Abstract
Summary
Adjusting for age, sex, and precipitating cause, the relative risk of death was increased following fractures at most skeletal sites.
Introduction
This study aims to determine long-term survival following fractures due to any cause at each skeletal site.
Methods
In a historical cohort study, 2,901 Olmsted County, MN, USA, residents ≥35 years old who experienced any fracture in 1989–1991 were followed passively for up to 22 years for death from any cause. Standardized mortality ratios (SMRs) compared observed to expected deaths.
Results
During 38,818 person-years of follow-up, 1,420 deaths were observed when 1,191 were expected (SMR, 1.2; 95 % CI, 1.1–1.3). The overall SMR was greatest soon after fracture, especially among the men, but remained elevated for over a decade thereafter. Adjusting for age and sex, relative death rates were greater for pathological fractures and less for severe trauma fractures compared to the fractures due to no more than moderate trauma. In the latter group, long-term mortality was increased following fractures at many skeletal sites. After further adjustment for precipitating cause, overall SMRs were elevated not only following fractures at the traditional major osteoporotic sites (i.e., distal forearm, proximal humerus, thoracic/lumbar vertebrae, and proximal femur) combined (SMR, 1.2; 95 % CI, 1.1–1.3) but also following all other fracture types combined (SMR 1.2; 95 % CI, 1.1–1.4), excluding the hand and foot fractures not associated with any increased mortality.
Conclusions
The persistence of increased mortality long after the occurrence of a fracture has generally been attributed to underlying comorbidity, but this needs to be defined in much greater detail if specific opportunities are to be identified for reducing the excess deaths observed.
Keywords: Cohort study, Epidemiology, Fractures, Population-based, Survival
Introduction
The likelihood of dying following a fracture is not only of obvious interest to the patient, but it is also important for assessing the societal burden of osteoporosis, as well as the potential benefits of osteoporosis prophylaxis. Since mortality is rarely attributed to the fracture on death certificates [1], most analyses have relied on survival estimates. Indeed, a large literature shows that survival is significantly impaired in the immediate aftermath of a hip fracture, especially in men, and does not recover over long-term follow-up [2]. The actual hazard of death at different points in follow-up is less certain, however, and we found previously that the relative death rate was no longer elevated compared to that expected after about 2 years in women and 7 years in men [3]. By contrast, another early study suggested that excess deaths following a spine fracture occurred later, rather than early, and that survival was unimpaired after a wrist fracture [1]. Despite the fact that most fractures in older individuals are partially attributable to osteoporosis [4], survival following many types of fracture has not been described in detail, even though fractures at skeletal sites other than the hip, spine, or wrist account for a substantial portion of all deaths following fracture [5, 6]. More information on skeletal site-specific survival would assist in generating hypotheses about the actual relation of these fractures to any subsequent deaths. The purpose of this study was to address this issue in a large cohort of adults residing in a community where fracture ascertainment is complete, including all fracture sites and causes, and where long-term follow-up is available.
Methods
The present analysis was based on long-term follow-up of a large population-based cohort of Olmsted County, MN, USA, adults with fractures [7]. Such research can be conducted here because medical care is virtually self-contained within the community, and there are relatively few providers. Most orthopedic care, for example, is provided by the Mayo Clinic, which has maintained a common medical record with its two affiliated hospitals in the community (Saint Marys and Rochester Methodist) for over 100 years. The Mayo Clinic dossier-type record thus contains both inpatient and outpatient data. The diagnoses and surgical procedures recorded in these records are indexed, including diagnoses made for outpatients seen in office or clinic consultations, emergency room visits, or nursing home care, as well as those recorded for hospital inpatients, at autopsy examination, and on death certificates. Medical records of the other providers who serve the local population, most notably the Olmsted Medical Center and its affiliated hospital, are also indexed and retrievable. Thus, details of almost all of the medical care provided to the residents of Olmsted County are available for study [8].
This unique medical records linkage system (the Rochester Epidemiology Project) was used to identify all fractures that occurred among residents ≥35 years old during the 3-year period, 1989–1991 [7], centered on the 1990 census of Olmsted County (a Metropolitan Statistical Area). Only a minority of fracture patients is hospitalized, but it was possible in our data system to identify those treated solely on an outpatient basis. The complete (inpatient and outpatient) medical records were reviewed for all local residents with any diagnosis attributable to rubrics 800 through 829 in the International Classification of Diseases [9]. Of 9,260 potential cases, record review was completed on all but 74 (0.8 %), who had not provided an authorization for review of their medical records for research [10]. The indexing system is very redundant, and we searched for fracture diagnoses made by any provider in any setting (i.e., emergency room, hospital, follow-up outpatient care, and nursing home) between 1 January 1988 through 31 December 1992, thus allowing an extra year on either side in order to identify all fractures that occurred during the study period. This same review served to exclude patients attended for complications of fractures that had actually occurred prior to the study period. Ascertainment of clinically evident fractures is believed to be complete [7]. All fractures were radiographically confirmed, but the original X-rays were not available for review. Thus, the diagnosis of vertebral fracture was accepted on the basis of a radiologist’s report of compression or collapse of one more thoracic or lumbar vertebrae. Fractures were classified according to etiology using information about each event that was recorded in the medical record: those caused by a specific pathological process (e.g., metastatic malignancy), those resulting from severe trauma (e.g., motor vehicle accident or a fall from greater than standing height), and those due to no more than moderate trauma (by convention, a fall from standing height or less).
