Abstract
Overweight and obese patients commonly suffer from depression and choice of depression therapy may alter weight. We conducted a cohort study to investigate whether obesity is associated with treatment choices for depression; and whether obesity is associated with appropriate duration of depression treatment and receipt of follow-up visits. Adults with a diagnosis of depression between January 1, 2006 and March 31, 2010 who had 1+ new episodes of an antidepressant medication and/or psychotherapy were eligible. Medication use, encounters, diagnoses, height, and weight were collected from health plan databases. We modeled receipt of the different therapies (medication and psychotherapy) by BMI and BMI trajectory during the 9-months prior to initiation of therapy using logistic regression models that accommodated correlation within provider and adjusted for covariates. We modeled BMI via a restricted cubic spline. Fluoxetine was the reference treatment option in the medication models. Lower BMI was associated with greater use of mirtazapine, and a declining BMI prior to treatment was associated with greater odds of initiating mirtazapine and paroxetine. Higher BMI was associated with greater odds of initiating bupropion even after adjustment for smoking status. Obese patients were less likely to receive psychotherapy and less likely to receive appropriate duration (180-days) of depression treatment compared to normal weight subjects. Our study provides evidence that BMI is considered when choosing therapy but associations were weak. Our results should prompt discussion about recommending and choosing depression treatment plans that optimize depression care and weight management concurrently. Differences in care and follow-up by BMI warrant additional research.
INTRODUCTION
Many overweight and obese people suffer from depression, and the impact of antidepressant drug treatment on body weight should be considered whenever depression treatment is initiated. In 2007–2008, the prevalence of obesity (defined as a body mass index (BMI) of ≥ 30 kg/m2) among US adults was almost 34%, (1) and depression is found in approximately 7% of obese adults.(2, 3) The causal pathway is likely bidirectional—obese adults are at greater risk of depression,(4–7) and vice versa. However, less evidence exists that depression leads to obesity,(7–10) and not all studies support an association between the two conditions.(11–13) There is also evidence that improvement in depression is associated with weight loss, (14) and weight loss is associated with improved mood and depressive symptoms. (15–17)
Notably, there is clinical evidence that the choice of antidepressant drug therapy may also influence changes in weight.(18, 19) Previous studies indicate that certain antidepressants (e.g. fluoxetine and bupropion) may reduce body weight, while others (e.g. paroxetine and mirtazapine) may increase body weight.(20–24) However, less evidence is available regarding the long-term impact of antidepressants on weight, and some associations appear to be transient.(19–24) With climbing rates of obesity (25, 26) and antidepressant agents now the most commonly prescribed drugs in the US,(27) this potential association has recently received renewed attention.(28) Any influence of antidepressants on weight, even in the short term, may be a cause for concern or an opportunity to positively influence obesity while treating depression.
The objective of this study was to investigate whether there is evidence that a patient’s body weight is considered when initiating treatment with antidepressant medications and/or psychotherapy in a large, integrated health plan and care delivery system in the Pacific Northwest. Because obesity has been associated with disparities in health care, such as lower rates of cancer screening (29) and less time exposed to physician education on health compared to normal weight patients,(30) we were also concerned that obese patients might be receiving lower quality of depression care when compared to normal weight patients. Therefore, we investigated whether patients with obesity were more or less likely to receive appropriate duration of depression treatment and receive an appropriate number of follow-up visits after initiating treatment.
METHODS
Setting
The study was conducted at Group Health (GH), an integrated health plan and care delivery system that provides comprehensive health care on a pre-paid basis to approximately 650,000 individuals in Washington State and parts of Idaho. GH contracts with the Group Health Physicians medical group to provide care within an integrated group practice (IGP) for approximately 70% of the plan’s enrollees. The remaining 30% receive care from non-GH provider networks located in geographic areas not served by GH medical centers. Information on health plan enrollment, health care use including diagnoses, procedures, pharmacy dispensings, and laboratory values are recorded and maintained in GH’s electronic databases. Since 2005, a fully-integrated electronic medical record (EMR) system documents all patient care at GH IGP clinics, including vitals signs such as height, weight, and body mass index (BMI). Because we were primarily interested in height, weight, and BMI for this study, only GH enrollees receiving care in the IGP were included.
GH provides specialty mental health care to its enrollees using both an IGP and a network model. Within the IGP, seven mental health clinics serve more densely populated areas in or near major cities. GH IGP patients also can receive mental health care from a network of contracted mental health providers, which includes both individual providers and clinics or groups. GH guidelines and provider training emphasize cognitive-behavioral therapy as part of first-line therapy for depressive disorders, and GH members may initiate outpatient mental health treatment either through self-referral (80% of initial treatment requests) or referral from a primary care physician (20% of requests). However, antidepressant drug treatment dominates psychotherapy as initial treatment of depression at GH, and 75% of new antidepressant prescriptions are written by primary care physicians.
The GH population closely resembles the underlying Washington state community with respect to age, race, and gender.(31) GH insurance plans vary considerably in the level of cost-sharing for outpatient primary care mental health care, but copayments for routine outpatient care and psychotherapy are similar to national averages. All non-Medicare GH plans include prescription drug coverage with nominal copayments. Prior studies suggest that GH enrollees obtain approximately 97% of their prescription medications at GH owned and contracted pharmacies.(31)
Population
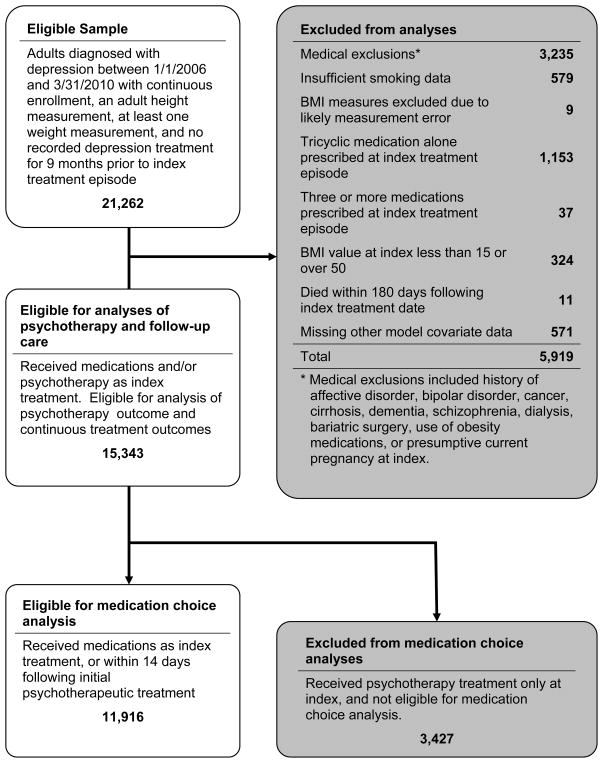
Men and women aged 18–65 years with a diagnosis of depressive disorder (ICD-9 = 296.2x, 296.3x, 311, or 300.4) anytime between January 1, 2006 and March 31, 2010 who also had at least one new episode for an antidepressant medication (defined as a dispensing for antidepressant medication without any other antidepressant medications or psychotherapy visits in the prior 9-months – See Appendix A for list of antidepressant medications) OR at least one new episode of psychotherapy (defined as occurrence of a psychotherapy visit without any other psychotherapy visits or receipt of antidepressant medications in the prior 9-months) in the GH IGP were eligible (Figure 1). Psychotherapy was defined as visits to a specialty mental health provider with a psychotherapy CPT code of 90801 through 90844. Index date was defined as the date of the first new antidepressant medication episode or psychotherapy episode. Only subjects with at least 9-months of continuous enrollment (<92 day gap) before index date, a plausible adult height measurement (48–84 inches), at least one plausible weight (85 to 600 lbs) and BMI (range, 15–50 kg/m2) value in the 9-months prior to index date, and survival for at least 180 days after index date were included. We excluded subjects with conditions or treatments known to be associated with significant weight fluctuations such as: diagnosis of cancer, psychotic disorders, cognitive impairment, cirrhosis, pregnancy, and kidney disease requiring dialysis, or prescribed an obesity drug treatment or undergone bariatric surgery. Subjects without information on smoking status, or who received 3+ antidepressants or only a tricyclic antidepressant at index date were also excluded. Tricyclics are primarily used for indications other than depression at GH - approximately 75% in 2011. After exclusions, our final sample size was 15,343 (Figure 1).
