days, and then the mice received intravenous infusions of 8×106 human T cells that were transduced with either anti-bcma2 or SP6. Tumors were measured with calipers every 3 days. The longest length and the length perpendicular to the longest length were multiplied to obtain the tumor size (area) in mm2. When the longest length reached 15 mm, mice were sacrificed. Animal studies were approved by the National Cancer Institute Animal Care and Use Committee.
Results
BCMA was expressed on multiple myeloma cell lines but not on several other types of cell lines
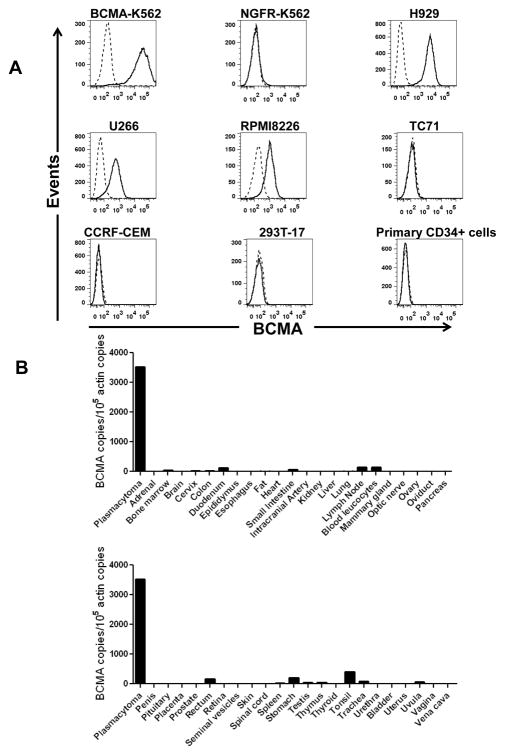
We used flow cytometry to characterize the expression of BCMA on the cell-surfaces of a variety of cells (Figure 1A). As expected, K562 cells transduced with the gene for BCMA expressed cell-surface BCMA. The multiple myeloma cell lines H929, U266, and RPMI8226 also expressed cell-surface BCMA. In contrast, NGFR-K562 cells, the sarcoma cell line TC71, the T-cell leukemia line CCRF-CEM, and the kidney cell line 293T-17 did not express cell-surface BCMA. Importantly, primary CD34+ hematopoietic cells lacked cell-surface BCMA expression.
Figure 1.
BCMA had a restricted pattern of expression. A, Cell-surface BCMA was detected on multiple myeloma cell lines, but BCMA was not detected on other cell lines. For all plots, the solid line represents staining with anti-BCMA antibodies, and the dashed line represents staining with isotype-matched control antibodies. BCMA was expressed by BCMA-K562 cells but not by NGFR-K562 cells. Flow cytometry staining revealed BCMA on the cell surface of the multiple myeloma cell lines H929, U266, and RPMI8226. BCMA was not detected on the sarcoma cell line TC71, on the T cell leukemia line CCRF-CEM, or on the kidney cell line 293T-17. Primary CD34+ hematopoietic cells lacked cell-surface BCMA expression. All plots are gated on live cells. The primary CD34+ cells plot is also gated on CD34+ cells. B, BCMA cDNA copies were measured in samples of the indicated normal tissues by qPCR. In addition, qPCR was performed on cDNA from cells of a plasmacytoma from Myeloma Patient 1 as a positive control. Neoplastic plasma cells made up 93% of the total cells in the sample. Actin copies were also measured by qPCR in all of the samples, and the results were expressed as the number of BCMA cDNA copies per 105 actin cDNA copies.
BCMA had a restricted mRNA expression pattern
A critical factor for any antigen being considered as a target for immunotherapies is the antigen’s expression pattern in normal tissues. BCMA has been reported to be expressed in plasma cells and in some B cells but to otherwise have limited expression (24–26, 35). To more completely assess the expression pattern of BCMA, we performed quantitative polymerase chain reaction (qPCR) on a panel of cDNA samples from a wide range of normal tissues (Figure 1B). As a positive control, we performed qPCR on cDNA from cells of a plasmacytoma that was resected from Myeloma Patient 1, a patient with advanced MM. Ninety-three percent of the cells from the plasmacytoma sample were plasma cells as determined by flow cytometry. The BCMA expression of the plasmacytoma sample was dramatically higher than the BCMA expression of any other tissue. Not surprisingly, BCMA cDNA copies were detected in several hematologic tissues such as blood leukocytes, bone marrow, spleen, lymph node, and tonsil. Low levels of BCMA cDNA copies were detected in the samples of testis and trachea. In addition, low levels of BCMA cDNA copies were detected in most gastrointestinal organs such as duodenum, rectum, and stomach. One possible explanation for BCMA expression in gastrointestinal organs and the trachea was the known presence of plasma cells and B cells in tissues such as lamina propria and Peyer’s Patches (41, 42).
