Abstract
Purpose
Mammographic breast density (MBD) is decreased by tamoxifen, but the effect of aromatase inhibitors (AI) is less clear.
Experimental Design
We enrolled early stage postmenopausal breast cancer patients initiating adjuvant AI therapy and ascertained mammograms before and at an average 10 months of AI therapy. We matched cases to healthy postmenopausal women (controls) from a large mammography screening cohort on age, baseline body mass index, baseline MBD and interval between mammograms. We estimated change in MBD using a computer-assisted thresholding program (Cumulus) and compared differences between cases and matched controls.
Results
In predominantly white women (96%), we found 14% of the 387 eligible cases had a MBD reduction of at least 5% after an average of 10 months of AI therapy. MBD reductions were associated with higher baseline MBD, AI use for more than 12 months and prior postmenopausal hormone use. Comparing each case to her matched control, there was no evidence of an association of change in MBD with AI therapy (median case-control difference among 369 pairs was −0.1% (10th and 90th percentile: −5.9%, 5.2%) p=0.51). Case-control differences were similar by type of AI (p’s 0.41 and 0.56); prior use of postmenopausal hormones (p=0.85); baseline MBD (p=0.55); or length of AI therapy (p=0.08).
Conclusions
In postmenopausal women treated with AIs, 14% of cases had a MBD reduction of >5%, but these decreases did not differ from matched controls. These data suggest that MBD is not a clinically useful biomarker for predicting the value of AI therapy in white postmenopausal women.
Keywords: aromatase inhibitors, mammographic density, breast density, biomarker
Introduction
Breast density, a mammographic reflection of the fat, epithelial and stromal composition of the breast has been shown to be a promising marker for breast cancer risk in healthy women, with a higher percentage of breast density (greater than 50%) associated with a 3–5 fold increase in risk of breast cancer compared to lower levels (1). High mammographic breast density or MBD at time of breast cancer diagnosis is also associated with increased risk of a local cancer recurrence (2, 3), and, among women with ductal carcinoma in situ, with an increased risk of a second breast cancer (4, 5).
Importantly, breast density appears modifiable. MBD has consistently been shown to increase with use of estrogen plus progestin menopausal therapy (6–8) and to decrease with exposure to tamoxifen (TAM) (9–12). However, these changes in MBD do not occur in all women, suggesting that the variability could reflect differential response to these therapies. In fact, women at high-risk for breast cancer within the IBIS-1 study who experienced at least 10% reduction in MBD while on TAM had a reduced risk of breast cancer (RR=0.51) compared to women who had no change in their densities (13); women on placebo who experienced similar reductions in MBD, however, did not have a decreased risk of breast cancer. A recent study in Korean women confirmed these findings among women in the adjuvant setting; a greater reduction in MBD with an average 13 months of TAM or aromatase inhibitor therapy was associated with recurrence-free survival. Compared to those experiencing the largest reduction in MBD (>10%), there were 1.33, 1.92 and 2.25 increased risks of recurrence with 5–10%, 0–5% and <0% reductions in MBD, respectively (14). These data suggest that change in MBD may be useful as a surrogate marker to identify women who may or may not benefit from certain endocrine therapies.
Aromatase inhibitors (AIs) are a class of pharmaceutical agents established as adjuvant therapy for postmenopausal women with early stage estrogen receptor (ER) and/progesterone receptor positive breast cancer (15). These agents are also beneficial in the prevention setting in terms of decreasing the incidence of primary breast cancers (16). AIs block the conversion of androstenedioneto estrone (E1) and testosterone to estradiol (E2) by cytochrome P450 (CYP) 19, aromatase, and have been shown to profoundly decrease levels of E1 and E2 in both serum and breast tissue (17–20). Our group recently demonstrated that overall aromatase expression was increased in tissue cores taken from dense regions compared to non-dense regions of the breasts of healthy women (21). This would suggest that MBD is influenced by aromatase expression and local estrogen synthesis, and potentially could be used as a biomarker to assess the impact of AI therapy.
