Abstract
The relationship between testosterone, well-being and mood is poorly understood. We investigated the effect of testosterone supplementation on mood, well-being, and self-reported health in men with erectile dysfunction (ED) and low serum testosterone levels. This was a randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov registration number NCT00512707) in which 140 men, 40 to 70-years, with ED and low serum testosterone levels were first optimized on sildenafil alone for 3 to 7-weeks and then randomized to receive either sildenafil plus testosterone gel (n = 70) or sildenafil plus placebo (n = 70) gel for 14-weeks. Using multiple imputations and generalized linear regression, we compared psychological changes in well-being, evaluated by the Psychological General Well-Being Index, and mood, evaluated by Derogatis Affects Balance Scale. Mood and well-being scores were similar between the two groups at baseline and did not substantially change during the administration of sildenafil or after randomization to testosterone. Our findings show that the addition of testosterone to sildenafil in men with ED and low serum testosterone levels was not associated with improvement in either well-being or mood.
Keywords: Erectile dysfunction, testosterone replacement, mood, affectivity balance, well-being, androgen deficiency
Introduction
In spite of the widely held perception that testosterone administration improves mood and well-being in men with low testosterone levels, the relationship between testosterone and psychological outcomes remains poorly understood. The data on the effects of testosterone on mood and well-being are limited and conflicting. Low testosterone levels have been associated weakly and inconsistently with depressed mood, fatigue, and reduced vigor in observational studies.(Araujo, et al., 2007; Barrett-Connor, et al., 1999) Open-label, uncontrolled trials of testosterone supplementation in men with low testosterone levels have suggested that testosterone administration improves mood and well-being.(Daniell, et al., 2006; Wang, et al., 2004; Wang, et al., 2011) However, randomized, placebo-controlled trials of testosterone have not confirmed these findings; three trials reported no significant differences in mood between testosterone and placebo groups, one trial reported inconsistent effects that were not sustained, and another reported significant benefit in improving minor depression.(Cavallini, et al., 2004; Pope, et al., 2010; Seidman, et al., 2001; Shores, et al., 2009; Steidle, et al., 2003)
ED is known to influence mood and well-being (Rosen, et al., 2004), and testosterone therapy is widely used alone and in combination with phosphodiesterase-5-inhibitors in men with ED and low testosterone levels. However, we do not know whether testosterone therapy improves mood and well-being in men with ED. Therefore, here we determined the effects of testosterone in comparison to placebo on mood, well-being, and self-reported health in men with ED and low testosterone levels. In this randomized, double-blind, placebo-controlled, and parallel group clinical trial, men with ED and low testosterone levels received an optimized dose of sildenafil before being randomized to receive either testosterone or placebo gel.(Buvat, et al., 2011; Shabsigh, et al., 2004) The primary findings of the trial focusing on erectile function have been reported recently (Spitzer, et al., 2012); this report describes the effects of adding testosterone to sildenafil on mood, well-being, and self-reported health in comparison to that of adding a placebo.
Materials and Methods
Subjects
The trial (ClinicalTrials.gov registration number NCT00512707) was approved by the Institutional Review Boards at Charles Drew University and Boston University, and all participants provided written informed consent. A Data and Safety Monitoring Board reviewed adverse events semiannually.
The trial’s design has been reported previously.(Spitzer, et al., 2012) Briefly, the principal eligibility criteria included ED as defined by a score of 25 or less on the erectile function domain of the International Index of Erectile Function, age between 40 and 70-years, and a serum testosterone measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) <11.5 nmol/L (330 ng/dL) and/or free testosterone <173 pmol/L (50 pg/mL).(Goldstein, et al., 1998; Rosen, et al., 1997) These criteria for serum testosterone levels were selected because they are below the lower limits of normal for young, healthy men.(Bhasin, et al., 2011) Prostate cancer, creatinine >180µmol/L (2mg/dL), hemoglobin A1c ≥8.5%, prostate specific antigen (PSA) >4ng/mL, blood pressure >160/100mmHg, myocardial infarction or stroke within six-months, or congestive heart failure, and use of androgens, antiandrogens, or nitrates comprised major exclusion criteria. Medications that influence androgen levels, such as high dose opiates, glucocorticoids, or antiepileptics, also excluded participation.