BCMA protein was expressed by MM cells. Except for plasma cells, BCMA protein expression was not detected in normal human organs
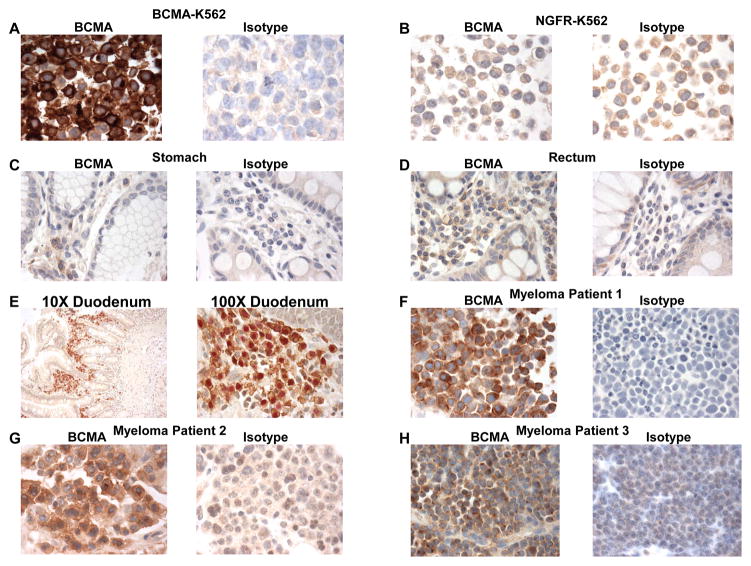
After demonstrating a very restricted expression pattern of BCMA RNA, we carried out an assessment of BCMA protein expression by immunohistochemistry (IHC). As expected, our anti-BCMA IHC staining procedure yielded strong staining of BCMA-K562 cells and a lack of staining with NGFR-K562 negative control cells (Figures 2A and 2B). We went on to evaluate BCMA protein expression in normal human organs. Except for plasma cells, we did not detect BCMA protein expression by the cells of any of the organs that we stained. We detected plasma cells expressing cell-surface BCMA in gastrointestinal organs. Examples of BCMA-expressing plasma cells that were detected in the stomach and rectum are shown in Figure 2C and 2D. MUM1 (multiple myeloma 1) is a protein that is expressed by many malignant and benign lymphocytes and by plasma cells (43). Except for lymphocytes and plasma cells, MUM1 is not expressed by normal gastrointestinal tissues (43). We performed IHC double staining of colon and duodenum with anti-BCMA and anti-MUM1and found that BCMA was expressed only by lymphoid cells and plasma cells (Figure 2E). BCMA expression by plasma cells probably accounts for the low levels of BCMA RNA detected in these organs because we did not detect BCMA expression by any of the other cells in these organs. We detected BCMA-expressing plasma cells in the tonsil (data not shown). The organs assessed by IHC and found to lack BCMA expression except for plasma cells included the following: adrenal, bladder, bone, eye, breast, cerebellum, cerebral cortex, fallopian tube, esophagus, stomach, small intestine, colon, rectum, heart, kidney, liver, lung, ovary, pancreas, parathyroid, pituitary, placenta, prostate, skin, spinal cord, spleen, skeletal muscle, testis, thymus, thyroid, trachea, cervix, uterine endometrium.
Figure 2.
IHC staining revealed BCMA expression by normal plasma cells and MM cells but not gastrointestinal epithelial cells. A, and B, BCMA-K562 cells exhibited strong BCMA expression while NGFR-K562 cells did not express BCMA. Isotype-matched control antibodies did not stain either cell line. Staining of sections of C, normal stomach and D, normal rectum showed BCMA-expressing plasma cells in the lamina propria but no BCMA expression by other cells of these organs. IHC staining was performed with both anti-BCMA and isotype-matched control antibodies on consecutive sections. E, Double staining of normal duodenum with anti-BCMA (surface, brown) and anti-MUM1(nuclear, red) revealed BCMA+ plasma cells and plasmacytoid cells. BCMA was not expressed by other cells of the duodenum. 10X and 100X views are shown. F, G, H, Staining of myeloma tissue sections from Myeloma Patients 1 through 3 showed cell-surface BCMA expression on the majority of neoplastic plasma cells. IHC staining was performed with BCMA and isotype-matched control antibodies on consecutive sections.
For a protein to be an appropriate target for CAR-expressing T cells aimed at MM, the protein must be expressed on the surface of MM cells. To assess BCMA expression by MM cells, we stained tissue sections from 3 different patients with MM (Figure 2F–H). These sections are shown in Figure 2. In all 3 of the samples, the neoplastic plasma cells expressed cell-surface BCMA.