Following additional approval by the Institutional Review Boards of Mayo Clinic and the Olmsted Medical Center, we used Rochester Epidemiology Project data resources to passively follow this inception cohort through their community medical records for all-cause mortality. The risk of death following fracture was evaluated by comparing the numbers of deaths observed to the numbers expected in this cohort during their follow-up, i.e., standardized mortality ratios (SMRs). Expected survival was based on annual Minnesota life tables. Ninety-five percent confidence intervals (95 % CI) for the SMRs were calculated assuming the expected rates are fixed and the observed deaths follow a Poisson distribution. Poisson regression was used to compare the SMRs over age, sex, and time following fracture. Adjusted SMRs for each fracture site were calculated by applying age, sex, and precipitating cause distribution case weights (based on the overall population) to the observed and expected values for each facture site. Analyses were run using R2.14 (http://www.R-project.org/).
Results
Over the 3-year study period, 1989–1991, 3,665 fractures were experienced by 2901 Olmsted County residents aged 35 years or older, 98 % of whom were white in accordance with the racial composition of the community in this age group (97 % white in 1990). Altogether, 2,362 patients (80 % of the women and 84 % of the men) experienced a single fracture during these 3 years, but 402 had two fractures, 90 had three, and 47 had four or more fractures each. This cohort was subsequently followed for up to 22 years (38,818 person-years), during which time 1,420 patients died; these 1,420 deaths exceeded the 1,191 deaths expected (SMR, 1.2; 95 % CI, 1.1–1.3). Of particular interest is the timing of these deaths following the incident fracture. This is illustrated in Fig. 1, where it is apparent that the risk of death was highest soon after fracture, especially in the men. Indeed, the 30-day SMR following the first fracture from any cause was 8.4 (95 % CI, 5.1–13) among the men compared to 3.9 (95 % CI, 2.4–6.0) among the women. Over the full duration of follow-up, however, the relative death rate was more similar for women and men (p=0.054). Moreover, adjusting for sex, the overall relative death rate did not vary significantly by age (p=0.135).
Fig. 1.
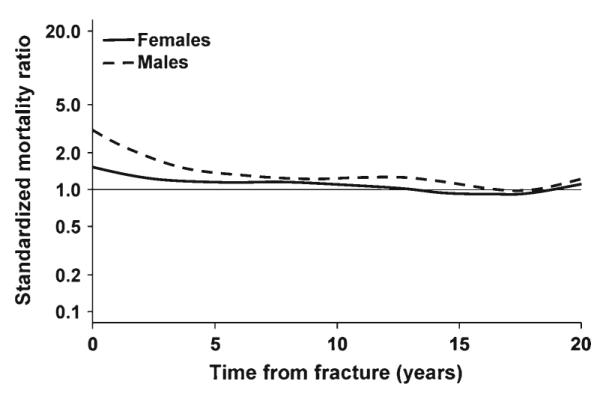
Standardized mortality ratio among 2,901 Olmsted County, MN, USA, women and men, adjusted for age, by time following any fracture in 1989–1991
After adjusting for sex and age at the time of fracture, the risk of death varied by cause of the fracture for those subjects with a documented cause (Fig. 2). Specifically, 68 fractures (2 % of the total) experienced by 30 people were due to a specific local pathological process (mostly metastatic prostate cancer, lung cancer, or multiple myeloma in the men and breast cancer or multiple myeloma in the women). Compared to expected, the patients with pathological fractures were at a 14-fold (95 % CI, 9.2–20) increased risk of death during the first 10 years of follow-up, with a 30-day SMR of 28 (95 % CI, 3.4–102). Due to the small number of pathological fractures, and the fact that some skeletal sites were not affected, we did not analyze mortality patterns for specific types of pathological fractures.
Fig. 2.