Study design
Men initially entered an open-label 3–7 week sildenafil optimization phase, in which sildenafil dose was individually titrated based on erectile response and tolerability. Participants were then randomized either to topical 1% testosterone gel (10-g daily) or placebo gel daily in 1:1 allocation during a 14-week intervention period using concealed randomization and permuted blocks with a block size of 4.
Adjustment of Testosterone Dose
Participants in both the testosterone and placebo groups had blood drawn by phlebotomists in the clinical research center 2 weeks after starting testosterone gel. A designated unblinded research physician reviewed the results of serum total testosterone levels. This unblinded physician was involved only in dose titration and did not participate in any other aspects of the trial. If a serum total testosterone level was outside the target range of 17.4 nmol/L (500 ng/dL) to 34.7 nmol/L (1000 ng/dL), the unblinded physician increased or decreased the dose of testosterone gel to either 15-g or 5-g daily. Men with testosterone levels within the target range continued testosterone 10-g topically daily.
Blinding
The investigating staff and the participants were blinded to intervention allocation; the group and testosterone dose assignment was known only to the Investigational Drug Pharmacist, who dispensed testosterone gel and sildenafil, and a designated physician, who performed the dose titration. To maintain blinding, the participants received 3-tubes of the gel daily; those assigned to the 10-g dose received daily two tubes containing testosterone gel and one tube containing the placebo gel, while those assigned to placebo group received three tubes of the placebo gel.
Safety Monitoring
Adverse events were assessed at each visit. Hematocrit and PSA levels were measured, and prostate exams were performed at weeks 8 and 14 of the intervention; these are previously described.(Spitzer, et al., 2012)
Outcome Measures
Well-being and mood were assessed using the Psychological General Well-Being Index (PGWBI), a 22-item questionnaire that evaluated six dimensions of self-reported wellness: positive well-being, depressed mood, anxiety, self control, general health, and vitality.(Daniell, et al., 2006; Dupuy, 1984) Higher scores in each dimension reflect increasing well-being. A global score was calculated as the sum of each domain score. Because the range of possible absolute scores for each domain of the PGWBI differed numerically, the scores were normalized to a 100% scale to facilitate comparison.
The Derogatis Affects Balance Scale (DABS) is a 40-item mood inventory and consists of 4 positive affect dimensions (joy, contentment, vigor, and affection) as well as 4 negative affect dimensions (anxiety, depression, guilt, and hostility).(Derogatis & Palmer, 1998) Each domain was calculated as the sum of 5-items and could range from 0 to 20, wherein higher scores indicate greater affectivity. PGWBI and DABS were administered at baseline, randomization, and during weeks 8 and 14 post randomization.
Statistical Methods
Statistical analyses and plots were performed in R version-2.15.1 (R Foundation, Vienna, Austria) and SAS version-9.3 (SAS Institute Inc, Cary, NC).(Wickham, 2009) Estimates of the effect of testosterone on change in outcomes between baseline and study end were obtained using linear regression analyses. We used the Multivariate Imputation by Chained Equations (MICE) algorithm to assign 25 values for each missing record, and to obtain effect estimates that combine results for all imputed data sets.(Van Buuren & Groothuis-Oudshoorn, 2011) In the linear model that generated the imputed values, baseline age, Body Mass Index (BMI), diabetes, hypertension, and race as well as previously reported measures of sexual function at randomization and study end (14 weeks) were included as covariates. Simple linear regression of observed (non-imputed) data evaluated the relationship between baseline serum total testosterone levels, change in well-being, and change in vigor. Results were considered statistically significant if null hypotheses could be rejected at the 0.05 level.