If aromatase expression is increased in mammographically dense tissue, we hypothesize aromatase inhibitors (AIs) will decrease overall MBD. The studies to date examining the influence of AIs on MBD are mixed (14, 22–28), with the majority finding no association (Table 1); however most of these studies had small cohorts of patients. The largest study to date of 175 women on AI therapy by Kim et al. showed small reductions in MBD with AI use (average 3% at one year of therapy); however, their study was not able to evaluate the influence of body mass index, had a high proportion of prior chemotherapy use, a younger population (median age 49), and did not consider type of adjuvant aromatase inhibitor therapy (14).
Table 1.
Studies of aromatase inhibitors and mammographic breast density in postmenopausal women
| Study | Population | Eligibility | Intervention | N | Mean Absolute Change | Average* Duration of therapy |
|---|---|---|---|---|---|---|
|
| ||||||
| Vachon, 2007 (22) | Postmenopausal cases from NCIC MA17 | Prior TAM use | Letrozole Placebo |
35 33 |
−0.3 −1.0 (p=0.58) |
12 months |
|
| ||||||
| Fabian, 2007 (23) | Postmenopausal women | Taking hormone replacement therapy | Letrozole | 42 | 0.40 | 6 months |
|
| ||||||
| Mousa, 2008 (27) | High risk postmenopausal women | Taking hormone replacement therapy | Letrozole None** |
18 22 |
−6.8 −1.4 (p=0.04) |
Median 24 months |
|
| ||||||
| Cigler, 2010 (24) | Postmenopausal cases and non-cases | > 25% density | Letrozole Placebo |
30 19 |
−1.74 −0.24 (p=0.61) |
12 months |
| 27 16 |
−0.01 −1.32 (p=0.61) |
24 months | ||||
|
| ||||||
| Cigler, 2011 (25) | Postmenopausal women | Any visible density | Exemestane Placebo |
36 34 |
−1.3 0.22 (p=0.59) |
6 months |
| 34 31 |
0.56 0.58 (p=0.96) |
12 months | ||||
| 24 19 |
−0.17 −2.93 (p=0.52) |
24 months | ||||
|
| ||||||
| Prowell, 2011 (26) | Postmenopausal cases | DCIS or Stage I–III cases | Anastrozole | 50 | +2% (p=0.87)*** | 6 months |
| < 6 months prior local therapy | 43 | −16% (p=0.08)*** | 12 months | |||
|
| ||||||
| Smith, 2012 (28) | Postmenopausal women | High risk | Letrozole | 16 | 8/16 had reduction | 6 months |
| 16 | 11/16 had reduction | 12 months | ||||
|
| ||||||
| Kim, 2012 (14) | Premenopausal and Postmenopausal cases in Korea | ER positive breast cancer cases receiving at least 2 years endocrine therapy | Any aromatase inhibitor | 175 | −3.1+6.3% | 13 months |
| Tamoxifen | 890 | −6.5+7.1% | ||||
|
| ||||||
| Current study | Postmenopausal breast cancer cases matched to healthy controls | Cases from AI trials | Exemestane or Anastrozole | 369 | −1.3 | 10 months |
| Controls from screening cohort | Controls | 369 | −1.1 (p=0.73) | |||
Average unless otherwise specified.
Study was retrospective in nature and identified a group of women on combination hormone therapy who were not on letrozole therapy.
Percent change= (100*(1-year percent density − baseline percent density)) instead of absolute change.
In this report, we present data from the largest study to date to examine the influence of AIs on MBD in postmenopausal women. We examine changes in MBD among women on three clinical trials of early stage breast cancer patients treated with an AI, evaluate factors associated with MBD changes, and compare them to MBD changes in an age-matched cohort of women undergoing routine mammography screening. We also examined whether our findings differ by the two classes of AI therapy, steroidal vs. non-steroidal AIs.
Methods
Women with early stage postmenopausal breast cancer enrolled onto NCIC Clinical Trials Group (NCIC CTG) study MA.27, North Central Cancer Treatment Group (NCCTG) N063I or Mayo Clinic (MC) MC0532 who provided consent for mammogram retrieval were eligible for this study. All women were receiving anastrozole (1 mg orally once daily) or exemestane (25 mg orally once daily) in the adjuvant setting. Eligibility was determined following mammogram retrieval. A woman was considered to be ineligible for this study if she had AI therapy for less than six months, bilateral breast cancer, breast implantation or surgery in non-cancerous breast; lacked a mammogram taken within 12 months of the start of AI; or lacked a mammogram taken after at least 6 months of AI use that was the same image format (film versus digital) as the pre-AI mammogram. This last criterion was put in place as percent density has been shown to differ with image formats (29). If multiple mammograms were available after the start of AI, the mammogram closest to the day of one year of AI use and was the same image format as the pre-AI mammogram (baseline mammogram) was used. This image is referred to here as the year one mammogram.