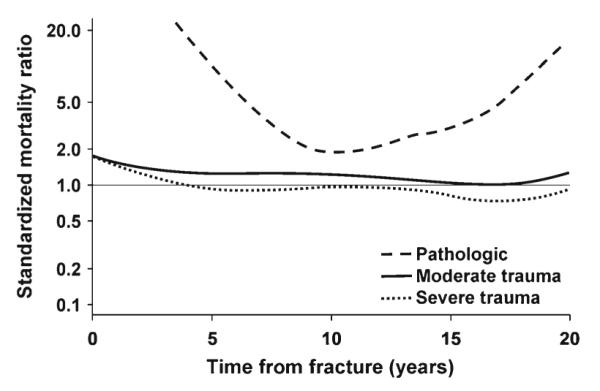
Standardized mortality ratio among 2,901 Olmsted County, MN, USA, residents following a fracture in 1989–1991, adjusted for age and sex, for fractures due to different precipitating events
By contrast, the overall risk of death in the first 10 years was no greater than expected (SMR, 1.0; 95 % CI, 0.9–1.2) among those whose fractures were due to severe trauma (Fig. 2). Altogether, 43 % of all fractures resulted from severe trauma: motor vehicle accidents in 308 (8 % of the total), falls from greater than standing height in 389 (11 %), recreational mishaps in 265 (7 %), and occupational and other injuries in 613 (17 %). Detailed mortality data by fracture site are shown in Table 1 for the severe trauma fractures. Generally, the risk of death was greater during the first year following fracture (p<0.001) compared to later time periods and, in that early interval, was greater among the men than the women (p<0.001). Indeed, the 30-day SMR was 16 (95 % CI, 7.9–28) among the men compared to 6.4 (95 % CI, 2.3–14) among the women. The relative death rate was elevated for most fracture types, although many SMRs were not statistically significant due to the relatively small number of deaths within the first year. Most site-specific SMRs were lower in the 1–5 year period following a severe trauma fracture, and few were statistically significant despite more deaths being observed in this longer time interval. None of the fracture site-specific SMRs was increased beyond 5 years after the fracture.
Table 1.
Relative (observed versus expected) death rate among Olmsted County, MN residents following a fracture due to severe trauma in 1989–1991, adjusted for age, by skeletal site, sex, and time interval after fracture
| <1 year |
1–5 years |
>5 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |||||||
| Fracture site | n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
| Skull/face | 2 | 7.4 (0.9–27) | 7 | 21 (8.3–43) | 1 | 0.7 (0.02–4.0) | 1 | 0.7 (0.02–3.7) | 4 | 0.6 (0.2–1.6) | 10 | 1.0 (0.5–1.9) |
| Hands/fingers | 2 | 1.2 (0.2–4.4) | 3 | 2.1 (0.4–6.1) | 4 | 0.7 (0.2–1.7) | 6 | 1.0 (0.4–2.1) | 14 | 0.9 (0.5–1.4) | 24 | 0.7 (0.5–1.1) |
| Distal forearm | 1 | 0.9 (0.02–5.2) | 1 | 3.5 (0.1–20) | 4 | 0.9 (0.2–2.2) | 0 | 0 (0–2.7) | 19 | 0.9 (0.5–1.4) | 6 | 0.9 (0.3–2.0) |
| Proximal humerus | 2 | 3.8 (0.5–14) | 0 | 0 (0–15) | 4 | 1.5 (0.4–4.0) | 2 | 2.8 (0.3–10) | 8 | 1.1 (0.5–2.2) | 4 | 2.4 (0.6–6.0) |
| Other arm | 2 | 4.7 (0.6–17) | 4 | 6.0 (1.6–15) | 1 | 1.2 (0.03–6.6) | 3 | 3.8 (0.8–11) | 4 | 1.0 (0.3–2.4) | 2 | 0.6 (0.1–2.0) |
| Clavicle/scapula/ sternum |
2 | 4.2 (0.5–15) | 5 | 11 (3.5–25) | 3 | 1.7 (0.4–5.0) | 2 | 1.3 (0.2–4.6) | 5 | 0.6 (0.2–1.3) | 8 | 1.0 (0.4–1.9) |
| Ribs | 2 | 1.4 (0.2–5.2) | 4 | 1.7 (0.5–4.4) | 11 | 2.7 (1.4–4.8) | 14 | 1.7 (0.9–2.9) | 10 | 0.7 (0.3–1.3) | 28 | 1.0 (0.6–1.4) |
| Vertebra | 3 | 2.0 (0.4–5.9) | 2 | 1.6 (0.2–5.6) | 6 | 1.2 (0.4–2.6) | 8 | 2.0 (0.8–3.8) | 16 | 0.9 (0.5–1.4) | 11 | 0.7 (0.3–1.2) |
| Other spine | 1 | 42 (1.1–232) | 1 | 6.8 (0.2–38) | 0 | 0 (0–31) | 1 | 1.4 (0.04–7.8) | 0 | 0 (0–3.8) | 7 | 2.3 (0.9–4.6) |
| Pelvis | 2 | 2.1 (0.2–7.6) | 2 | 14 (1.7–50) | 6 | 2.3 (0.8–5.0) | 2 | 5.2 (0.6–19) | 6 | 0.8 (0.3–1.8) | 1 | 0.5 (0.01–2.9) |
| Hip | 4 | 4.7 (1.3–12) | 3 | 5.3 (1.1–15) | 4 | 1.2 (0.3–3.0) | 2 | 1.4 (0.2–4.9) | 15 | 1.7 (0.95–2.8) | 4 | 1.1 (0.3–2.8) |