Sample Size Calculation
The sample size of 140 participants was first guided by an effect size estimate for the primary outcome, the International Index of Erectile Function Erectile Function Domain.(Spitzer, et al., 2012) Additionally, the sample size was calculated to have at least 80% power to detect a difference in secondary outcomes with effect sizes of 0.5 or greater. For example, assuming a standard deviation of 4.0 (20%) for the PGWBI positive well-being dimension, this study involving 140 men would have at least 80% power to detect a 1.9 (9.5%) or greater difference between testosterone and placebo groups.(Daniell, et al., 2006)
Results
Flow of Subjects through the Trial
1379 men were screened by phone for eligibility, and 701 were screened in person.(Spitzer, et al., 2012) Of those deemed ineligible after the in-person screening, 74% were excluded due to having a serum testosterone exceeding 11.5nmol/L (330 ng/dL). 152 received sildenafil monotherapy during an optimization phase, however 12 were not randomized because they failed to attend the scheduled study visits, took an exclusionary medication, had a primary sexual disorder other than ED, developed a gastrointestinal bleed, or did not have a sexual partner. 140 men were randomized either to sildenafil and testosterone gel (n=70) or sildenafil and placebo gel (n=70). In the testosterone arm, 5 were lost to follow-up, 2 withdrew due to skin irritation, 2 were discontinued due to developing hematocrit exceeding 54%, and 1 withdrew due to the smell of the gel. In the placebo arm, 11 were lost to follow up and 1 withdrew due to skin irritation. Thus, 60 and 58 participants in the testosterone and placebo arms, respectively, completed the trial.
Baseline Characteristics of the Participants
Mean age was 55-years, and 97% of men described themselves as heterosexual. The two groups were similar in their baseline characteristics, such as age, BMI, and prevalence of co-morbid conditions (Table). Baseline mean serum total testosterone levels were 8.6 nmol/L (248 ng/dL) and 8.8 nmol/L (254 ng/dL) in the testosterone and placebo groups, respectively. PGWBI and DABS scores were similar in the two arms at baseline (Table).
The Effect of Testosterone on Mood and Well-being in Men with Erectile Dysfunction in a Randomized, Placebo-Controlled Trial
| Table Baseline Demographics, Well-Being and Mood | ||
|---|---|---|
| Demographics |
Testosterone (n=70) |
Placebo (n=70) |
| Age (years) | 55.1 (8.3) | 54.6 (8.5) |
| Race – no. (%) | ||
| Black | 28 (41%) | 33 (47%) |
| White | 36 (53%) | 32 (46%) |
| Other | 4 (6%) | 5 (7%) |
| Body Mass Index (kg/m2) | 31.5 (6.4) | 32.7 (6.0) |
| Diabetes Mellitus – no. (%) | 13 (21%) | 14 (22%) |
| Hypertension – no. (%) | 29 (45%) | 27 (40%) |
| Cardiovascular Disease – no. (%) | 35 (50%) | 32 (46%) |
| Serum Testosterone | ||
| Total Testosterone (ng/dL) | 248 (62) | 254 (68) |
| Psychological General Well-Being Index Score (%) | ||
| Positive Well-being | 62 (17) | 61 (19) |
| Depressed Mood | 86 (14) | 83 (15) |
| General Health | 73 (16) | 73 (17) |
| Anxiety | 75 (15) | 72 (18) |
| Self Control | 85 (13) | 84 (14) |
| Global Score | 73 (12) | 72 (14) |
| Derogatis Affects Balance Scale Dimension | ||
| Joy | 13.0 (3.2) | 12.9 (4.3) |
| Contentment | 13.1 (2.9) | 12.8 (3.6) |
| Vigor | 12.2 (3.2) | 12.3 (4.1) |
| Affection | 14.4 (3.6) | 14.2 (3.8) |
| Depression | 3.9 (3.3) | 4.7 (3.7) |
| Anxiety | 5.4 (3.5) | 6.2 (3.1) |
| Guilt | 4.1 (3.7) | 4.4 (3.9) |
| Hostility | 4.5 (3.5) | 4.8 (3.7) |
Baseline characteristics are displayed as mean (standard deviation). Scores are reported prior to receipt of sildenafil, testosterone or placebo. Psychological General Well-Being Index scores may range from 0 to 100%, with higher scores reflecting improved well-being. Derogatis Affects Balance Scale dimension scores may range from 0 to 20, with higher scores reflecting greater affectivity of the respective dimension.