Information on prior chemotherapy, prior postmenopausal hormone therapy use (estrogen alone or combination estrogen and progesterone therapy), baseline weight and weight gain was available from self-reported questionnaires or in- person interviews. Knowledge of the blinded treatment arm (either anastrozole or exemestane) was also made available for patients enrolled to NCIC CTG MA27.
Because MBD naturally declines in postmenopausal women as they age, it was essential to have a comparison population closely matched to the cases initiating AI therapy. Controls were drawn from participants in the Mayo Mammography Health Study (MMHS), a cohort of women having routine screening mammography at the Mayo Clinic in Rochester, MN, who had at least one screening mammogram prior to their enrollment mammogram (30). The MMHS enrolled over 50% of the female residents of Minnesota, Wisconsin, and Iowa having screening mammography between October, 2003 and September, 2006 at the Mayo Clinic, who were over the age of 35, and had no prior history of breast cancer. To be considered as a potential control, a women had to have had a screening mammogram at least 12 months prior to enrollment in the MMHS (baseline mammogram) that was the same format as the mammograms taken at the time of enrollment into MMHS (the one year mammogram). Matching was done using a greedy algorithm providing the closest match available to the case. For each case, a control was chosen whose age at her baseline mammogram was within 5 years of the age of the case at her baseline mammogram; body mass index category was the same as the case (underweight/normal vs. overweight/obese); interval between baseline and one-year mammograms was within 120 day of that of the case; baseline mammographic density was within 10% quantitative MBD of the case (31).
Mammogram collection and density estimation
All baseline (pre-AI) and one-year mammograms were digitized using an Array Digitizer with 12 bit grayscale depth. The pixel size was 0.130 × 0.130 mm2 for both the 18 × 24 cm2 and 24 × 30 cm2 films. The craniocaudal (CC) views of the noncancerous breast (or corresponding breast side in controls) at both time points were digitized on each woman and all mammograms were scrubbed of patient identifiers. Batch files were created so that baseline and year one images could be displayed simultaneously on side-by-side screens for assessment. The baseline and one-year images were randomly assigned to left or right sided screen. A 5% repeat set of images was included within each batch file for assessment of reliability.
Percent breast density (dense area divided by total area X 100) was estimated using a computer-assisted thresholding program that has routinely been used in several studies of breast density, including our own (32–35). Percent density was assessed on batch files consisting of paired mammograms from each case and their matched control over a four month period by one evaluator (FFW) trained by Norman F. Boyd, MD (University of Toronto, Canada), an acknowledged leader in the field of MBD. Our evaluator has over ten years’ experience in density estimation, consistently demonstrates associations with breast cancer using this density measure (34, 36, 37) and has shown high intraclass correlation with Dr. Boyd on over 600 images (data not shown).
Briefly, two thresholds are set by a trained programmer; one separates the breast from the background and the second separates dense from non-dense tissue. Both images were viewed simultaneously and assessed for percent density, allowing the evaluator to flag pairs for which adequate comparisons could not be made (i.e. how breast capture at two time points were substantially different). To assess the intra-reader reliability in terms of determining change in percent density, the evaluator read pre and post-treatment images from 46 women at two different sessions. Bland Altman plots were constructed to compare estimates of change at these two sessions. The difference in the change in breast density between the first and second sessions was within 5% for all, but 3 of the 46 women (mean 6.5%: 95%CI: 0.0%–13.7%)
Statistical analysis
We first examined all cases on AI therapy to assess what factors are associated with the increased likelihood of a 5% or more reduction in percent MBD (event) using multivariate logistic regression.
We next performed analyses to examine whether there were differences in the one-year change in density between the women on AI therapy and their matched controls. A paired t-test was used to assess whether the difference in the change in MBD over one year between the case and her matched control was significantly different than zero for the following patient cohorts: (1) all the matched pairs; (2) the matched pairs where both the case and her control had a baseline mammographic density of at least 10%; (3) the matched pairs on exemestane; (4) the matched pairs on anastrozole; (5) matched pairs where the case was on AI therapy for at least 12 months and (6) matched pairs who had the same status of prior postmenopausal hormone therapy (HT) use (either both never or both ever). SAS 9.2 (Cary, NC) was used for all statistical analyses.