Appendix A.
Adjusted and unadjusted psychotherapy receipt odds ratio estimates by patient characteristics, n=15,343*
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| BMI at index | ||||||||
| 18.5 | 1.14 (1.07, 1.23) | 1.11 (1.05, 1.18) | ||||||
| 25 | 1 (referent) | 1 (referent) | ||||||
| 30 | 0.90 (0.87, 0.93) | 0.92 (0.89, 0.95) | ||||||
| 35 | 0.81 (0.77, 0.85) | 0.85 (0.81, 0.89) | ||||||
| 40 | 0.73 (0.68, 0.78) | 0.77 (0.72, 0.83) | ||||||
| 45 | 0.66 (0.59, 0.73) | 0.71 (0.63, 0.80) | ||||||
|
| ||||||||
| BMI trajectory in 9 months prior to index | ||||||||
| Declining | 1.12 (1.00, 1.26) | 1.10 (0.99, 1.23) | ||||||
| Stable | 1 (referent) | 1 (referent) | ||||||
| Increasing | 0.80 (0.70, 0.92) | 0.83 (0.73, 0.95) | ||||||
| Unknown | 1.19 (1.10, 1.29) | 1.20 (1.11, 1.29) | ||||||
|
| ||||||||
| Female | 0.77 (0.70, 0.85) | 0.73 (0.67, 0.79) | ||||||
|
| ||||||||
| Age group | ||||||||
| 18 to 25 | 1.52 (1.33, 1.75) | 1.54 (1.32, 1.79) | ||||||
| 26 to 35 | 1.55 (1.36, 1.78) | 1.61 (1.39, 1.87) | ||||||
| 36 to 45 | 1.28 (1.16, 1.41) | 1.32 (1.19, 1.47) | ||||||
| 46 to 55 | 1.09 (1.01, 1.19) | 1.13 (1.05, 1.23) | ||||||
| 56 to 65 | 1 (referent) | 1 (referent) | ||||||
|
| ||||||||
| Current or former smoker | 0.68 (0.60, 0.77) | 0.62 (0.55, 0.71) | ||||||
|
| ||||||||
| Anxiety diagnosis | 1.83 (1.68, 2.00) | 1.86 (1.72, 2.02) | ||||||
|
| ||||||||
| Sleep disorder diagnosis | 0.99 (0.80, 1.23) | 1.12 (0.85, 1.47) | ||||||
Among 15,343 study subjects who contributed to this analysis, 5,382 were defined to be psychotherapy recipients, having either received psychotherapy as their index treatment (N=3,427), or within 30 days of an index medication treatment (N=1,955).
Abbreviations: OR=odds ratio; CI=confidence interval; BMI=body mass index
Figure 1.
Study flowchart.
Data Collection
We identified demographic and enrollment information, prescription medication use, health care encounters, and medical conditions from GH electronic health care databases. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes were used to identify medical and psychiatric conditions. Height, weight, and smoking status were determined from EMR-entered data fields. In the GH IGP, clinic staff obtains weight and height measurements during routine clinical care and enter these data into the EMR. Care standards indicate that weights should be obtained at each outpatient visit without extra clothing and shoes. Prior research at GH indicates that weight and height measures routinely obtained in clinical care are highly correlated with those obtained by trained research staff and may be used in research studies without statistical correction.(32)
Exposure
The main exposures of interest were BMI at index and BMI trajectory in the 9 months prior to index. The weight closest to the index date was taken as the index weight, and the mode of all adult heights was taken as the index height. Of the 15,343 subjects included in our analyses, 58% were weighed on their index date. Of the remaining 42%, the most recent weight measurement occurred between 1 and 275 days before index, with a median of 40 days.
BMI trajectory in the 9-months prior to index was categorized as increasing (≥3% gain in BMI), decreasing (≥3% loss in BMI), stable (<3% absolute change in BMI), or unknown. When calculating BMI trajectory, we required a minimum of 2 BMI measures that were at least 30 days apart. Trajectories were categorized as unknown for individuals with only one weight measurement or without measures taken at least 30 days apart; however, these individuals were retained in our models because it is common for providers to make decisions about treatment without clear documentation of recent changes in weight. The median number of days between BMI values was 32. The median change in BMI over the 9-months prior to index was 0.0% overall, 5.0% among the BMI increasing group, and −5.3% among the BMI decreasing group.
Outcomes
The first outcome of interest was initial treatment choice for depression. Because providers and patients make separate decisions for each type of treatment, we modeled the medication decision and psychotherapy decision separately. Based on prior published studies, medication treatments of interest were classified according to their likely impact on subsequent changes in body weight: antidepressants that may reduce weight (fluoxetine and bupropion), may increase weight (mirtazapine and paroxetine), or are likely to be weight neutral – other selective serotonin reuptake inhibitors (SSRI) (citalopram, escitalopram, fluvoxamine, and sertraline) and other serotonin 2 antagonist and reuptake inhibitors (SARI) (nefazodone or trazodone alone).
The second set of outcomes were based on HEDIS performance measures for depression care, which are widely-used by health plans in the United States as indicators of patient care quality. These included 1) continuous treatment with either medication or psychotherapy through 84 and 180 days after index date; and 2) having three or more visits to mental health or primary care provider(s) in the first 84 days after index.(33) To determine the end date of a continuous medication treatment episode, all antidepressant medications of interest dispensed from index date to end of follow-up period were taken from the pharmacy data. Consecutive dispensings for any antidepressant with overlaps of one or more days were combined into single episodes of use. The days supply from the dispensing record was multiplied by a compliance factor of 80% to determine how long each dispensing would normally last (run-out date). The treatment episode ended on the run-out date of the last antidepressant dispensed in the episode. Psychotherapy visits were collapsed into single episodes of treatment if gaps between visits were ≤ 60 days. Continuous treatment through 84 and 180 days was defined to be uninterrupted treatment by any combination of psychotherapy or medication treatment episodes that in combination spanned the entire period from index through 84 or 180 days, respectively.