Compliance
Compliance to gel therapy, which was measured by counting used gel tubes, was 90% and 89% for testosterone and placebo groups, respectively.(Spitzer, et al., 2012)
Hormone Levels
At the end of 14 weeks of treatment, serum total testosterone levels were 22.5nmol/L (649ng/dL) and 11.8nmol/L (339ng/dL) for the testosterone and placebo groups, respectively. Change in total testosterone levels during the intervention was significantly greater for the testosterone arm vs. placebo arm (difference between testosterone and placebo mean changes 8.3 nmol/L; 95% CI 3.2, 13.4; P = 0.001).(Spitzer, et al., 2012)
Missingness
Figures 1 and 2 display the number of participants contributing observed data at time points throughout the study. 9.7% of PGWBI and DABS scores were imputed due to missingness.
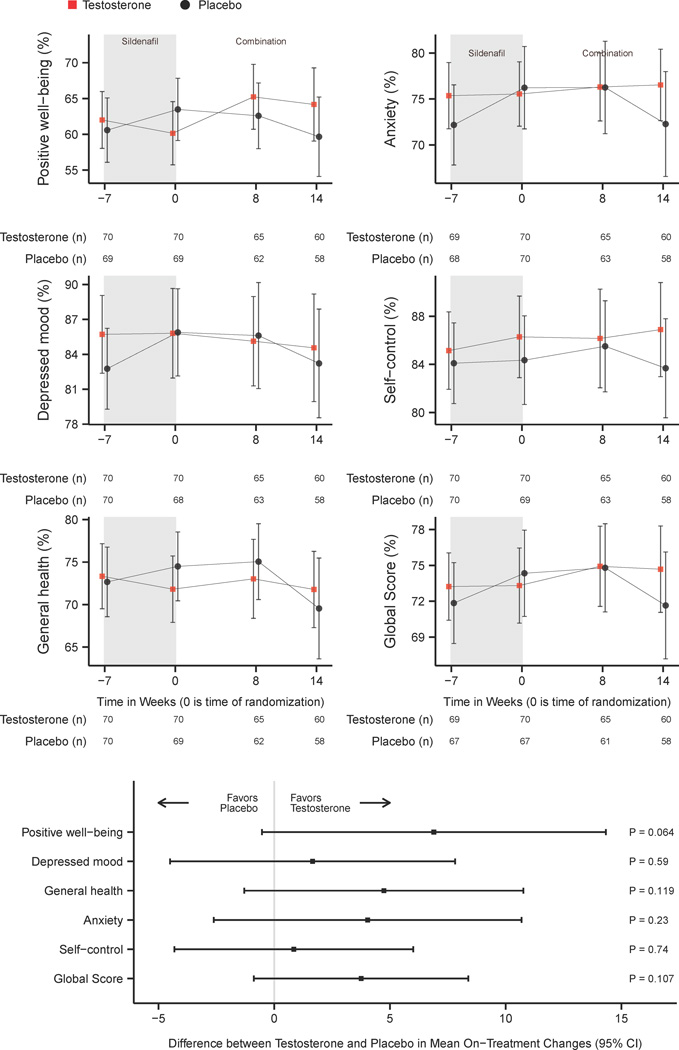
Figure 1. Change in Psychological General Well-Being Index Scores with Sildenafil and Testosterone or Sildenafil and Placebo.
CI = Confidence Interval
Figure 1 displays observed (non-imputed) means and 95% confidence intervals for Psychological General Well-Being Index positive well-being, depressed mood, general health, anxiety, self-control, and global score at baseline, after being optimized on sildenafil citrate (randomization), and during weeks 8 and 14 after randomization to combination sildenafil and testosterone or sildenafil and placebo. These data have been normalized to a 100% scale to facilitate comparison. Tables report the number of participants included at each data point among the testosterone and placebo groups. Multiple imputed estimates of the differences in score changes over 14 weeks between testosterone and placebo groups with 95% confidence intervals are shown for domain and global scores. Higher scores in each scale correspond with greater well-being.