| Other leg | 5 | 2.7 (0.9–6.3) | 3 | 2.8 (0.6–8.1) | 7 | 1.0 (0.4–2.2) | 5 | 1.1 (0.3–2.5) | 33 | 0.9 (0.6–1.3) | 20 | 1.1 (0.7–1.7) |
| Feet/toes | 3 | 1.2 (0.2–3.5) | 2 | 1.1 (0.2–6.5) | 5 | 0.5 (0.2–1.1) | 2 | 0.4 (0.1–1.4) | 40 | 0.8 (0.6–1.1) | 22 | 0.9 (0.6–1.4) |
| Any severe trauma | 15 | 1.3 (0.8–2.2) | 20 | 2.3 (1.4–3.6) | 43 | 1.0 (0.7–1.4) | 37 | 1.1 (0.8–1.6) | 147 | 0.9 (0.8–1.04) | 126 | 0.9 (0.7–1.1) |
Note that 146 fractures of uncertain cause (experienced by 134 people) were excluded from this analysis
Number of deaths in the interval
Standardized mortality ratio (SMR), adjusted for age, and 95 % confidence interval (95 % CI). Statistically significant associations are italicized
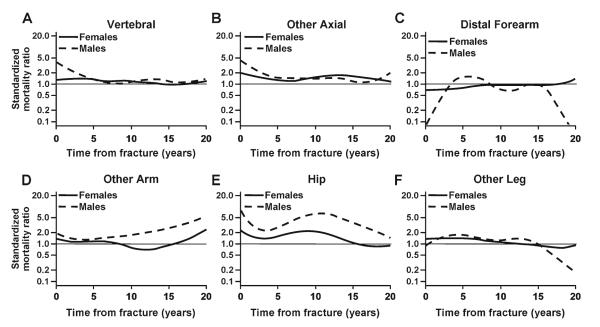
Since increased mortality following pathological fractures and those due to severe trauma might be expected, the remainder of the analysis focused on the fractures due to no more than moderate trauma, where the relative death rate remained elevated over most of the follow-up period (Fig. 2). Altogether, 1,876 fractures (51 % of the total) were attributed to minimal or moderate trauma, including 448 fractures where no specific traumatic event was recognized (e.g., fractures that occurred in the course of daily activities and those found incidentally); the latter accounted for the majority (56 %) of fractures of thoracic/lumbar vertebrae. Falls from a standing height or less were responsible for 1,428 cases, representing 67 % of upper limb fractures, 57 % of lower limb fractures, and 39 % of fractures overall. The pattern of deaths following the fractures due to no more than moderate trauma is delineated in Fig. 3 by fracture type (note that some skeletal sites were collapsed into larger groups for this purpose). Thus, adjusting for age, the elevated risk of death following a moderate trauma vertebral fracture remained elevated in both women and men throughout follow-up (Fig. 3a), as did the relative death rate following other fractures of the axial skeleton (Fig. 3b). No overall increased risk of death was seen following a distal forearm fracture (Fig. 3c), whereas a greater risk of death was seen in men compared to women following other fractures of the upper limb (Fig. 3d), excluding hand and finger fractures. Likewise, relative death rates were greater following a hip fracture among the men (Fig. 3e), but sex-specific estimates were more comparable following other lower limb fractures (Fig. 3f), excluding foot and toe fractures.
Fig. 3.
Standardized mortality ratio among 2901 Olmsted County, MN, USA, residents following a fracture due to no more than moderate trauma in 1989–1991, adjusted for age, by fracture site, and sex
Detailed data for the specific fractures attributed to moderate trauma are delineated in Table 2. The relative risk of death within the first year was greater among men than women with a moderate trauma fracture (p=0.002), with a 30-day SMR of 5.9 (95 % CI, 2.7–11) among the men compared to 3.9 (95 % CI, 2.3–6.2) among the women. However, the male disadvantage declined in the 1–5-year follow-up period (p=0.040) and disappeared after 5 years (p=0.271). Moreover, unlike those due to severe trauma, SMRs remained elevated throughout follow-up for many types of fractures. Since moderate trauma fractures were more common, more deaths were observed and more of these SMRs were statistically significantly increased.
Table 2.