Covariates
Potential confounders of interest were obtained from electronic health care databases and included age, anxiety disorder, sleep disorders, and smoking status.
Statistical Analyses
Descriptive statistics
We calculated the mean and standard deviation of subjects’ age and BMI at index, and frequency distributions for categorical variables for the 15,343 eligible study subjects. Individuals who were first treated with psychotherapy and also received antidepressant medications within 14 days of their initial treatment were categorized into the corresponding medication category for the descriptive statistics and medication choice model.
Psychotherapy model
To examine the relationship between BMI and receipt of psychotherapy as part of a new treatment episode of depression, we modeled the receipt of psychotherapy among all 15,343 eligible subjects using generalized estimating equations (GEE) models that accommodated correlation within care provider.(34) Models regressed a binary indicator of psychotherapy receipt within the first 30 days of a new treatment episode using indicator variables to adjust for sex, age group (18–25, 26–35, 36–45, 46–55, or 56–65), BMI trajectory category (declining, stable, increasing, or unknown), history of anxiety disorder or sleep disorder at index, and having self-reported smoking within 9 months preceding the index treatment episode. To avoid restrictive assumptions on the association between BMI and receipt of psychotherapy, we modeled BMI continuously with a restricted cubic spline with a single internal knot at 32.5, the approximate midpoint of the BMI distribution.(35) This yields a specification of the association based on 2 parameters; the null hypothesis of no association can then be evaluated via a joint Wald test with 2 degrees of freedom.
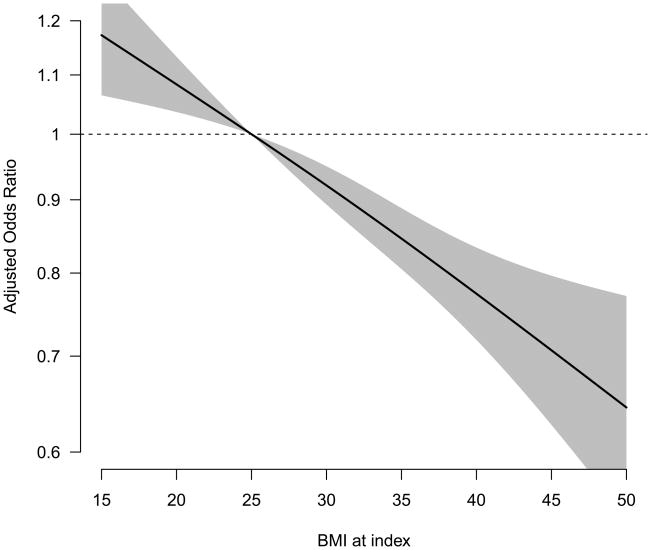
Receipt of psychotherapy was regressed on each of the covariates above separately for unadjusted models, and together in a single fully-adjusted model. To calculate odds ratio estimates associated with specific BMI values, we obtained the BMI spline parameter and covariance matrix estimates from the fitted models. We calculated, for individual BMI values ranging from 15 to 50, the psychotherapy receipt odds ratio associated with each BMI value, along with its point-wise 95% confidence interval, relative to a referent BMI value of 25. We plotted these estimated odds ratios, producing a curve which takes on a value of 1 at the referent BMI value, indicated by the dashed horizontal line (Figure 2). The odds of psychotherapy receipt for patients with BMI values for which the grey confidence band excludes the horizontal dotted line differ significantly from the odds for patients with a BMI of 25. For reporting purposes, we chose a few odds ratio estimates and their respective confidence intervals from each plot (see the results section). Our GEE models used a logit link function. Estimates were obtained assuming an independence working correlation structure, with all inference based on the robust “sandwich” variance estimator.(36)
Figure 2.
Adjusted psychotherapy receipt odds ratios associated with various BMI values, relative to a referent BMI value of 25.
Medication choice model
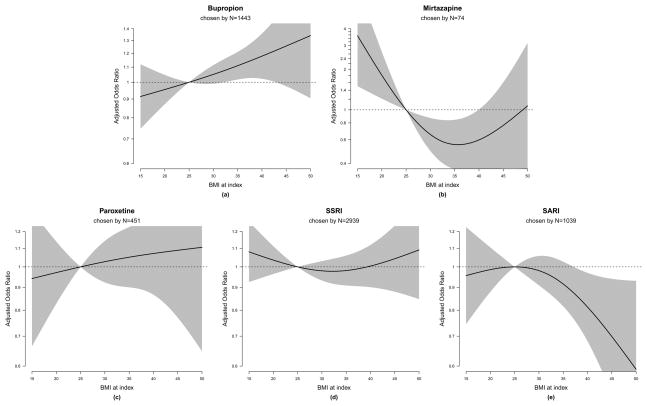
We modeled initial medication choice among 11,916 subjects who received antidepressant medications within 14 days of the index date. We took medication choice to be a nominal multinomial outcome with categories: fluoxetine, bupropion, mirtazapine, paroxetine, other SSRI, or other SARI. Individuals who only received psychotherapy for their depression treatment (N=3,427) were excluded from this analysis. We fit simultaneous logistic regression models of treatment choice, specifying fluoxetine as the baseline outcome category,(37) adjusting for the same set of covariates as discussed above, with the addition of an indicator of concurrent psychotherapy treatment. Fluoxetine was selected as the referent category for several reasons: 1) it is associated with weight loss, 2) it is the most commonly prescribed antidepressant in our population, and 3) it was listed as the GH formulary “preferred” drug during the years of our study. We followed the approach of Kuss and McLerran to estimate simultaneous logistic models that accommodate correlation within provider.(38) As for the psychotherapy analysis, we modeled BMI via a restricted cubic spline with a single knot at BMI of 32.5. This yields a specification of the BMI/treatment choice association based on 10 parameters (two for each of the five non-fluoxetine treatment categories). To evaluate the null hypothesis of no association, we performed a joint Wald test with 10 degrees of freedom. For presentation of results, we generated plots of each medication choice odds ratios associated with BMI values, and chose a few specific BMI values to report odds ratios for in the results section (Figure 3).
Figure 3.
Adjusted medication choice odds ratios associated with various BMI values, relative to a referent BMI value of 25, over a referent medication choice of fluoxetine.