BCMA-specific CARs were expressed on transduced T cells
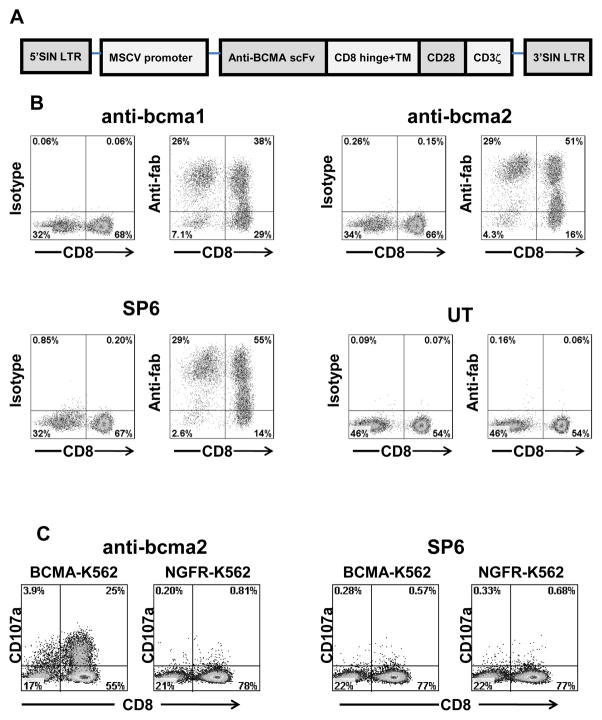
BCMA is expressed on multiple myeloma cells, and it has a restricted expression pattern in normal tissues (Figure 1 and Figure 2). Due to the expression pattern of BCMA, we reasoned that BCMA would be an appropriate target for CAR-expressing T cells. To further assess the suitability of BCMA as a target for CAR-expressing T cells, we designed two CARs. Each CAR contained an scFv derived from one of two mouse anti-human-BCMA monoclonal antibodies (39). We named the two CARs anti-bcma1 and anti-bcma2. The CARs contained the hinge and transmembrane regions of the human CD8α molecule, the signaling moiety of the CD28 costimulatory molecule, and the signaling domains of the CD3ζ molecule (Figure 3A). We also designed a negative-control CAR named SP6. The SP6 CAR contained the variable regions of the hapten-specific SP6 monoclonal antibody (10). Except for the different variable regions, the sequence of the SP6 CAR was identical to the sequences of anti-bcma1 and anti-bcma2. We ligated DNA encoding each of the CARs that we designed into a self-inactivating lentiviral vector, which has been described in detail previously (40). Replication-incompetent lentiviruses encoding the CARs were used to transduce human T cells. After transductions, we found high levels of cell surface expression of anti-bcma1, anti-bcma2, and SP6 on the transduced T cells (Figure 3B).
Figure 3.
Anti-BCMA CARs were expressed on the surface of T cells. A, A general diagram of anti-BCMA CAR fusion proteins is shown (LTR, long terminal repeat; MSCV, mouse stem cell virus; SIN, self-inactivating). From the N-terminus to the C-terminus, the anti-BCMA CARs all included an anti-BCMA scFv, the hinge and transmembrane (TM) regions of the CD8α molecule, the cytoplasmic portion of the CD28 molecule, and the cytoplasmic portion of the CD3ζ molecule. B, T cells from Donor B were transduced with lentiviral vectors encoding one of three CARs or left untransduced (UT). Expression of two anti-BCMA CARs, which were designated anti-bcma1 and anti-bcma2, was detected by flow cytometry 5 days after the transduction. Expression of the control CAR SP6 was also detected. As controls, isotype-matched antibody staining was included for each cell type. The plots are gated on CD3+ lymphocytes. The numbers on the plots are the percentages of cells in each quadrant. This is one of seven experiments with similar results. C, T cells were transduced with either the anti-BCMA CAR anti-bcma2 or the negative-control CAR SP6. The cells were cultured with either the BCMA-expressing cell line BCMA-K562 or the BCMA-negative cell line NGFR-K562 for 4 hours. After transduction with anti-bcma2, T cells degranulated in a BCMA-specific manner. SP6-transduced T cells from the same donors did not degranulate in a BCMA-specific manner. The plots are gated on live CD3+ lymphocytes. The numbers on the plots are the percentages of cells in each quadrant. This is one of three experiments with similar results.
Anti-BCMA-CAR-transduced T cells degranulated and produced cytokines specifically in response to BCMA-expressing target cells
Anti-BCMA-CAR-transduced T cells upregulated CD107a specifically in response to stimulation with BCMA-expressing target cells (Figure 3C). This indicates BCMA-specific degranulation of the T cells, which is a requirement for perforin-mediated cytotoxicity (44).
We assessed the ability of anti-BCMA-CAR-transduced T cells to produce cytokines in response to BCMA. T cells transduced with either anti-bcma1 or anti-bcma2 produced large amounts of IFNγ when they were cultured overnight with the BCMA-expressing target cell line BCMA-K562, but the CAR-transduced T cells only produced background levels of IFNγ when they were cultured with the negative-control target cell line NGFR-K562 (Table 1). T cells transduced with anti-BCMA CARs produced large amounts of IFNγ when they were cultured overnight with BCMA-expressing multiple myeloma cell lines. In contrast, the anti-BCMA CARs produced only background levels of IFNγ when they were cultured with a variety of BCMA-negative target cell lines. T cells transduced with the SP6 negative-control CAR produced only low levels of IFNγ when cultured with any of the cell lines. The SP6 CAR specifically produced IFNγ when stimulated with cells coated with its target, the hapten 2,4,6-trinitrophenyl (data not shown). T cells expressing either anti-bcma1 or anti-bcma2 were functionally similar. Compared to anti-bcma1-transduced T cells, anti-bcma2-transduced T cells consistently exhibited slightly stronger and more specific recognition of BCMA-expressing target cells in ELISA, CD107a, and other functional assays; therefore, we used anti-bcma2 in our remaining experiments.
Table 1.
Anti-BCMA-CAR-transduced T cells specifically recognized BCMA
| BCMA-expressing targets** | NGFR-K562 | BCMA-negative targets | T Cells Alone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BCMA-K562 | H929 | RPMI-8226 | CCRF-CEM | A549 | TC71 | 293T | |||
| Effector Cells* | |||||||||
| anti-bcma1 | 15392 | 11306 | 5335 | 76 | 76 | 52 | 65 | 54 | 112 |
| anti-bcma2 | 25474 | 23120 | 10587 | 62 | 67 | 32 | 31 | 28 | 41 |
| SP6 | 32 | 60 | 149 | 27 | 28 | 21 | 361 | 73 | 27 |
| Untransduced T cells | <12 | <12 | <12 | <12 | <12 | <12 | <12 | 12 | <12 |
| Targets alone | <12 | <12 | <12 | <12 | <12 | <12 | <12 | 13 | |
Effector cells were T cells from a patient with multiple myeloma (Myeloma Patient 4). The T cells were transduced with the indicated CAR or left untransduced.