Relative (observed versus expected) death rate among Olmsted County, MN residents following a fracture due to no more than moderate trauma in 1989–1991, adjusted for age, by skeletal site, sex and time-interval after fracture
| <1 year |
1-5 years |
>5 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |||||||
| Fracture site | n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
n a | SMR (95 % CI)b |
| Skull/face | 1 | 1.6 (0.04–8.8) | 5 | 11 (3.7–26) | 3 | 1.2 (0.2–3.5) | 3 | 2.7 (0.6–7.8) | 4 | 0.5 (0.1–1.3) | 2 | 1.6 (0.2–5.8) |
| Hands/fingers | 0 | 0 (0–2.7) | 1 | 0.8 (0.02–4.7) | 4 | 0.7 (0.2–1.7) | 4 | 1.2 (0.3–3.0) | 27 | 1.0 (0.7–1.5) | 8 | 1.2 (0.5–2.3) |
| Distal forearm | 5 | 0.7 (0.2–1.7) | 0 | 0 (0–5.9) | 23 | 0.8 (0.5–1.2) | 3 | 1.4 (0.3–4.1) | 105 | 1.1 (0.9–1.3) | 5 | 0.6 (0.2–1.4) |
| Proximal humerus | 4 | 1.3 (0.4–3.3) | 3 | 4.1 (0.8–12) | 15 | 1.3 (0.7–2.1) | 3 | 1.1 (0.2–3.2) | 36 | 1.6 (1.1–2.2) | 15 | 3.0 (1.7–5.0) |
| Other arm | 4 | 2.0 (0.6–5.2) | 0 | 0 (0–6.6) | 5 | 0.6 (0.2–1.3) | 4 | 1.6 (0.4–4.0) | 25 | 0.8 (0.6–1.2) | 7 | 2.1 (0.8–4.3) |
| Clavicle/scapula/ sternum |
1 | 1.1 (0.03–6.2) | 5 | 5.1 (1.6–12) | 7 | 2.1 (0.8–4.3) | 4 | 2.2 (0.6–5.5) | 10 | 2.6 (1.2–4.8) | 5 | 4.9 (1.6–11) |
| Ribs | 11 | 1.6 (0.8–2.9) | 6 | 1.7 (0.6–3.7) | 34 | 1.7 (1.2–2.4) | 16 | 1.4 (0.8–2.3) | 42 | 1.5 (1.1–2.0) | 33 | 1.1 (0.8–1.6) |
| Vertebra | 23 | 1.3 (0.8–2.0) | 14 | 2.9 (1.6–4.8) | 99 | 1.7 (1.4–2.0) | 24 | 1.7 (1.1–2.6) | 126 | 1.2 (0.96–1.4) | 26 | 1.0 (0.7–1.5) |
| Other spine | 2 | 18 (2.2–65) | 1 | 4.8 (0.1–27) | – | No cases | 1 | 9.2 (0.2–51) | – | No cases | 1 | 14 (0.4–77) |
| Pelvis | 10 | 2.0 (0.98–3.8) | 4 | 5.1 (1.4–13) | 24 | 1.4 (0.9–2.1) | 3 | 1.5 (0.3–4.4) | 35 | 1.4 (0.95–1.9) | 3 | 0.9 (0.2–2.7) |
| Hip | 28 | 1.9 (1.2–2.7) | 14 | 5.5 (3.0–9.3) | 60 | 1.2 (0.9–1.5) | 13 | 2.0 (1.05–3.4) | 79 | 1.5 (1.2–1.9) | 12 | 2.9 (1.5–5.0) |
| Other leg | 8 | 1.5 (0.6–2.9) | 2 | 0.8 (0.1–2.8) | 27 | 1.5 (1.01–2.2) | 14 | 1.9 (1.05–3.2) | 57 | 1.1 (0.9–1.5) | 17 | 1.0 (0.6–1.6) |
| Feet/toes | 1 | 0.7 (0.02–3.8) | 1 | 6.5 (0.2–36) | 4 | 0.6 (0.2–1.5) | 0 | 0 (0–5.0) | 23 | 0.9 (0.6–1.4) | 6 | 2.4 (0.9–5.2) |
| Any moderate trauma | 75 | 1.5 (1.2–1.8) | 44 | 2.7 (2.0–3.6) | 223 | 1.2 (1.05–1.4) | 77 | 1.6 (1.2–2.0) | 465 | 1.2 (1.1–1.3) | 130 | 1.3 (1.1–1.5) |
Note that 146 fractures of uncertain cause (experienced by 134 people) were excluded from this analysis
Number of deaths in the interval
Standardized mortality ratio (SMR), adjusted for age, and 95 % confidence interval (95 % CI). Statistically significant associations are italicized
Even after adjusting for age, sex, and precipitating cause (i.e., specific pathology, severe trauma, no more than moderate trauma), overall SMRs were elevated for most types of fractures (Table 3). Indeed, the relative risk of death following fractures at the traditional major osteoporotic sites (i.e., distal forearm, proximal humerus, vertebra, proximal femur combined) was the same (SMR, 1.2; 95 % CI, 1.1–1.3) as that for all other fracture types combined (SMR, 1.2; 95 % CI, 1.1–1.4), excluding the hand and foot fractures that were not associated with any increased mortality.