Quality/continuity of depression care models
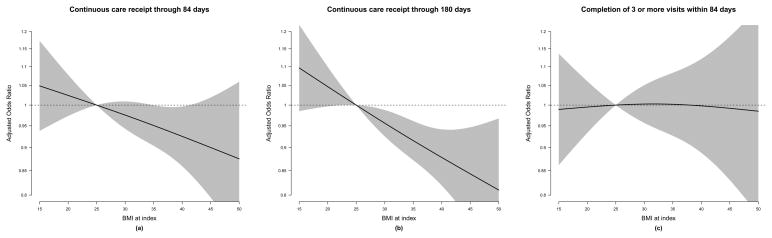
To examine the relationship between BMI and the quality of depression care among all 15,343 subjects, we first identified individuals who had undergone continuous depression treatment for 84 days and for 180 days after the index treatment date. We derived binary indicators of continuous treatment through 84 and 180 days post index date, and an indicator of having made at least 3 in-person visits within the first 84 days following the index treatment. We modeled each of these binary outcomes using the same approach as the psychotherapy and medication receipt analyses described above, and produced plots as before (Figure 4).
Figure 4.
Adjusted follow up care receipt odds ratios associated with various BMI values, relative to a referent BMI value of 25.
All analyses were conducted using SAS 9.2 software, SAS Institute Inc., Cary, NC, USA.
RESULTS
Subject characteristics by index treatment group are presented in Table 1. We identified a total of 15,343 subjects who initiated a new depression treatment episode between January 2006 and March 2010. Fluoxetine was the most common antidepressant medication initiated (39% of index treatments), followed by “other SSRIs” (19%), of which citalopram was the predominant medication used. Trazadone accounted for nearly all of “other SARI” medications prescribed. Psychotherapy alone was the index treatment in 22% of subjects but an additional 12–23% of subjects in each medication category received concomitant psychotherapy. The age of subjects at index (mean 43 years) and sex (66% females) were relatively similar across the treatment groups except that mirtazapine users consisted of slightly more males and “other SSRI” users more females. Average BMI at index was 29 kg/m2 and over 39% were considered obese according to the World Health Organization definition of BMI>30 kg/m2. There was little variability in the BMI distributions across treatment groups except slightly lower BMIs among initiators of mirtazapine. Subjects initiating mirtazapine also appeared to differ from the rest of the cohort by having a higher likelihood of decreasing weight trajectory in the 9 months prior to index, current or former smoker status, anxiety disorder diagnosis, and non-commercial insurance (9.5% insured by Medicare). Other notable differences in potential confounders included a higher proportion of smokers in the bupropion treatment group, higher anxiety disorder diagnoses in the paroxetine group, and higher sleep disorder diagnoses in the “other SARI” group.
Table 1.
Characteristics of eligible patients at index treatment episode, by index drug treatment, or first drug prescribed within 14 days of an index psychotherapy treatment.
| Patient characteristics | All | Psychotherapy Only | Fluoxetine | Bupropion | Mirtazapine | Paroxetine | Other SSRI* | Other SARI** |
|---|---|---|---|---|---|---|---|---|
| N | 15343 | 3427 | 5970 | 1443 | 74 | 451 | 2939 | 1039 |
| Female | 10193 (66.4%) | 2170 (63.3%) | 4006 (67.1%) | 950 (65.8%) | 41 (55.4%) | 298 (66.1%) | 2072 (70.5%) | 656 (63.1%) |
|
| ||||||||
| Age at index, mean (SD) | 43.03 (13.05) | 41.3 (13.15) | 43.12 (13.26) | 43.69 (12.05) | 45.65 (12.6) | 44.54 (12.51) | 43.04 (12.96) | 46.37 (12.48) |
|
| ||||||||
| Year of index treatment | ||||||||
| 2006 | 4997 (32.6%) | 1051 (30.7%) | 2203 (36.9%) | 524 (36.3%) | 18 (24.3%) | 151 (33.5%) | 667 (22.7%) | 383 (36.9%) |
| 2007 | 4260 (27.8%) | 876 (25.6%) | 1766 (29.6%) | 405 (28.1%) | 24 (32.4%) | 142 (31.5%) | 725 (24.7%) | 322 (31%) |
| 2008 | 3471 (22.6%) | 822 (24%) | 1230 (20.6%) | 291 (20.2%) | 22 (29.7%) | 96 (21.3%) | 808 (27.5%) | 202 (19.4%) |
| 2009 | 2615 (17%) | 678 (19.8%) | 771 (12.9%) | 223 (15.5%) | 10 (13.5%) | 62 (13.7%) | 739 (25.1%) | 132 (12.7%) |
|
| ||||||||
| BMI at index, mean (sd) | 29.14 (6.68) | 28.41 (6.45) | 29.35 (6.73) | 29.82 (6.84) | 27.29 (6.68) | 29.39 (6.7) | 29.26 (6.82) | 29.15 (6.35) |
|
| ||||||||
| BMI category | ||||||||
| Under 18.5 | 184 (1.2%) | 38 (1.1%) | 69 (1.2%) | 15 (1%) | 6 (8.1%) | 7 (1.6%) | 36 (1.2%) | 13 (1.3%) |
| [18.5, 25) | 4539 (29.6%) | 1179 (34.4%) | 1662 (27.8%) | 384 (26.6%) | 26 (35.1%) | 125 (27.7%) | 877 (29.8%) | 286 (27.5%) |
| [25, 30) | 4610 (30%) | 1018 (29.7%) | 1855 (31.1%) | 416 (28.8%) | 19 (25.7%) | 130 (28.8%) | 848 (28.9%) | 324 (31.2%) |
| [30, 35) | 3096 (20.2%) | 654 (19.1%) | 1189 (19.9%) | 330 (22.9%) | 15 (20.3%) | 99 (22%) | 574 (19.5%) | 235 (22.6%) |