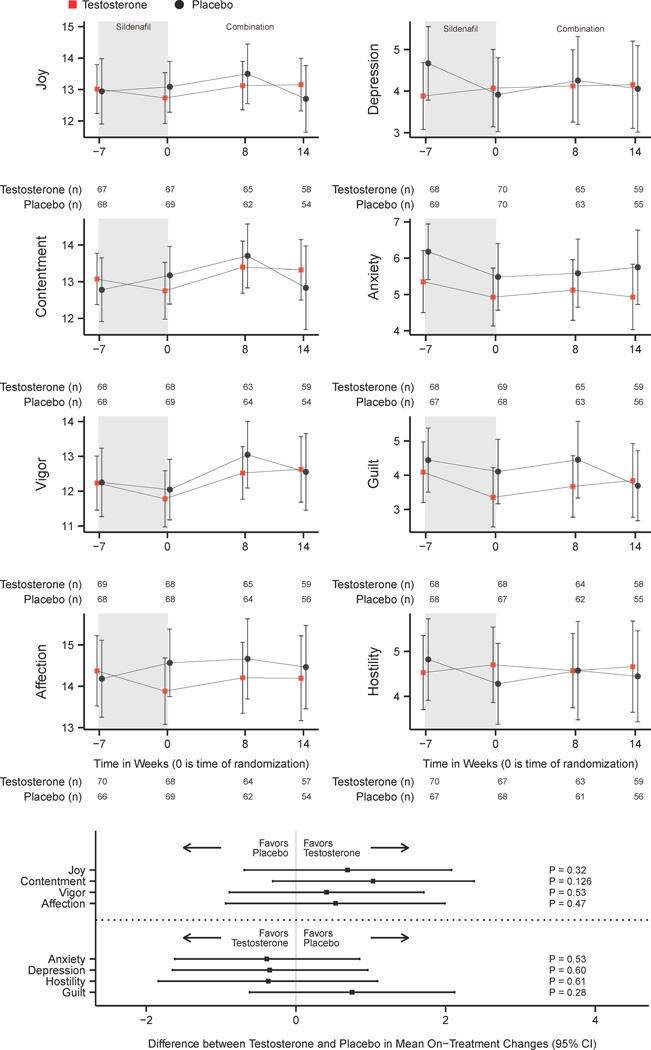
Figure 2. Change in Derogatis Affects Balance Scale Scores with Sildenafil and Testosterone or Sildenafil and Placebo.
CI = Confidence Interval
Figure 2 displays observed (non-imputed) means and 95% confidence intervals for Derogatis Affects Balance Scale mean affect scores at baseline, after being optimized on sildenafil citrate (randomization), and during weeks 8 and 14 after randomization to combination sildenafil and testosterone or sildenafil and placebo. Tables report the number of participants included at each data point among the testosterone and placebo groups. Affect scores may range from 0 to 20, and higher scores correspond with increased affectivity. Multiple imputed estimates of the differences in score changes over 14 weeks between testosterone and placebo groups with 95% confidence intervals are shown for each domain.
Outcomes
PGWBI scores did not change substantially with the addition of sildenafil alone (Figure 1). PGWBI domains and global well-being score did not demonstrate any significant differences between the testosterone and the placebo groups while receiving testosterone and sildenafil or placebo and sildenafil (Figure 1).
Self-rated mood scores did not change substantially in either group during the open-label sildenafil phase (Figure 2). Similarly, after randomization, testosterone and placebo arms did not reveal any statistically significant differences in any domain of positive or negative affect (Figure 2).