Table 3.
Relative (observed versus expected) death rate among Olmsted County, MN residents following a fracture in 1989–1991, adjusted for age, sex, and precipitating cause, by skeletal site
| Fracture sitea | n a | SMR (95 % CI)b |
|---|---|---|
| Skull/face | 43 | 1.2 (0.8–1.6) |
| Hand/fingers | 97 | 0.8 (0.6–1.03) |
| Distal forearm | 170 | 0.9 (0.8–1.1) |
| Proximal humerus | 98 | 1.5 (1.1–1.8) |
| Other arm | 63 | 0.9 (0.6–1.1) |
| Clavicle/scapula/sternum | 60 | 1.7 (1.2–2.2) |
| Ribs | 222 | 1.4 (1.2–1.7) |
| Vertebra | 371 | 1.3 (1.1–1.4) |
| Other spine | 18 | 2.9 (1.3–4.4) |
| Pelvis | 100 | 1.4 (1.1–1.7) |
| Hip | 243 | 1.5 (1.3–1.7) |
| Other leg | 201 | 1.2 (0.98–1.4) |
| Feet/toes | 107 | 0.8 (0.6–0.96) |
| Any | 1370 | 1.1 (1.1–1.2) |
Note that 146 fractures of uncertain cause (experienced by 134 people) were excluded from this analysis
Number of deaths
Standardized mortality ratio (SMR) and 95 % confidence interval (95 % CI). Statistically significant associations are italicized
Discussion
Wide variation by sex, age, and precipitating cause among fractures at different skeletal sites has made it difficult to summarize the overall risk of subsequent mortality. In this population-based study, we were able to adjust for the underlying increased risk of death with age, for the greater likelihood of dying following fractures among men compared to women and for the fact that survival was worse following pathological fractures and better following severe trauma fractures compared with fractures due to no more than moderate trauma. The result is an estimated 10 % overall increase in the relative risk of all-cause mortality following any fracture among unselected adults in the general population. As expected, most of the major osteoporotic fracture sites were associated with increases in long-term mortality, but the relative death rate was just as great following all other types of fracture (excluding hands and feet) combined. The latter observation suggests that more attention should be paid to potential contributing causes (e.g., secondary osteoporosis) of these other fractures.
As also expected, the increased mortality was most evident in the first year following fracture, especially for fractures attributed to severe trauma where the 30-day SMR was 10 (95 % CI, 6.0–17). No elevation in overall mortality was evident in this group beyond the first year of follow-up. However, 65 % of all excess deaths observed in the cohort as a whole occurred more than a year following fracture, and these later deaths are less likely due to the fracture per se. Instead, they have often been attributed to underlying comorbidity [11]. In the case of the pathological fractures, the association with comorbidity, in this case malignancy, may be an indirect one where fracture is a manifestation of advanced, metastatic disease that ultimately proves fatal. By contrast, no comparably tidy explanation is evident for the moderate trauma fractures, where the contribution of comorbidity to pathogenesis may vary by fracture type. This requires in-depth study in order to identify the particular comorbidity clusters that are associated with specific fracture events. Such information might then provide the insights needed to design targeted interventions to reduce excess deaths.
Our results for hip fractures, specifically, are consistent with most previous reports insofar as mortality risk was greater among men than women and was highest in the year following fracture [2]. Although early deaths have been attributed to physiological stresses of the injury itself or to various complications [11], we showed previously that mortality was not elevated among men with a hip fracture but no significant comorbidity and increased with the comorbidity score [12]. In this context, comorbidity has generally been linked to frailty and an increased risk of falling, which dominates low bone density with respect to hip fracture pathogenesis [13]. Such frailty-related comorbidity presumably accounts for the continued increased risk of death long after hip fracture [14], and adjustment for comorbidity eliminated this increase in one study [15]. In our investigation, the SMR remained elevated for up to 15 years among the women after adjusting for age, sex, and etiology, which is consistent with other results [2]. However, the overall SMR following hip fracture was 1.5, which was not much greater than that observed for several other types of fractures (e.g., ribs, pelvis) that have received less attention in the literature.