| [35, 40) | 1717 (11.2%) | 326 (9.5%) | 703 (11.8%) | 160 (11.1%) | 3 (4.1%) | 52 (11.5%) | 369 (12.6%) | 104 (10%) |
| 40 and over | 1197 (7.8%) | 212 (6.2%) | 492 (8.2%) | 138 (9.6%) | 5 (6.8%) | 38 (8.4%) | 235 (8%) | 77 (7.4%) |
|
| ||||||||
| BMI trajectory in 9 months prior to index | ||||||||
| Decreasing (≥3% loss) | 2331 (15.2%) | 515 (15%) | 887 (14.9%) | 199 (13.8%) | 16 (21.6%) | 85 (18.8%) | 453 (15.4%) | 176 (16.9%) |
| Stable | 5946 (38.8%) | 1293 (37.7%) | 2335 (39.1%) | 521 (36.1%) | 19 (25.7%) | 166 (36.8%) | 1162 (39.5%) | 450 (43.3%) |
| Increasing (≥3% gain) | 2171 (14.1%) | 400 (11.7%) | 910 (15.2%) | 237 (16.4%) | 8 (10.8%) | 50 (11.1%) | 423 (14.4%) | 143 (13.8%) |
| Unknown | 4895 (31.9%) | 1219 (35.6%) | 1838 (30.8%) | 486 (33.7%) | 31 (41.9%) | 150 (33.3%) | 901 (30.7%) | 270 (26%) |
|
| ||||||||
| Current smoker, or smoked in last 9 months | 3757 (24.5%) | 632 (18.4%) | 1346 (22.5%) | 644 (44.6%) | 22 (29.7%) | 128 (28.4%) | 731 (24.9%) | 254 (24.4%) |
|
| ||||||||
| Concurrent psychotherapy*** | 5382 (35.1%) | 3427 (100%) | 1014 (17%) | 176 (12.2%) | 17 (23%) | 69 (15.3%) | 542 (18.4%) | 137 (13.2%) |
|
| ||||||||
| Sleep disorder diagnosis | 332 (2.2%) | 84 (2.5%) | 113 (1.9%) | 27 (1.9%) | 2 (2.7%) | 9 (2%) | 62 (2.1%) | 35 (3.4%) |
|
| ||||||||
| Anxiety disorder diagnosis | 3127 (20.4%) | 907 (26.5%) | 941 (15.8%) | 198 (13.7%) | 28 (37.8%) | 142 (31.5%) | 737 (25.1%) | 174 (16.7%) |
|
| ||||||||
| Health plan | ||||||||
| Basic Health | 563 (3.7%) | 119 (3.5%) | 206 (3.5%) | 62 (4.3%) | 1 (1.4%) | 13 (2.9%) | 121 (4.1%) | 41 (3.9%) |
| Commercial | 13549 (88.3%) | 3086 (90%) | 5286 (88.5%) | 1270 (88%) | 59 (79.7%) | 406 (90%) | 2541 (86.5%) | 901 (86.7%) |
| Medicaid | 226 (1.5%) | 28 (0.8%) | 94 (1.6%) | 21 (1.5%) | 1 (1.4%) | 5 (1.1%) | 61 (2.1%) | 16 (1.5%) |
| Medicare | 377 (2.5%) | 96 (2.8%) | 117 (2%) | 36 (2.5%) | 7 (9.5%) | 11 (2.4%) | 73 (2.5%) | 37 (3.6%) |
| Private | 628 (4.1%) | 98 (2.9%) | 267 (4.5%) | 54 (3.7%) | 6 (8.1%) | 16 (3.5%) | 143 (4.9%) | 44 (4.2%) |
|
| ||||||||
| Follow-up visits by 84 days after index date | ||||||||
| None | 4048 (26.4%) | 989 (28.9%) | 994 (16.6%) | 499 (34.6%) | 40 (54.1%) | 154 (34.1%) | 714 (24.3%) | 658 (63.3%) |
| One | 5704 (37.2%) | 919 (26.8%) | 2532 (42.4%) | 557 (38.6%) | 15 (20.3%) | 192 (42.6%) | 1251 (42.6%) | 238 (22.9%) |
| Two | 2816 (18.4%) | 523 (15.3%) | 1407 (23.6%) | 229 (15.9%) | 9 (12.2%) | 64 (14.2%) | 535 (18.2%) | 49 (4.7%) |
| Three or more | 2775 (18.1%) | 996 (29.1%) | 1037 (17.4%) | 158 (10.9%) | 10 (13.5%) | 41 (9.1%) | 439 (14.9%) | 94 (9%) |
|
| ||||||||
| Continuous treatment for at least 84 days | 9957 (64.9%) | 2539 (74.1%) | 3956 (66.3%) | 742 (51.4%) | 48 (64.9%) | 290 (64.3%) | 1943 (66.1%) | 439 (42.3%) |
|
| ||||||||
| Continuous treatment for at least 180 days | 7351 (47.9%) | 1916 (55.9%) | 2933 (49.1%) | 508 (35.2%) | 40 (54.1%) | 201 (44.6%) | 1419 (48.3%) | 334 (32.1%) |
Other SSRI included Citalopram (N=2058), Sertraline (N=651), and Venlafaxine (N=177), Escitalopram (N=43), Fluvoxamine (N=10)
Other SARI included Trazodone (N=1034) and Nefazodone (N=5)*
Concurrent psychotherapy is defined here as the receipt of psychotherapy within 30 days of the beginning of the index treatment episode.
Abbreviations: SD= standard deviation; BMI= body mass index
BMI and receipt of psychotherapy
Higher BMI values at index were associated with significantly lower odds of receiving psychotherapy within the first 30 days of a new depression treatment episode. (P<0.0001; Figure 2 and Appendix A). Compared to a patient with a BMI of 25, a patient with a BMI of 30 was estimated to have 8% lower odds of receipt of psychotherapy (adjusted OR=0.92; 95% CI: 0.89, 0.95); similarly, a patient with a BMI of 40 was estimated to have a 23% lower odds of receipt of psychotherapy (adjusted OR=0.77; 95% CI: 0.72, 0.83). Having an upward BMI trajectory in the 9-months prior to index date was associated with lower odds of psychotherapy (OR=0.83; 95% CI: 0.73, 0.95) compared to subjects with stable BMI. Unknown BMI trajectory was associated with increased odds of initiating psychotherapy (OR=1.20; 95% CI: 1.11, 1.29).
BMI and initial medication choice
Results for fully adjusted models are presented in Figure 3 and Appendix B; results based on unadjusted analyses were substantively similar. Overall, the joint Wald test indicated a statistically significant (adjusted) association between BMI and medication choice (P=0.0023).
Appendix B.