Baseline Serum Total Testosterone Levels and Positive Well-being
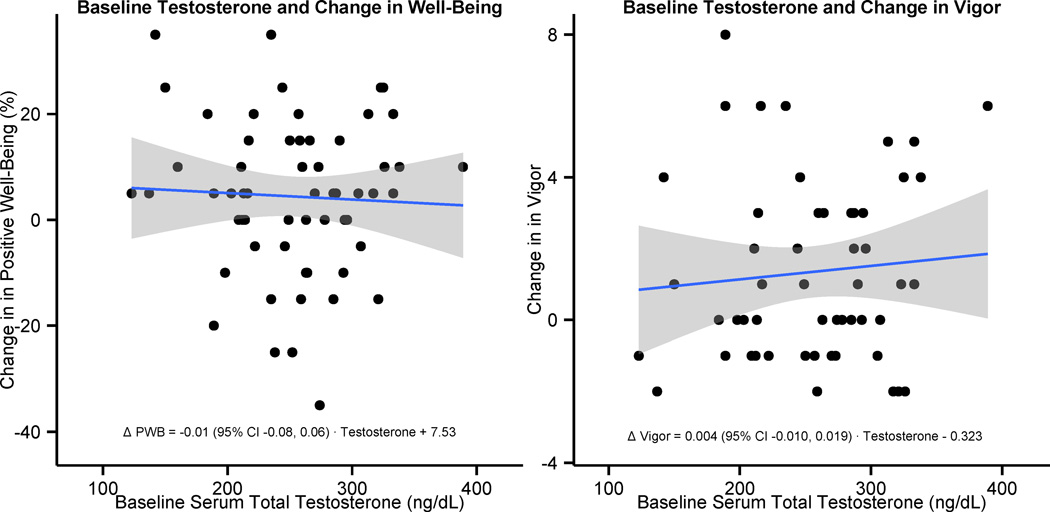
Baseline serum total testosterone levels did not predict on-treatment changes in PGWBI positive well-being domains among those receiving testosterone therapy (β-estimate -0.01; 95% CI -0.08, 0.06; P = 0.72; Figure 3). Similarly, baseline serum total testosterone levels did not predict change in DABS vigor domains (β-estimate 0.004; 95% CI -0.010, 0.019; P = 0.55; Figure 3).
Figure 3. Baseline Serum Total Testosterone Levels Among Men Receiving Testosterone Does Not Predict Change in Well-being or Vigor.
PWB = Positive Well-being; CI = Confidence Interval
A scatter plot displays the relationship between observed (non-imputed) baseline serum total testosterone levels and on-treatment changes in Psychological General Well-Being Index positive well-being domains over 14 weeks among men randomized to testosterone therapy on the left (n = 60). The relationship between observed baseline serum total testosterone and change in Derogatis Affects Balance Scale Vigor domain among men receiving testosterone therapy is displayed on the right (n = 57). Regression lines with 95% confidence intervals overlay the data, and the equations for these lines are printed at the bottom of each plot.
Discussion
These data overall suggest that addition of testosterone gel to an optimized dose of sildenafil in men with ED and low serum testosterone was not associated with improvement in sense of well-being and mood. Analyses using multiple imputations revealed no significant differences between testosterone and placebo groups during the intervention in any aspect of mood or well-being. Moreover, changes in well-being and vigor did not vary significantly with baseline serum total testosterone levels.
Although sildenafil administration during the open-label phase was associated with substantial improvement in erectile function and sexual activity, these improvements in sexual function were not associated with significant changes in mood and well-being.(Goldstein, et al., 1998; Rosen, et al., 2002) This is somewhat surprising in light of the known association between erectile dysfunction, mood and affect.(Rosen, et al., 2004) It is possible that the domains of mood and well-being that are associated with erectile function and sexual activity may not be captured by the instruments that were used in this trial. It is also possible that sexual function may vary independently of mood and well-being.