Comorbid conditions have also been implicated in the elevated death rate following vertebral fracture [11], as observed in this and most other studies [1, 6, 16–24]. Compared to hip fractures, however, falls are less often the precipitating event for spine fractures [25]; the distribution of comorbidities varies correspondingly, with more emphasis on metabolic diseases and corticosteroid use that are commonly associated with low bone density [26]. Although low bone density itself is independently associated with reduced survival [27], the presence of a vertebral fracture further exacerbates the risk of death [28], which may persist even in the absence of serious comorbid conditions [23].
By contrast, most studies have found no elevated risk of death following a distal forearm fracture [1, 17, 20, 29, 30], consistent with our results. This has generally been attributed to the fact that such fractures often occur among relatively healthy middle-aged individuals who fall while walking [4]. In a much older population of patients (mean age, 77 years) treated in a hospital system, survival 7 years after a distal forearm fracture was only 80 % of that expected, although the increased likelihood of death was confined to those with significant comorbidities [31].
With respect to the other fracture sites, some studies have lumped many skeletal sites together and found that overall survival was impaired [5, 6, 24, 29, 32], as did we in the present study. However, our more detailed analyses showed that the increased risk of mortality was most closely associated with fractures of the axial skeleton (clavicle/scapula/sternum, ribs, thoracic/lumbar vertebrae, other spine, pelvis), along with proximal humerus fractures, but not fractures of the hands/fingers, upper arm (excluding the proximal humerus), or the feet/toes. Slight increases in the overall SMR for skull/face fractures and fractures of the leg (excluding the hip) were not statistically significant. Others have found decreased survival following fractures of the proximal humerus [20, 24, 29, 30, 32, 33], ribs [29], pelvis [33, 34], and sites in the leg excluding the hip [33]. As seen here, no excess deaths have been observed following fractures of the foot and ankle [29]. The role that underlying comorbidity might play in any long-term mortality associated with these other fractures is unclear.
This investigation had a number of strengths, including the use of a population-based cohort comprised of all community residents age 35 years or over who experienced any fracture (not just osteoporotic fractures) from any cause (not just moderate trauma) in the 3-year period 1989–1991 [7]. These unselected subjects were then followed passively from the time of the first fracture of each type during the study period, though not necessarily the first-ever lifetime fracture of that type, for up to 22 years through the medical records linkage system of the Rochester Epidemiology Project, which provides access to the records of essentially all providers of medical care to local residents [8]. Consequently, except for fractures which never come to clinical attention (e.g., some fractures of the vertebrae and ribs), fracture ascertainment should be complete, and the denominator population is well characterized. Because of the demographic makeup of the community, however, there were only a small number of fractures among nonwhites, and any resulting estimates for them would be unstable. However, the majority of age-related fractures in this country occur in the white population, and hip fracture incidence rates from this community are comparable to estimates of hip fracture incidence for US whites generally [35].
Conclusions
Most of the outcome-related literature has focused on clinical management of fractures, where short-term morbidity and case fatality are the primary endpoints. This study, as expected, also found increased mortality in the year following most types of fracture, especially those due to severe trauma. However, the customary emphasis on immediate outcomes of fracture care is called into question by the fact that the majority of the excess deaths occurred after the first year. Indeed, the relative risk of death remained elevated long after many of the fractures that were attributed to no more than moderate trauma. Although it is generally assumed that these late deaths are related to underlying comorbidity of some sort, this needs to be tested more explicitly. In order to design possible interventions to improve survival, it is important to identify the specific comorbid conditions that relate to the different types of fracture and also to test whether these conditions vary by age or sex.
Acknowledgments
This work was supported by research grant P01 AG04875 and made possible by the Rochester Epidemiology Project (R01 AG034676) from the National Institute on Aging, US Public Health Service. The authors wish to thank Mrs. Judy Bruen and Mrs. Leona Bellrichard for data collection and Mrs. Mary Roberts for assistance in preparing the manuscript.
Footnotes
Conflict of interest None.