Adjusted antidepressant medication choice odds ratios (and 95% CI) relative to the choice of Fluoxetine, n=11,916*
| N | Index medication**
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Bupropion | Mirtazapine | Paroxetine | Other SSRI | Other SARI | ||||
| 1,443 | 74 | 451 | 2,939 | 1,039 | ||||
|
| ||||||||
| Adjusted OR (95% CI)*** | ||||||||
| BMI value (most recent) | ||||||||
| 18.5 | 0.94 (0.83, 1.07) | 2.20 (1.29, 3.76) | 0.96 (0.78, 1.19) | 1.05 (0.95, 1.15) | 0.98 (0.84, 1.14) | |||
| 25 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |||
| 30 | 1.05 (0.99, 1.11) | 0.66 (0.49, 0.87) | 1.03 (0.92, 1.14) | 0.98 (0.93, 1.03) | 0.98 (0.91, 1.06) | |||
| 35 | 1.11 (1.02, 1.21) | 0.55 (0.36, 0.84) | 1.05 (0.9, 1.23) | 0.98 (0.91, 1.06) | 0.92 (0.82, 1.03) | |||
| 40 | 1.18 (1.02, 1.36) | 0.60 (0.36, 1.01) | 1.07 (0.87, 1.32) | 1.00 (0.90, 1.12) | 0.81 (0.68, 0.97) | |||
| 45 | 1.26 (0.98, 1.62) | 0.77 (0.37, 1.60) | 1.09 (0.77, 1.54) | 1.04 (0.88, 1.24) | 0.70 (0.52, 0.94) | |||
|
| ||||||||
| BMI trajectory over last 9 mo. | ||||||||
| Declining | 0.94 (0.79, 1.11) | 1.87 (0.99, 3.50) | 1.31 (1.00, 1.72) | 0.99 (0.88, 1.10) | 1.01 (0.84, 1.22) | |||
| Stable | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |||
| Increasing | 1.18 (0.99, 1.40) | 1.32 (0.55, 3.14) | 0.80 (0.57, 1.12) | 0.95 (0.84, 1.08) | 0.86 (0.70, 1.04) | |||
| Unknown | 1.19 (1.05, 1.34) | 2.15 (1.35, 3.44) | 1.20 (0.96, 1.51) | 1.01 (0.91, 1.11) | 0.75 (0.63, 0.89) | |||
|
| ||||||||
| Concurrent psychotherapy | 0.74 (0.62, 0.88) | 1.24 (0.68, 2.24) | 0.83 (0.64, 1.07) | 1.15 (1.01, 1.29) | 0.77 (0.61, 0.98) | |||
|
| ||||||||
| Current or former smoker | 2.62 (1.99, 3.46) | 1.05 (0.66, 1.67) | 1.09 (0.87, 1.37) | 0.91 (0.81, 1.02) | 0.91 (0.77, 1.06) | |||
|
| ||||||||
| Anxiety at index | 0.67 (0.55, 0.81) | 2.63 (1.53, 4.50) | 2.14 (1.71, 2.68) | 1.67 (1.46, 1.90) | 0.88 (0.73, 1.07) | |||
|
| ||||||||
| Sleep disorder at index | 0.93 (0.62, 1.39) | 1.46 (0.32, 6.71) | 0.85 (0.42, 1.72) | 1.04 (0.79, 1.37) | 1.53 (1.05, 2.23) | |||
Of 15,343 study subjects, 3,427 psychotherapy patients who received no medications within 14 days of index treatment were excluded from this analysis, leaving 11,916 to contribute to this analysis.
Treatment categories are defined by the medication associated with the index treatment, or by the first medication received within 14 days of an index psychotherapy treatment. Estimates here are interpretable as the medication choice odds ratio, relative to a choice of Fluoxetine, associated with the factors shown.
Estimates shown are adjusted for sex and age group
Abbreviations: OR= odds ratio; CI= confidence interval; BMI= body mass index
The estimated (adjusted) odds of choosing bupropion over fluoxetine increased monotonically as a function of BMI (Figure 3a). For example, a patient with a BMI of 35 was estimated to have 11% higher odds of choosing bupropion over fluoxetine (adjusted OR=1.11; 95% CI: 1.02, 1.21) compared to a patient with a BMI of 25. The odds of initiating bupropion were also higher among subjects with an increasing BMI trajectory compared to those with stable BMI (OR=1.18; 95% CI: 0.99, 1.40). The association between higher BMI and choice of bupropion therapy over fluoxetine was similar in sensitivity analyses of non-smokers only (data not shown).
The odds of choosing mirtazapine over fluoxetine decreased with increasing BMI, until approximately a BMI of 35 (Figure 3b and Appendix B). Compared to a patient with BMI of 25, the odds of choosing mirtazapine over fluoxetine were 2.2 times higher for a patient with BMI of 18.5 (OR=2.20; 95% CI: 1.29, 3.76); higher BMI was associated or suggestive of reduced odds of initiating mirtazapine over fluoxetine (e.g., OR=0.66; 95% CI: 0.49, 0.87 for BMI=30 vs. 25; OR=0.60; 95% CI: 0.36, 1.01 for BMI=40 vs. 25). Odds of initiating both mirtazapine and paroxetine were higher among subjects with declining BMI (OR=1.87; 95% CI: 0.99, 3.50 for mirtazapine; OR=1.31; 95% CI: 1.00, 1.72 for paroxetine) compared to the stable BMI group, but no association was found between BMI at index and initiation of paroxetine (Figure 3c).
There was no significant relationship observed between BMI and the odds of initiating “other SSRIs” relative to fluoxetine (Figure 3d) but very high BMI was associated with a lower odds of choosing “other SARIs” (predominantly trazadone) (e.g., OR=0.81; 95% CI: 0.68, 0.97 for BMI=40; OR=70; 95% CI: 0.52, 0.94 for BMI=45) compared to the BMI=25 group (Figure 3e). An unknown BMI trajectory in the 9 months prior to initiation of therapy was associated with higher odds of initiating mirtazapine (OR=2.15; 95% CI: 1.35, 3.44) and bupropion (OR=1.19; 95% CI: 1.05, 1.34), and lower odds of initiating “other SARIs” (OR=0.75; 95% CI: 0.63, 0.89) relative to fluoxetine (Appendix B).
BMI and quality/continuity of depression care
Higher BMI at index was suggestive of a trend toward lower odds of having continuous depression treatment at 84 days post index date, but this finding was not statistically significant (P=0.11; Figure 4, left panel). However, we observed significantly lower odds of continuous depression treatment with increasing BMI at 180 days after index (P=0.0007; Figure 4, center panel). There was no significant relationship between BMI at index and the odds of having 3 or more visits to primary care or mental health providers within 84 days of index (P=0.98; Figure 4, right panel), but a declining BMI trajectory in the 9-months prior to index was associated with a greater odds of making 3 or more visits within 84 days of index compared to those with stable BMI trajectory (OR=1.19; 95% CI, 1.04–1.36) (Appendix C).
Appendix C.