The absence of changes in mood and well-being with the addition of testosterone to sildenafil among men with low testosterone levels and ED is also surprising. The administration of highly supraphysiologic doses of testosterone has been anecdotally associated with aggression; a randomized, placebo-controlled, cross-over study of testosterone 150 to 600 mg intramuscularly once weekly in young, healthy men noted increases in aggressive and punitive responses while playing a computer game.(Kouri, et al., 1995) Physiologic replacement doses of topical testosterone in open-label clinical trials of 4-months and 3-years duration have noted significant improvements in self-reported mood.(Wang, et al., 2004; Wang, et al., 2011) In contrast, randomized, placebo-controlled trials have not demonstrated consistent improvements in mood in men with low serum testosterone levels and depressed mood, reduced libido, or fatigue. In one trial, testosterone gel 7.5-g daily for 3-months resulted in significantly greater remission of minor depression (53% vs. 19% of men receiving testosterone or placebo, respectively; P = 0.041).(Shores, et al., 2009) Other trials have demonstrated temporary effects or no effect at all. Supplementation with testosterone 160 mg by mouth daily improved depression scores at 3-months but not at 6-months in a trial comparing testosterone, carnitine and placebo.(Cavallini, et al., 2004) Treatment with testosterone gel at 50 or 100 mg topically daily for 3-months did not change mood in comparison to placebo.(Steidle, et al., 2003) Testosterone 200 mg intramuscularly for 6-weeks in did not change depression scores among men with depression and low serum testosterone levels vs. placebo.(Seidman, et al., 2001) Similarly, 6-weeks of testosterone gel did not significantly change depression scores among men with low serum testosterone and depression treated with serotonergic antidepressants.(Pope, et al., 2010) The results of this trial add to evidence supporting no effect of testosterone supplementation on mood and well-being.
The duration of testosterone therapy may be important for its effects on mood. This trial’s duration was 14-weeks, and it is possible that a longer duration of therapy might have shown some benefit. Moreover, men in this trial were selected primarily because of ED and not mood disorder.(Shores, et al., 2009) This might have impacted these findings.
This first randomized trial to study the effects of testosterone on affect and well-being in men with ED and low testosterone levels has several strengths. First, the trial featured a double-blind, parallel-group, randomized, and placebo-controlled design. 140 participants were randomized contributing to a robust sample size and >80% statistical power to detect clinically meaningful differences in the outcomes. The dose of testosterone was adjusted to raise testosterone levels into the midnormal range. Multiple imputations were used to preserve randomization despite missing data and helped to limit the effect of confounders.
The trial has some limitations as well. The trial evaluated the effects of the addition of testosterone to sildenafil. The effects of testosterone alone on mood and affects balance may differ from those of testosterone administered in conjunction with sildenafil. More than half of participants were obese, which negatively associates with testosterone levels.(Bhasin, et al., 2010; Hall, et al., 2008; Travison, et al., 2007) Effects of testosterone therapy on mood in leaner men may differ from effects in this more obese population. The participants in this trial had low testosterone levels not due to a known organic cause. Therefore, these findings should not be extrapolated to men with classical hypogonadism due to known diseases of the hypothalamus, pituitary, and the testis. Although mood, well-being and affect were not the primary outcome of this trial, these outcomes were pre-specified in the study’s hypotheses and protocol a priori and the sample size was based on consideration of achieving >80% statistical power to detect clinically meaningful differences in the outcomes, described in this manuscript. The ongoing multicenter Testosterone Trial (T Trial; clinical trials registration #NCT00799617) with an estimated enrollment of 800 men will likely help to further clarify the complex relationship between testosterone therapy and well-being.
Conclusions
The addition of testosterone gel to an optimized dose of sildenafil in men with ED and low serum testosterone was not associated with improvement in sense of well-being or mood.
Acknowledgements
A grant from the National Institute of Child Health and Human Development (5R01HD047722) primarily supported this research. The Boston University Clinical and Translational Science Institute (1UL1RR025771) and Boston Claude D. Pepper Older Americans Independence Center (grant 5P30AG031679 from the National Institute of Aging) also provided funding for this study. The authors thank the members of the Data and Safety Monitoring Board (Abraham Morgentaler, MD [Chair]; Leonard Marks, MD; Andre Guay, MD; and Ridwan Shabsigh, MD) and the staff of the Boston University Clinical and Translational Science Institute for their contribution.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Author Contributions: Shalender Bhasin designed this trial. Shalender Bhasin and Shehzad Basaria implemented the protocol and collected data from participants. Statistical analyses were conducted by Thomas Travison, Maithili Davda and Matthew Spitzer. Matthew Spitzer wrote the first draft of the manuscript. Leonard Derogatis contributed expertise on analyses of affect balance. All authors reviewed, edited, and approved the final manuscript.