The indicated target cells were combined with the effector cells for an overnight incubation and an IFNγ ELISA was performed. All units are pg/mL IFNγ
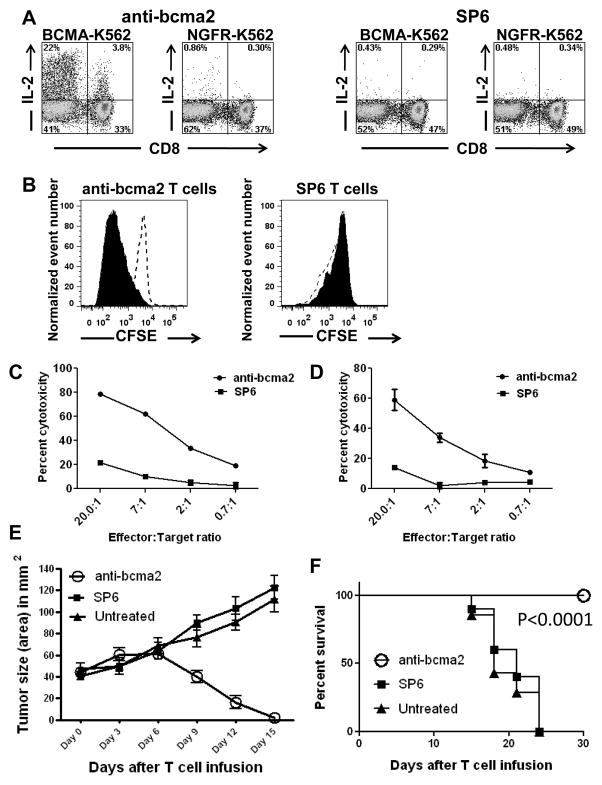
We successfully cultured and transduced the T cells of Myeloma Patient 4, a patient who had received 13 prior cycles of therapy for her myeloma. Six days after the cultures were initiated, anti-bcma2 was detected on 69% of the T cells. Large populations of anti-bcma2-transduced T cells from Myeloma Patient 4 produced IL-2, TNF, and IFNγ in a BCMA-specific manner after a 6-hour stimulation with BCMA-expressing target cells (Figure 4A, and Supplemental Figure 1).
Figure 4. T cells expressing anti-BCMA CARs produced cytokines, proliferated, and killed in a BCMA-specific manner. Anti-BCMA CAR-transduced T cells eradicated tumors in vivo.
A, T cells from Myeloma Patient 4 were transduced with either anti-bcma2 or the negative-control CAR SP6. Four days later, the CAR-transduced T cells were cultured for 6 hours with either the BCMA-expressing cell line BCMA-K562 or the BCMA-negative cell line NGFR-K562. Large fractions of the T cells transduced with anti-bcma2 produced IL-2 when cultured with BCMA-K562. Only small numbers of anti-bcma2-transduced T cells produced IL-2 when they were cultured with NGFR-K562. Only small numbers of SP6-transduced T cells produced IL-2 when cultured with either BCMA-K562 or NGFR-K562. The plots are gated on CD3+ lymphocytes. The numbers on the plots are the percentages of cells in each quadrant. This is one of three experiments with similar results. B, T cells from Donor B were transduced with either anti-bcma2 or SP6. The T cells were then labeled with CFSE. The T cells were cultured with either irradiated BCMA-K562 cells or irradiated NGFR-K562 cells. IL-2 was not included in the cultures. Four days later, the T cells were analyzed by flow cytometry. The plots are gated on CAR-expressing T cells. The CFSE fluorescence was less intense in anti-bcma2-expressing T cells that were cultured with BCMA-K562 cells (solid) than in anti-bcma2-expressing T cells that were cultured with NGFR-K562 cells (dashed, open). This indicates that anti-bcma2-expressing T cells proliferated specifically in response to BCMA. For SP6-expressing T cells, CFSE fluorescence intensity was similar for T cells cultured with either BCMA-K562 or NGFR-K562. This is one of two experiments with similar results. Anti-bcma2-transduced T cells from Donor A specifically killed the multiple myeloma cell lines C, H929 and D, RPMI8226 in 4-hour cytotoxicity assays at various effector:target cell ratios. T cells transduced with the negative control CAR SP6 caused much lower levels of cytotoxicity at all effector:target ratios. For all effector:target ratios, the cytotoxicity was determined in duplicate, and the results are displayed as the mean +/− the standard error of the mean. E, Mice were injected intradermally with RPMI8226 cells and tumors were allowed to grow for 17 to 19 days. On day 0, the mice received intravenous infusions of 8×106 T cells that were transduced with either anti-bcma2 or the negative control CAR SP6. Another group of mice was left untreated. The mice all had established tumors at the time of T-cell infusion. One-hundred percent of mice receiving infusions of anti-bcma2 had rapid and complete regressions of their tumors. In contrast, all mice receiving infusions of SP6-transduced T cells and all mice left untreated had progressive enlargement of their tumors. F, Tumors did not recur in mice receiving anti-bcma2-transduced T cells for the duration of the experiment. All mice receiving anti-bcma2-transduced T cells survived and were healthy for the duration of the experiment. All mice receiving SP6-transduced T cells or left untreated died with progressive tumors. The P<0.0001 refers to the comparison of anti-bcma2 and SP6. E and F show combined results of 3 experiments (anti-bcma2, n=10; SP6, n=10; untreated, n=7).