Contributor Information
L. J. Melton, III, Division of Epidemiology, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, USA.
S. J. Achenbach, Biomedical Statistics and Informatics, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA
E. J. Atkinson, Biomedical Statistics and Informatics, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA
T. M. Therneau, Biomedical Statistics and Informatics, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, MN, USA
S. Amin, Division of Rheumatology, Department of Internal Medicine, College of Medicine, College of Medicine, Mayo Clinic, Rochester, MN, USA
References
- 1.Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137:1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 2.Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Therneau TM, Larson DR. Long-term trends in hip fracture prevalence: the influence of hip fracture incidence and survival. Osteoporos Int. 1998;8:68–74. doi: 10.1007/s001980050050. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Melton LJ., 3rd Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 6.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 7.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.International Classification of Diseases 9th revision, clinical modification. vol 1. Commission on Professional and Hospital Activities; Ann Arbor: 1978. ICD-9-CM. [Google Scholar]
- 10.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997;337:1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 11.Teng GG, Curtis JR, Saag KG. Mortality and osteoporotic fractures: is the link causal, and is it modifiable? Clin Exp Rheumatol. 2008;26:S125–S137. [PMC free article] [PubMed] [Google Scholar]
- 12.Poór G, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Determinants of reduced survival following hip fractures in men. Clin Orthop. 1995;319:260–265. [PubMed] [Google Scholar]
- 13.Järvinen TLM, Sievänen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336:124–126. doi: 10.1136/bmj.39428.470752.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32:468–473. doi: 10.1016/s8756-3282(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 15.Tosteson AN, Gottlieb DJ, Radley DC, Fisher ES, Melton LJ., 3rd Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18:1463–1472. doi: 10.1007/s00198-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail AA, O’Neill TW, Cooper C, Finn JD, Bhalla AK, Cannata JB, Delmas P, Falch JA, Felsch B, Hoszowski K, Johnell O, Diaz-Lopez JB, Lopez Vaz A, Marchand F, Raspe H, Reid DM, Todd C, Weber K, Woolf A, Reeve J, Silman AJ. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 1998;8:291–297. doi: 10.1007/s001980050067. [DOI] [PubMed] [Google Scholar]
- 17.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 18.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 19.Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Rodriguez-Garcia M, Cannata-Andia JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14:520–524. doi: 10.1007/s00198-003-1405-4. [DOI] [PubMed] [Google Scholar]
- 20.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15:38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]
- 21.Hasserius R, Karlsson MK, Jonsson B, Redlund-Johnell I, Johnell O. Long-term morbidity and mortality after a clinically diagnosed vertebral fracture in the elderly—a 12- and 22-year follow-up of 257 patients. Calcif Tissue Int. 2005;76:235–242. doi: 10.1007/s00223-004-2222-2. [DOI] [PubMed] [Google Scholar]
- 22.Trone DW, Kritz-Silverstein D, von Muhlen DG, Wingard DL, Barrett-Connor E. Is radiographic vertebral fracture a risk factor for mortality? Am J Epidemiol. 2007;166:1191–1197. doi: 10.1093/aje/kwm206. [DOI] [PubMed] [Google Scholar]
- 23.Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90:1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 24.Morin S, Lix LM, Azimaee M, Metge C, Caetano P, Leslie WD. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int. 2011;22:2439–2448. doi: 10.1007/s00198-010-1480-2. [DOI] [PubMed] [Google Scholar]
- 25.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 26.Lowe H, Shane E. Osteoporosis associated with illnesses and medications, Chapter 52. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3rd edn Elsevier; Amsterdam: 2008. pp. 1283–1305. [Google Scholar]
- 27.Mussolino ME, Gillum RF. Low bone mineral density and mortality in men and women: the Third National Health and Nutrition Examination Survey linked mortality file. Ann Epidemiol. 2008;18:847–850. doi: 10.1016/j.annepidem.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, Santora AC, 2nd, Black DM. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 29.Browner WS, Pressman AR, Nevitt MC, Cummings SR. Mortality following fractures in older women. The Study of Osteoporotic Fractures. Arch Intern Med. 1996;156:1521–1525. [PubMed] [Google Scholar]
- 30.Shortt NL, Robinson CM. Mortality after low-energy fractures in patients aged at least 45 years old. J Orthop Trauma. 2005;19:396–400. doi: 10.1097/01.bot.0000155311.04886.7e. [DOI] [PubMed] [Google Scholar]
- 31.Rozental TD, Branas CC, Bozentka DJ, Beredjiklian PK. Survival among elderly patients after fractures of the distal radius. J Hand Surg Am. 2002;27:948–952. doi: 10.1053/jhsu.2002.36995. [DOI] [PubMed] [Google Scholar]
- 32.Piirtola M, Vahlberg T, Lopponen M, Raiha I, Isoaho R, Kivela SL. Fractures as predictors of excess mortality in the aged-a population-based study with a 12-year follow-up. Eur J Epidemiol. 2008;23:747–755. doi: 10.1007/s10654-008-9289-4. [DOI] [PubMed] [Google Scholar]
- 33.Barrett JA, Baron JA, Beach ML. Mortality and pulmonary embolism after fracture in the elderly. Osteoporos Int. 2003;14:889–894. doi: 10.1007/s00198-003-1494-0. [DOI] [PubMed] [Google Scholar]
- 34.Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83:1141–1144. doi: 10.1302/0301-620x.83b8.11709. [DOI] [PubMed] [Google Scholar]
- 35.Melton LJ, 3rd, Kearns AE, Atkinson EJ, Bolander ME, Achenbach SJ, Huddleston JM, Therneau TM, Leibson CL. Secular trends in hip fracture incidence and recurrence. Osteoporos Int. 2009;20:687–694. doi: 10.1007/s00198-008-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]