Adjusted odds ratio estimates by patient characteristics of receiving continuous antidepressant treatment through 84 and 180 days and completion of at least 3 in-person visits within the first 84 days of treatment initiation, n=15,343*
| 84 days continuous treatment | 180 days continuous treatment | 3+ in-person visits within first 84 days | |
|---|---|---|---|
|
| |||
| Adjusted OR (95% CI)** | |||
| BMI value (most recent) | |||
| 18.5 | 1.03 (0.96, 1.11) | 1.06 (0.99, 1.14) | 0.99 (0.91, 1.08) |
| 25 | 1 (referent) | 1 (referent) | 1 (referent) |
| 30 | 0.98 (0.94, 1.01) | 0.95 (0.92, 0.99) | 1.01 (0.96, 1.06) |
| 35 | 0.95 (0.91, 1.00) | 0.91 (0.87, 0.96) | 1.01 (0.94, 1.10) |
| 40 | 0.93 (0.86, 1.01) | 0.87 (0.81, 0.93) | 1.02 (0.90, 1.15) |
| 45 | 0.91 (0.77, 1.07) | 0.83 (0.71, 0.96) | 1.03 (0.83, 1.28) |
|
| |||
| BMI trajectory over last 9 mo. | |||
| Declining | 1.01 (0.92, 1.12) | 1.00 (0.92, 1.08) | 1.19 (1.04, 1.36) |
| Stable | 1 (referent) | 1 (referent) | 1 (referent) |
| Increasing | 1.03 (0.93, 1.14) | 1.03 (0.92, 1.14) | 0.92 (0.83, 1.03) |
| Unknown | 1.18 (1.08, 1.27) | 1.12 (1.04, 1.21) | 1.12 (1.02, 1.23) |
|
| |||
| Female | 0.87 (0.80, 0.94) | 0.89 (0.83, 0.95) | 0.91 (0.83, 1.00) |
|
| |||
| Age group | |||
| 18 to 25 | 0.90 (0.80, 1.01) | 0.99 (0.89, 1.10) | 1.00 (0.87, 1.14) |
| 26 to 35 | 0.98 (0.83, 1.15) | 0.99 (0.85, 1.16) | 1.20 (1.03, 1.41) |
| 36 to 45 | 0.86 (0.75, 0.99) | 0.88 (0.78, 0.99) | 1.08 (0.93, 1.25) |
| 46 to 55 | 0.85 (0.77, 0.94) | 0.92 (0.84, 1.01) | 1.07 (0.97, 1.19) |
| 56 to 65 | 1 (referent) | 1 (referent) | 1 (referent) |
|
| |||
| Current or former smoker | 0.70 (0.63, 0.77) | 0.75 (0.69, 0.82) | 0.70 (0.62, 0.79) |
|
| |||
| Anxiety at index | 1.00 (0.91, 1.09) | 0.87 (0.81, 0.93) | 0.98 (0.80, 1.20) |
|
| |||
| Sleep disorder at index | 1.23 (1.00, 1.50) | 1.04 (0.86, 1.26) | 0.80 (0.62, 1.03) |
Of 15,343 patients who contributed data to these analyses, 9,957 received continuous care through 84 days, 7,351 received continuous care through 180 days, and 2,775 had completed 3 or more visits within 84 days.
Odds ratio estimates are adjusted for sex, age group, BMI trajectory in the 9 months prior to index, smoking status, anxiety disorder, and sleep disorder, each measured at the beginning of the index treatment episode.
Abbreviations: OR=odds ratio; CI=confidence interval; BMI=body mass index
DISCUSSION
Depression is common in obese patients,(24, 25) and there is evidence from prior clinical trials that different antidepressant drug treatments can have positive and negative impacts on body weight.(20–24) More is known about this effect over the short versus long-term.(19) In this large, population-based sample of adults initiating treatment for depression, we found some evidence that a patients’ current BMI or recent changes in BMI may be influencing provider and patient choice of antidepressant drug treatments but the evidence was weak and the 95% confidence intervals were not consistently significant or were barely significant. Since antidepressants can influence body weight and there is no evidence to warrant the choice of one second-generation antidepressant over another on the basis of differences in efficacy,(39) a stronger relationship between antidepressant choice and BMI may be appropriate in some patients. However, other differences in side effects and tolerability must also be factored into treatment decisions. For example, mirtazapine is not a customary first line treatment for depression due to its strong associations with paresthesias and somnolence,(40) and therefore prescribing mirtazapine as first line on the basis of optimizing weight may be questionable. Nevertheless, we found some evidence that decisions about use of bupriopion and mirtazipine may be influenced by the patient’s body weight at the time of treatment initiation. Further research is needed to understand whether these treatment decisions translate into long-term difference in body weight trajectory.
Our study revealed additional evidence that obese patients may be receiving different depression care than similar patients with normal weight. We found strong evidence that obese patients are significantly less likely to receive psychotherapy as part of a new treatment episode for depression. Our study cannot offer insights into why this disparity is present, but we hypothesize several possible mechanisms including some bias on the part of both providers and patients regarding the efficacy of counseling in obese patients, views against counseling, and possible impact of multiple co-morbid health conditions on access to or time for psychotherapy. We also found evidence that obese patients were less likely to receive or adhere to appropriate duration (at the 180-day time point) of depression treatment. Receiving six-months of depression treatment has been recognized by HEDIS as an important benchmark for quality of care. Again, the current study does not offer insights into why obese patients are more likely to receive suboptimal depression treatment. It is possible that weight gain with some antidepressant medications could lead to a greater rate of treatment discontinuation by patients, but further research is needed to test this hypothesis.
The current study has a number of limitations that should be considered when interpreting the results. While GH enrollees are representative of the underlying community served by the health plan, the study was conducted at one health plan and in a single region of the US. GH enrollees tend to be Caucasian and of a higher socioeconomic status than the general US population. As such, we cannot be certain whether results will generalize to other regions, clinical settings, or populations. Estimates indicate that approximately 97% of enrollees receive their medications at GH pharmacies,(31) but misclassification of antidepressant medication use due to factors such as receiving prescription medications elsewhere and non-compliance cannot be ruled out. We also cannot rule out misclassification of psychotherapy if subjects received therapy from outside providers who did not submit claims for payment. The capture of weight and height data on enrollees relies on an office visit to a provider or setting that routinely captures such information. For example, it is unlikely that patients are weighed when visiting a behavioral health provider versus patients are routinely weighed in primary care. Anthropometric measurements such as weight and height are therefore subject to potential bias due to non-random missing data. We recognize that other co-morbidities and behaviors, such as binge eating disorder and smoking cessation, may influence the choice of antidepressant therapy. We attempted to adjust for important confounders and conducted sensitivity analyses among non-smokers but residual confounding cannot be ruled out. The data do not allow us to consider the role of binge eating in choice of depression treatment because formal diagnoses are rarely recorded (e.g., fewer than 0.1% of enrollees treated for depression at GH had a diagnosis code for eating disorder not otherwise specified in 2011). Last but importantly, all outcomes studied reflect both provider and patient behavior and our data do not allow us to distinguish between the two.
Despite some limitations, this is the first study to our knowledge that evaluates whether providers are considering a patient’s body weight when initiating treatment with antidepressant medications and/or psychotherapy. We drew the sample from a large, defined population with stable membership that receives almost all their care within a delivery system with sophisticated electronic records on height and weight. This near complete capture of information was an ideal setting for the research question; however, the study warrants replication in other settings and populations.
Our study findings offer a first step toward understanding and promoting the consideration of body weight when prescribing antidepressants. As obesity reaches epidemic proportions in the US (1) and often co-exists with depression,(2, 3) our study may be used to promote discussion about recommending and choosing depression treatment plans that optimize depression care and weight management concurrently. We hope this study also raises awareness and prompts further research on barriers to recommending psychotherapy to patients with high BMIs and patients with other factors identified as being associated with lower odds of receiving psychotherapy (i.e., smokers and women) as well as targeted interventions to improve continuation of therapy and appropriate follow-up care for depression.
Acknowledgments
Funding/Support: This research was conducted by the Group Health Research Institute. This project was funded under grant R01 MH083671 from the National Institute of Mental Health (NIMH) – Principle investigator: D. Arterburn. The authors of this report are responsible for its content.
Role of the Sponsor: The authors of this article are responsible for its content. No statement may be construed as the official position of the NIMH. The funder had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; or preparation and approval of the manuscript.