Anti-BCMA-CAR-transduced T cells proliferated in response to BCMA
We assessed anti-bcma2-transduced T cells for the ability to proliferate when stimulated with BCMA-expressing target cells. We cultured CFSE-labeled, anti-bcma2-transduced T cells with either target cells that expressed BCMA or target cells that did not express BCMA (Figure 4B). Anti-bcma2-transduced T cells specifically proliferated when stimulated with BCMA-expressing target cells. As expected, there was no BCMA-specific proliferation when T cells transduced with the SP6 negative-control CAR were assessed. At the beginning of the proliferation assay reported in Figure 4B, 0.8×106 anti-bcma2-expressing T cells were cultured with either BCMA-K562 cells or NGFR-K562 cells. After 4 days of culture, 2.7×106 anti-bcma2-expressing T cells were present in the cultures containing BCMA-K562 cells while only 0.6×106 anti-bcma2-expressing T cells were present in the cultures containing NGFR-K562 cells. This BCMA-specific increase in the absolute number of anti-bcma2-expressing T cells confirms the results of the CFSE proliferation assay.
Anti-BCMA-CAR-transduced T cells killed multiple myeloma cell lines
T cells transduced with anti-bcma2 specifically killed the BCMA-expressing multiple myeloma cell lines H929 and RPMI8226 in 4-hour cytotoxicity assays (Figures 4C and 4D). SP6-transduced T cells exhibited much lower levels of cytotoxicity against these cell lines.
Anti-BCMA-CAR-transduced T cells eradicate tumors in vivo
We established RPMI8226 human multiple myeloma cell line tumors in immunodeficient mice. We allowed sizable tumors to develop over 17 to 19 days, and then we treated the mice with a single intravenous infusion of anti-bcma2-transduced human T cells. The anti-bcma2-transduced T cell infusion cured 100% of the mice, with dramatic regressions of all tumors occurring between day 6 and day 15 after the T cell infusion (Figures 4E and 4F). In contrast, tumors continued to increase in size in all mice receiving infusions of T cells expressing a negative-control CAR designated SP6. The mice receiving infusions of anti-bcma2-transduced T cells had no signs of toxicity during this experiment.
Soluble BCMA protein does not interfere with anti-BCMA CAR function
BCMA can be found in the serum of humans, and serum BCMA levels are elevated in patients with MM (45). Other investigators have previously shown that CAR-expressing T cells were not blocked from recognizing target cells by soluble protein in vitro or in vivo (46). To determine if anti-bcma2 is blocked by soluble BCMA, we performed ELISA assays in which anti-bcma2-transduced T cells were cultured with BCMA-expressing target cells. Graded concentrations of BCMA protein were added to the culture medium. BCMA protein concentrations of more than 10-fold higher than the median BCMA levels found in the serum of multiple myeloma patients did not block recognition of BCMA target cells by anti-bcma2-transduced T cells in vitro (Supplemental Figure 2); furthermore, we found that RPMI8226 cells secreted BCMA, and mice bearing RPMI8226 tumors had easily detectable serum human BCMA (Supplemental Figure 3), so serum BCMA did not preclude effective treatment of BCMA+ tumors in mice (Figures 4E and 4F).
Anti-BCMA-CAR-transduced T cells recognized and killed primary multiple myeloma cells
We detected BCMA expression on neoplastic plasma cells of Myeloma Patients 1 through 3 by IHC (Figure 2). We also assessed cell-surface BCMA expression on primary MM cells from Myeloma Patients 1, 5, and 6 by flow cytometry, and we found uniform cell-surface BCMA expression on the plasma cells of all 3 patients. Overall, we found cell-surface BCMA expression on MM cells from 5 of 5 unique patients by either IHC or flow cytometry. Examples of flow cytometry staining for BCMA are shown in Figures 5A and 5C.
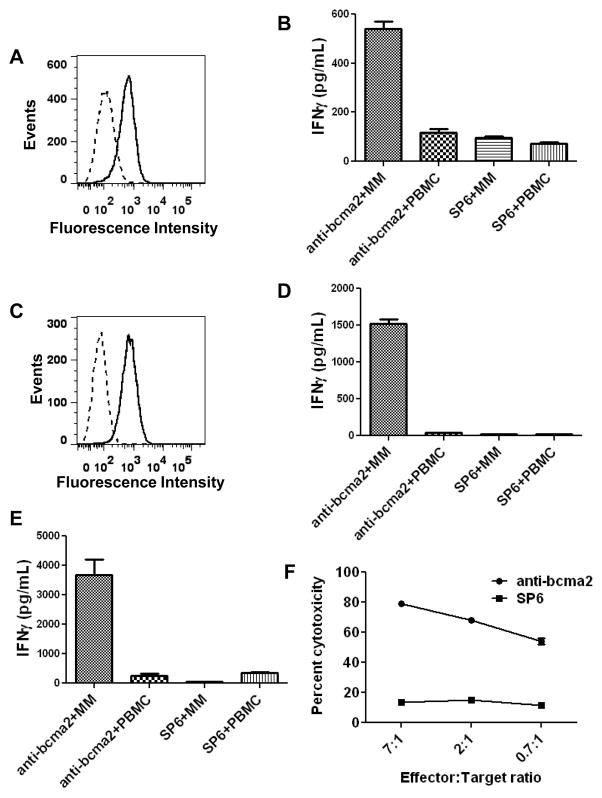
Figure 5. Anti-BCMA-CAR-transduced T cells specifically recognized and killed BCMA-expressing primary multiple myeloma cells.