Results
A total of 574 postmenopausal breast cancer patients starting adjuvant AI therapy were consented to participate in this study, where 505 patients (88.0%) were enrolled on the NCIC CTG MA27; 57 (9.9%) on MC0532 and 12 (2.1%) on NCCTG N063I. After mammogram retrieval process, 187 women were found to be ineligible due to: lacking a mammogram taken at least 6 months following start of AI (89 pts.) or at most 12 months prior to starting an AI (2 pts.); discontinuing AI treatment prior to a mammogram being taken (17 pts.); and having mammograms in different formats (digital vs. film) at the two time periods (79 pts.). A total of 387 cases were eligible and used in case-only analyses. However, controls could not be matched for 18 of these women, thus, a total of 369 matched pairs from MA27 (n=342), NCCTG N063I (n=7) and MC0532 (n=20) were used for case-control comparisons (Table 2).
Table 2.
Characteristics of cases on AI therapy and matched healthy controls across the three studies
| Descriptive Factor | MA.27 (n=342 matches) | N063I (n=7 matches) | MC0532 (n=20 matches) | Overall (n=369 matches) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Case n (%) | Control n (%) | Case n (%) | Control n (%) | Case n (%) | Control n (%) | Case n (%) | Control n (%) | |
|
| ||||||||
| Baseline BMI | ||||||||
| Normal/Underweight | 82 (24%) | 82 (24%) | 1 (14%) | 1 (14%) | 4 (20%) | 4 (20%) | 87 (24%) | 87 (24%) |
| Overweight/Obese | 260 (76%) | 260 (76%) | 6 (86%) | 6 (86%) | 16 (80%) | 16 (80%) | 282 (76%) | 282 (76%) |
|
| ||||||||
| Year 1 BMI | ||||||||
| Normal/Underweight | 68 (21%) | 88 (26%) | 2 (29%) | 1 (14%) | 2 (13%) | 4 (20%) | 72 (20%) | 93 (25%) |
| Overweight/Obese | 262 (79%) | 253 (74%) | 5 (71%) | 6 (86%) | 13 (87%) | 16 (80%) | 280 (80%) | 275 (75%) |
| No Data | 12 | 1 | 0 | 0 | 5 | 0 | 17 | 1 |
|
| ||||||||
| Age At Baseline | ||||||||
| Range | 45–93 | 41–91 | 58–79 | 54–79 | 47–83 | 49–79 | 45–93 | 41–91 |
| Median | 63 | 63 | 75 | 74 | 61 | 61.5 | 63 | 63 |
|
| ||||||||
| Prior HRT Use | ||||||||
| Ever | 179 (52%) | 178 (52%) | 3 (43%) | 5 (71%) | 13 (65%) | 11 (55%) | 195 (53%) | 194 (53%) |
| Never | 163 (48%) | 164 (48%) | 4 (57%) | 2 (29%) | 7 (35%) | 9 (45%) | 174 (47%) | 175 (47%) |
|
| ||||||||
| Prior Chemotherapy | ||||||||
| Yes | 113 (33%) | NA | 0 | NA | 3 (15%) | NA | 116 (31%) | NA |
| No | 229 (77%) | 7 (100%) | 17(85%) | 253 (69%) | ||||
|
| ||||||||
| Treatment Arm | ||||||||
| Exemestane | 178 (52%) | NA | NA | NA | NA | NA | NA | NA |
| Anastrozole | 164 (48%) | NA | NA | NA | NA | NA | NA | NA |
|
| ||||||||
| Time On Therapy (Months) | ||||||||
| Mean | 10.3 | NA | 9.1 | NA | 11.6 | NA | 10.3 | NA |
| Range | 6.0–17.9 | NA | 6.9–11.6 | NA | 7.2–23.3 | NA | 6.0–23.3 | NA |
| 6–12 Months | 258 (75%) | NA | 7 (100%) | NA | 14 (70%) | NA | 279 (76%) | NA |
| 12+ Months | 84 (25%) | NA | 0 (0%) | NA | 6 (30%) | NA | 90 (24%) | NA |
|
| ||||||||
| Interval Between Mammograms (Days) Range | 196–838 | 267–834 | 245–455 | 305–467 | 285–738 | 293–806 | 196–838 | 267–834 |
| Median | 396 | 400 | 368 | 368 | 400 | 397 | 398 | 399 |