Footnotes
Financial Disclosures: None reported
Author Contributions:
Study concept and design: Arterburn, Bogart, Boudreau, Haneuse, and Simon.
Acquisition of data: Arterburn, Bogart, Boudreau, Theis, and Westbrook
Analysis and interpretation of data: Arterburn, Bogart, Boudreau, and Haneuse
Drafting of the manuscript: Arterburn, Bogart, and Boudreau
Critical revision of the manuscript for important intellectual content: Arterburn, Bogart, Boudreau, Haneuse, Simon, and Westbrook.
Statistical analysis: Bogart and Haneuse
Obtaining funding: Arterburn, Boudreau, Haneuse, Simon
Administrative, technical, or material support: Arterburn, Theis, and Westbrook
Study supervision: Arterburn and Westbrook
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biological psychiatry. 2003;54:330–7. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 3.Faith MS, Matz PE, Jorge MA. Obesity-depression associations in the population. J Psychosom Res. 2002;53:935–42. doi: 10.1016/s0022-3999(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 4.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 5.Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS. Waist Circumference, Abdominal Obesity, and Depression among Overweight and Obese U.S. Adults: National Health and Nutrition Examination Survey 2005–2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. International journal of obesity (2005) 2006;30:513–9. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- 7.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 8.Faith MS, Butryn M, Wadden TA, Fabricatore A, Nguyen AM, Heymsfield SB. Evidence for prospective associations among depression and obesity in population-based studies. Obes Rev. 2011;12:e438–53. doi: 10.1111/j.1467-789X.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 9.Patten SB, Williams JV, Lavorato DH, Khaled S, Bulloch AG. Weight gain in relation to major depression and antidepressant medication use. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- 11.Patten SB, Williams JV, Lavorato DH, Brown L, McLaren L, Eliasziw M. Major depression, antidepressant medication and the risk of obesity. Psychother Psychosom. 2009;78:182–6. doi: 10.1159/000209349. [DOI] [PubMed] [Google Scholar]
- 12.Hach I, Ruhl UE, Klotsche J, Klose M, Jacobi F. Associations between waist circumference and depressive disorders. J Affect Disord. 2006;92:305–8. doi: 10.1016/j.jad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Ho RC, Niti M, Kua EH, Ng TP. Body mass index, waist circumference, waist-hip ratio and depressive symptoms in Chinese elderly: a population-based study. Int J Geriatr Psychiatry. 2008;23:401–8. doi: 10.1002/gps.1893. [DOI] [PubMed] [Google Scholar]
- 14.Simon GE, Rohde P, Ludman EJ, et al. Association between change in depression and change in weight among women enrolled in weight loss treatment. General hospital psychiatry. 2010;32:583–9. doi: 10.1016/j.genhosppsych.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing RR, Epstein LH, Marcus MD, Kupfer DJ. Mood changes in behavioral weight loss programs. J Psychosom Res. 1984;28:189–96. doi: 10.1016/0022-3999(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 16.Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol. 1994;62:165–71. doi: 10.1037//0022-006x.62.1.165. [DOI] [PubMed] [Google Scholar]
- 17.Schowalter M, Benecke A, Lager C, et al. Changes in depression following gastric banding: a 5- to 7-year prospective study. Obes Surg. 2008;18:314–20. doi: 10.1007/s11695-007-9316-7. [DOI] [PubMed] [Google Scholar]
- 18.Ricca V, Mannucci E, Di Bernardo M, Rizzello SM, Cabras PL, Rotella CM. Sertraline enhances the effects of cognitive-behavioral treatment on weight reduction of obese patients. J Endocrinol Invest. 1996;19:727–33. doi: 10.1007/BF03347875. [DOI] [PubMed] [Google Scholar]
- 19.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–72. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 20.Aronne LJ, Segal KR. Weight gain in the treatment of mood disorders. J Clin Psychiatry. 2003;64 (Suppl 8):22–9. [PubMed] [Google Scholar]
- 21.Berkowitz RI, Fabricatore AN. Obesity, psychiatric status, and psychiatric medications. Psychiatr Clin North Am. 2005;28:39–54. vii–viii. doi: 10.1016/j.psc.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh R, Franco K. Managing weight gain as a side effect of antidepressant therapy. Cleve Clin J Med. 2003;70:614, 6, 8. doi: 10.3949/ccjm.70.7.614. passim. [DOI] [PubMed] [Google Scholar]
- 23.Kachur SG, Hannan CL, Ward KE. Antidepressant-induced weight gain. Med Health R I. 2005;88:359–61. [PubMed] [Google Scholar]
- 24.Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: a review. Obes Rev. 2004;5:115–21. doi: 10.1111/j.1467-789X.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 27.Paulose-Ram R, Safran MA, Jonas BS, Gu Q, Orwig D. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16:560–70. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]
- 28.Keith SW, Redden DT, Katzmarzyk PT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. International journal of obesity (2005) 2006;30:1585–94. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 29.Ludman EJ, Ichikawa LE, Simon GE, et al. Breast and cervical cancer screening specific effects of depression and obesity. Am J Prev Med. 2010;38:303–10. doi: 10.1016/j.amepre.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertakis KD, Azari R. The impact of obesity on primary care visits. Obes Res. 2005;13:1615–23. doi: 10.1038/oby.2005.198. [DOI] [PubMed] [Google Scholar]
- 31.Saunders KDR, Stergachis A. Group Health Cooperative. In: Pharmacoepidemiology. BLS, editor. John Wiley and Sons; West Sussez, England: 2005. pp. 223–39. [Google Scholar]
- 32.Arterburn DE, Ichikawa L, Ludman E, et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2:277–81. doi: 10.1016/j.orcp.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson B. HEDIS antidepressant medication management measures and performance-based measures: an opportunity for improvement in depression care. Am J Manag Care. 2007;13:S98–102. [PubMed] [Google Scholar]
- 34.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 35.Hastie TJ. Generalized additive models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. At&T Bell Laboratories; Yonkers, NY: 1992. pp. 249–308. [Google Scholar]
- 36.Diggle P, Heagerty P, Liang K, Zeger SL. Analysis of Longitudinal Data. 2. Oxford University Press; Oxford: 2002. [Google Scholar]
- 37.Agresti A. Analysis of ordinal categorical data. John Wiley & Sons, Inc; Hoboken, NJ: 1984. [Google Scholar]
- 38.Kuss O, McLerran D. A note on the estimation of the multinomial logistic model with correlated responses in SAS. Comput Methods Programs Biomed. 2007;87:262–9. doi: 10.1016/j.cmpb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Gartlehner G, Hansen RA, Morgan LC, et al. Comparative Benefits and Harms of Second-Generation Antidepressants for Treating Major Depressive Disorder: An Updated Meta-analysis. Ann Intern Med. 2011;155:772–85. doi: 10.7326/0003-4819-155-11-201112060-00009. [DOI] [PubMed] [Google Scholar]
- 40.Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]