A, Flow cytometry staining for BCMA (solid line) and isotype-matched control staining (dashed line) revealed BCMA expression on the surface of primary bone marrow multiple myeloma cells from Myeloma Patient 5. The plot is gated on CD38high CD56+ plasma cells, which made up 40% of the bone marrow cells. B, Unmanipulated myeloma-containing bone-marrow cells (MM) from Myeloma Patient 5 or PBMC from Myeloma Patient 5 were cocultured overnight with allogeneic T cells from Donor C. The T cells had been transduced with either anti-bcma2 or the negative control CAR SP6. After the coculture, an IFNγ ELISA was performed. C, Flow cytometry for BCMA (solid line) and isotype-matched control staining (dashed line) revealed BCMA expression on the surface of MM cells from a plasmacytoma of Myeloma Patient 1. The plot is gated on plasma cells, which made up 93% of the total plasmacytoma cells. D, Unmanipulated myeloma cells (MM) from a plasmacytoma of Myeloma Patient 1 or PBMC from Myeloma Patient 1 were cocultured overnight with allogeneic T cells from Myeloma Patient 4. The T cells were transduced with either anti-bcma2 or the negative control CAR SP6. The PBMC from Myeloma Patient 1 did not contain BCMA+ cells. After the coculture, an IFNγ ELISA was performed. E, Unmanipulated myeloma cells (MM) from a plasmacytoma of Myeloma Patient 1 or PBMC from Myeloma Patient 1 were cultured overnight with autologous anti-bcma2-transduced T cells or autologous SP6-transduced T cells, and an IFNγ ELISA was performed. F, Myeloma cells from a plasmacytoma of Myeloma Patient 1 were specifically killed by autologous anti-bcma2-transduced T cells at low effector:target ratios while autologous SP6-transduced T cells caused only a low level of cytotoxicity of the myeloma cells in a 4-hour cytotoxicity assay. For all effector:target ratios, the cytotoxicity was determined in duplicate, and the results are displayed as the mean +/− the standard error of the mean.
We conducted a series of experiments to show that T cells transduced with anti-bcma2 could specifically recognize primary MM cells. Plasma cells that uniformly expressed cell-surface BCMA made up 40% of the cells in a bone marrow sample from Myeloma Patient 5 (Figure 5A). Allogeneic anti-bcma2-transduced T cells from Donor C produced IFNγ after coculture with the unmanipulated myeloma-containing bone marrrow cells of Myeloma Patient 5 (Figure 5B). Anti-bcma2-transduced T cells from the same allogeneic donor produced much less IFNγ when they were cultured with PBMC from Myeloma Patient 5. In addition, SP6-transduced T cells from Donor C did not specifically recognize the myeloma-containing bone marrow of Myeloma Patient 5. Other investigators have previously reported that normal PBMC did not contain cells that expressed BCMA (35). We assessed the PBMC of Myeloma Patient 5 for BCMA expression by flow cytometry. We found that the PBMC did not contain BCMA-expressing cells except for a small population of CD56+CD38high cells that made up approximately 0.75% of the PBMC. This population probably consisted of circulating MM cells.
Plasma cells made up 93% of the cells from a plasmacytoma resected from Myeloma Patient 1, and these primary plasma cells uniformly expressed BCMA (Figure 5C). BCMA expression was also detected in the neoplastic plasma cells of Myeloma Patient 1 by qPCR and by immunohistochemistry (Figure 1B and Figure 2). Anti-bcma2-transduced T cells from Myeloma Patient 4 produced IFNγ when cultured with the allogeneic, unmanipulated MM cells of Myeloma Patient 1 (Figure 5D). Anti-bcma2-transduced T cells from Myeloma Patient 4 did not produce significant amounts of IFNγ when cultured with PBMC from Myeloma patient 1. SP6-transduced T cells from Myeloma Patient 4 did not produce significant amounts of IFNγ when they were cultured with either MM cells or PBMC from Myeloma Patient 1. The PBMC of Myeloma Patient 1 did not express BCMA as measured by flow cytometry (data not shown).
We successfully cultured and transduced the T cells of Myeloma Patient 1, who had received 8 prior cycles of myeloma therapy. Eight days after the cultures were initiated, anti-bcma2 was detected on 65% of the T cells. The anti-bcma2-transduced T cells from Myeloma Patient 1 produced IFNγ specifically in response to autologous MM cells (Figure 5E). SP6-transduced T cells from Myeloma Patient 1 did not recognize autologous MM cells. Neither anti-bcma2-transduced T cells nor SP6-transduced T cells from Myeloma Patient 1 recognized autologous PBMC. Anti-bcma2-transduced T cells from Myeloma Patient 1 specifically killed autologous MM cells at low effector to target ratios; in contrast, SP6-transduced T cells from Myeloma Patient 1 exhibited low levels of cytotoxicity against autologous MM cells (Figure 5F).