References
- Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: The rancho bernardo study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D'Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the framingham heart study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvat J, Montorsi F, Maggi M, Porst H, Kaipia A, Colson MH, Cuzin B, Moncada I, Martin-Morales A, Yassin A, Meuleman E, Eardley I, Dean JD, Shabsigh R. Hypogonadal men nonresponders to the pde5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (tadtest study) J Sex Med. 2011;8:284–293. doi: 10.1111/j.1743-6109.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- Cavallini G, Caracciolo S, Vitali G, Modenini F, Biagiotti G. Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Urology. 2004;63:641–646. doi: 10.1016/j.urology.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Daniell HW, Lentz R, Mazer NA. Open-label pilot study of testosterone patch therapy in men with opioid-induced androgen deficiency. J Pain. 2006;7:200–210. doi: 10.1016/j.jpain.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Palmer A. The derogatis affects balance scale. In: Zalaquett CP, Wood RJ, editors. Evaluating stress a book of resources, Vol. 2. Scarecrow Press; 1998. pp. 89–117. [Google Scholar]
- Dupuy HJ. The psychological general well-being (pgwb) index. In: Wenger NK, editor. Assessment of quality of life in clinical trials of cardiovascular therapies. New York: Le Jacq Publications; 1984. pp. 170–183. [Google Scholar]
- Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil study group. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- Hall SA, Esche GR, Araujo AB, Travison TG, Clark RV, Williams RE, McKinlay JB. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab. 2008;93:3870–3877. doi: 10.1210/jc.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40:73–79. doi: 10.1016/0376-8716(95)01192-7. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Amiaz R, Brennan BP, Orr G, Weiser M, Kelly JF, Kanayama G, Siegel A, Hudson JI, Seidman SN. Parallel-group placebo-controlled trial of testosterone gel in men with major depressive disorder displaying an incomplete response to standard antidepressant treatment. J Clin Psychopharmacol. 2010;30:126–134. doi: 10.1097/JCP.0b013e3181d207ca. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Cappelleri JC, Gendrano N., 3rd The international index of erectile function (iief): A state-of-the-science review. Int J Impot Res. 2002;14:226–244. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (iief): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Seidman SN, Menza MA, Shabsigh R, Roose SP, Tseng LJ, Orazem J, Siegel RL. Quality of life, mood, and sexual function: A path analytic model of treatment effects in men with erectile dysfunction and depressive symptoms. Int J Impot Res. 2004;16:334–340. doi: 10.1038/sj.ijir.3901197. [DOI] [PubMed] [Google Scholar]
- Seidman SN, Spatz E, Rizzo C, Roose SP. Testosterone replacement therapy for hypogonadal men with major depressive disorder: A randomized, placebo-controlled clinical trial. J Clin Psychiatry. 2001;62:406–412. doi: 10.4088/jcp.v62n0602. [DOI] [PubMed] [Google Scholar]
- Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol. 2004;172:658–663. doi: 10.1097/01.ju.0000132389.97804.d7. [DOI] [PubMed] [Google Scholar]
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression) J Clin Psychiatry. 2009;70:1009–1016. doi: 10.4088/jcp.08m04478. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, Mazer NA, Knapp PE, Hanka S, Lakshman KM, Ulloor J, Zhang A, Orwoll K, Eder R, Collins L, Mohammed N, Rosen RC, Derogatis L, Bhasin S. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: A parallel, randomized trial. Ann Intern Med. 2012;157:681–691. doi: 10.7326/0003-4819-157-10-201211200-00004. [DOI] [PubMed] [Google Scholar]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. Aa2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–555. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in r. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (androgel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- Wang C, Ilani N, Arver S, McLachlan RI, Soulis T, Watkinson A. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin Endocrinol (Oxf) 2011;75:836–843. doi: 10.1111/j.1365-2265.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- Wickham H. Ggplot2: Elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]