Discussion
Because current therapies for MM are rarely curative, new therapies for this disease are clearly needed (1, 2). In particular, there is a need for therapies to eradicate residual malignant cells that persist in patients who obtain remissions with current treatments. Many monoclonal antibodies that could potentially be useful therapies for MM are currently being evaluated in clinical trials, but most of these antibodies target antigens that are expressed on both essential normal cells and myeloma cells (5, 7). Severe toxicities have occurred after infusions of genetically modified T cells targeting antigens expressed by normal epithelial tissues; therefore, when designing CAR-expressing T-cell therapies, it is prudent to avoid targeting antigens that are expressed on the cell-surfaces of normal epithelial cells (47, 48). Identification of new antigens that are expressed by MM cells but not by normal essential cells is a critical step for development of effective immunotherapies for MM.
We chose to investigate the TNF-receptor-superfamily-member BCMA as a possible target for CAR-expressing T cells. CAR-expressing T cells recognize cell-surface antigens (9); therefore, an antigen targeted by CAR-expressing T cells should ideally be expressed on the cell surfaces of malignant cells but not on the cell surfaces of essential normal cells. BCMA has been previously detected on the cell surfaces of MM cells by other investigators (26–28). In early studies, BCMA RNA was detected in a limited number of normal tissues (24, 25). BCMA RNA was not expressed by normal human T cells or myeloid cells (35). BCMA has been reported to be expressed by fibroblast-like synovial cells from patients with the autoimmune disease rheumatoid arthritis, but BCMA was not expressed by synovial cells from patients with osteoarthritis (49). BCMA mRNA has been previously detected in the gut-associated lymphoid tissues, which are known to contain B cells and plasma cells (41, 50). We measured BCMA transcript expression in a wide range of normal tissues and found that BCMA mRNA expression was quite limited (Figure 1B). We also determined that BCMA was not expressed on the cell surfaces of primary CD34+ hematopoietic cells (Figure 1A). Finally, we conducted an IHC analysis of BCMA protein expression in the major human organs. We did not detect BCMA protein expression by any cells except plasma cells (Figure 2). Normal B-lineage cells are often eradicated from patients receiving infusions of anti-CD19-CAR-expressing T cells (12, 17). Patients lacking B cells can remain free of infections when the patients receive infusions of supplemental immunoglobulins (12, 17), so BCMA expression on normal plasma cells and some normal B cells does not preclude targeting BCMA as a therapy for MM.
After showing that BCMA has a limited tissue expression pattern, we designed CARs that incorporated the variable regions of anti-BCMA monoclonal antibodies (Figure 3). T cells transduced with lentiviral vectors encoding these CARs gained the ability to perform a variety of in vitro functions in a BCMA-specific manner and to eradicate established BCMA+ tumors in vivo (Figures 3–5, Table 1). Both CD4+ and CD8+ T cells exhibited BCMA-specific activation Figures (3C and 4A). Compared to CD8+ T cells, a higher percentage of CD4+ T cells produced IL-2 (Figure 4A). The anti-BCMA-CAR-transduced T cells produced IFNγ when stimulated with primary MM cells and killed primary MM cells (Figure 5).
Evidence that BCMA might be a target for anti-myeloma immunity in humans has been reported (28). Allogeneic stem cell transplantation (AlloSCT) is sometimes used to treat MM (4). In patients with relapsed or persistent MM after AlloSCT, infusions of unmanipulated allogeneic lymphocytes from the original transplant donor can sometimes induce complete remissions (4, 28). Two patients who obtained complete remissions of relapsed MM after infusions of allogeneic donor lymphocytes developed serum anti-BCMA antibodies only after the donor lymphocyte infusions (28). The serum of both of these patients killed BCMA-expressing target cells in vitro by complement-mediated lysis and antibody-dependent cellular cytotoxicity (28).
In conclusion, BCMA is a promising target for CAR-transduced T cells. We have presented evidence that BCMA is not expressed by normal essential cells, and we detected uniform BCMA protein expression by immunohistochemistry or flow cytometry on MM cells of 5 out of 5 patients that were assessed. We designed lentiviral vectors encoding CARs that specifically recognized BCMA. T cells transduced with these CARs gained the ability to carry out BCMA-specific functions. Adoptive transfer of anti-BCMA-CAR-transduced T cells could potentially be an appropriate approach to test in clinical trials enrolling patients with advanced MM.
Supplementary Material
Translational Relevance.
One way to improve outcomes of patients with multiple myeloma (MM) might be to develop effective immunotherapies targeting antigens expressed by MM cells. T cells expressing chimeric antigen receptors (CARs) that target the B-cell antigen CD19 have potent in vivo activity. Unfortunately, a suitable target for CAR-expressing T-cell therapy of MM has not been previously identified because most proteins expressed on MM cells are also expressed on essential normal cells. We assessed B-cell maturation antigen (BCMA) as a possible target for CAR-expressing T cells. Our results show that BCMA is expressed uniformly on the malignant plasma cells of many patients with MM. BCMA expression was not detected on essential normal cells. We designed the first anti-BCMA CARs to be reported. T cells expressing these CARs could recognize and destroy primary human MM cells. These findings are the first steps toward clinical trials of anti-BCMA CAR-expressing T cells.
Acknowledgments
This work was supported by intramural funding of the Center for Cancer Research, National Cancer Institute, NIH.
This work was supported by National Institutes of Health, National Cancer Institutes intramural funding.
Footnotes
A patent application covering anti-BCMA chimeric antigen receptors was filed by the NIH. JNK is the inventor on this patent. There are no other conflicts of interest.
References
- 1.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clinical Cancer Research. 2011;17:1264–77. doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Treatment of multiple myeloma. Nature Reviews Clinical Oncology. 2011;8:479–91. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. New England Journal of Medicine. 2011;364:1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 4.Salit RB, Bishop MR. Reduced-intensity allogeneic hematopoietic stem cell transplantation for multiple myeloma: A concise review. Clinical Lymphoma, Myeloma and Leukemia. 2011;11:247–52. doi: 10.1016/j.clml.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Lonial S, Jakubowiak AJ, Harousseau JL, Anderson KC. Monoclonal antibodies in the treatment of multiple myeloma. British Journal of Haematology. 2011;154:745–54. doi: 10.1111/j.1365-2141.2011.08790.x. [DOI] [PubMed] [Google Scholar]
- 6.Yi Q. Novel immunotherapies. Cancer Journal. 2009;15:502–10. doi: 10.1097/PPO.0b013e3181c51f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van De Donk NWCJ, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia. 2012;26:199–213. doi: 10.1038/leu.2011.214. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nature Reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kershaw MH, Teng MWL, Smyth MJ, Darcy PK. Supernatural T cells: Genetic modification of T cells for cancer therapy. Nature Reviews Immunology. 2005;5:928–40. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 10.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biology of Blood and Marrow Transplantation. 2010;16:1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter DLBL, Kalos M, et al. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. The New England Journal of Medicine. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. Journal of Clinical Investigation. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–44. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 16.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15.[see comment] Nature Medicine. 2003;9:279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheadle EJ, Hawkins RE, Batha H, O’Neill AL, Dovedi SJ, Gilham DE. Natural expression of the CD19 antigen impacts the long-term engraftment but not antitumor activity of CD19-specific engineered T cells. Journal of Immunology. 2010;184:1885–96. doi: 10.4049/jimmunol.0901440. [DOI] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–86. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R, Bhaskar A, Kumar L, Sharma A, Jain P. Flow cytometric immunophenotyping and minimal residual disease analysis in multiple myeloma. American Journal of Clinical Pathology. 2009;132:728–32. doi: 10.1309/AJCP1GYI7EHQYUYK. [DOI] [PubMed] [Google Scholar]
- 23.Lin P, Owens R, Tricot G, Wilson CS. Flow Cytometric Immunophenotypic Analysis of 306 Cases of Multiple Myeloma. American Journal of Clinical Pathology. 2004;121:482–8. doi: 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- 24.Laabi Y, Gras MP, Carbonnel F, Brouet JC, Berger R, Larsen CJ, et al. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO Journal. 1992;11:3897–904. doi: 10.1002/j.1460-2075.1992.tb05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laabi Y, Gras MP, Brouet JC, Berger R, Larsen CJ, Tsapis A. The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Research. 1994;22:1147–54. doi: 10.1093/nar/22.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood. 2004;103:689–94. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 27.Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, et al. Neutralizing B-cell-activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clinical Cancer Research. 2007;13:5903–9. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- 28.Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E, et al. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105:3945–50. doi: 10.1182/blood-2004-11-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. Journal of Experimental Medicine. 2000;192:129–35. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL: A tutorial on B cell survival. Annual Review of Immunology. 2003:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 32.Kalled SL. The role of BAFF in immune function and implications for autoimmunity. Immunological Reviews. 2005;204:43–54. doi: 10.1111/j.0105-2896.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 33.Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Research. 2006;66:6675–82. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA Is Essential for the Survival of Long-lived Bone Marrow Plasma Cells. Journal of Experimental Medicine. 2004;199:91–7. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng LG, Sutherland APR, Newton R, Qian F, Cachero TG, Scott ML, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. Journal of Immunology. 2004;173:807–17. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 36.Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Molecular and Cellular Biology. 2001;21:4067–74. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shutga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 38.Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. Journal of Immunotherapy. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalled SL, Hsu Y. Anti-BCMA Antibodies. International Publicaton Number WO 2010/104949 A2 World Intellectual Property Organization Patent. Filing date 10 March 2010.
- 40.Yang S, Dudley ME, Rosenberg SA, Morgan RA. A simplified method for the clinical-scale generation of central memory-like CD8+ T cells after transduction with lentiviral vectors encoding antitumor antigen T-cell receptors. Journal of Immunotherapy. 2010;33:648–58. doi: 10.1097/CJI.0b013e3181e311cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunological Investigations. 2010;39:303–55. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 42.Soutar CA. Distribution of plasma cells and other cells containing immunoglobulin in the respiratory tract of normal man and class of immunoglobulin contained therein. Thorax. 1976;31:158–66. doi: 10.1136/thx.31.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natkunam Y, Warnke RA, Montgomery K, Falini B, Van De Rijn M. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Modern Pathology. 2001;14:686–94. doi: 10.1038/modpathol.3880373. [DOI] [PubMed] [Google Scholar]
- 44.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nature Medicine. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. British Journal of Haematology. 2012;158:727–38. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 46.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Molecular Therapy. 2012;20:633–43. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DAN, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Molecular Therapy. 2011;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagatani K, Itoh K, Nakajima K, Kuroki H, Katsuragawa Y, Mochizuki M, et al. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by APRIL. Arthritis and Rheumatism. 2007;56:3554–63. doi: 10.1002/art.22929. [DOI] [PubMed] [Google Scholar]
- 50.Barone F, Patel P, Sanderson JD, Spencer J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunology. 2009;2:495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.