|
| ||||||||
| Baseline Density (%) | ||||||||
| Mean (SD) | 17.6 (11.6) | 16.0 (12.0) | 17.5 (13.9) | 16.3 (15.7) | 20.5 (9.8) | 18.6 (9.7) | 17.8 (11.6) | 16.2 (11.9) |
| 0–10 % | 101 (29%) | 129 (38%) | 3 (43%) | 4 (57%) | 3 (15%) | 5 (25%) | 107 (29%) | 138 (37%) |
| 10–20% | 115 (34%) | 100(29%) | 2 (29%) | 1 (14%) | 6 (30%) | 5 (25%) | 123 (33%) | 106 (29%) |
| 20–30% | 69 (20%) | 66 (19%) | 0 (0%) | 0 (0%) | 6 (30%) | 8 (40%) | 75 (20%) | 74 (20%) |
| 30%+ | 57 (17%) | 47 (14%) | 2 (29%) | 2 (29%) | 5 (25%) | 2 (10%) | 64 (17%) | 51 (14%) |
|
| ||||||||
| Change in Density (%) | ||||||||
| Mean (SD) | −1.2 (3.8) | −1.1 (3.4) | −0.1 (2.8) | −1.1 (2.2) | −3.5 (4.4) | −1.0 (2.8) | −1.3 (3.9) | −1.1 (3.3) |
| 15%+ Decrease | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) | 0 (0%) |
| 10–14.9% Decrease | 5 (1%) | 5 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (1%) | 5 (1%) |
| 5–9.9% Decrease | 38 (11%) | 29 (8%) | 0 (0%) | 0 (0%) | 7 (35%) | 1 (5%) | 45 (12%) | 30 (8%) |
| Within 5% | 282 (82%) | 298 (87%) | 6 (86%) | 7 (100%) | 12 (60%) | 19 (95%) | 300 (81%) | 324 (88%) |
| 5–9.9% Increase | 13 (4%) | 8 (2%) | 1 (14%) | 0 (0%) | 1 (5%) | 0 (0%) | 15 (4%) | 8 (2%) |
| 10–14.9% Increase | 1 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (<1%) | 1 (<1%) |
| 15%+ Increase | 1 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (<1%) | 1 (<1%) |
|
| ||||||||
| Race (%) | ||||||||
| African American | 4 (1%) | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (1%) | 2 (1%) |
| American Indian | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (<1%) | 0 (0%) |
| Asian | 7 (2%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (2%) | 1 (<1%) |
| Caucasian | 329 (96%) | 333 (97%) | 7 (100%) | 7 (100%) | 20 (100%) | 19 (95%) | 356 (96%) | 359 (97%) |
| Unknown/Not Reported | 1 (<1%) | 6 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 1 (<1%) | 7 (2%) |
Of the 387 postmenopausal breast cancer women receiving an average of 10 months of adjuvant AI therapy, 14% (n=56) experienced at least a 5% decrease in their MBD. This increased to 20% for the 280 cases who had a baseline percent density of at least 10%. Multivariate analyses showed the likelihood of experiencing a reduction of at least 5% in MBD with AI therapy was increased for cases with a baseline density of 15% or more (OR: 10.4; 95% CI: 4.0–26.9: p <0.0001); who had 12 months or more of AI treatment (OR: 3.18; 95% CI: 1.68–6.03: p =0.0004) and had any prior HT exposure (OR: 1.95; 95% CI: 1.03–3.67: p =0.04). Baseline BMI, change in BMI between baseline and year 1 mammograms; age, time between mammograms and treatment arm were not associated with a reduction in MBD with AI use [Data not shown].
Table 2 describes characteristics of the 369 cases and matched controls used for the paired analyses, by parent trial and overall. Three fourths of the women were overweight or obese. Pre-AI MBD was less than 10% for 29% of cases (37% controls) and 30% or more for 17% of cases (14% controls). Prior use of postmenopausal hormones was balanced between the cases and controls (53%).
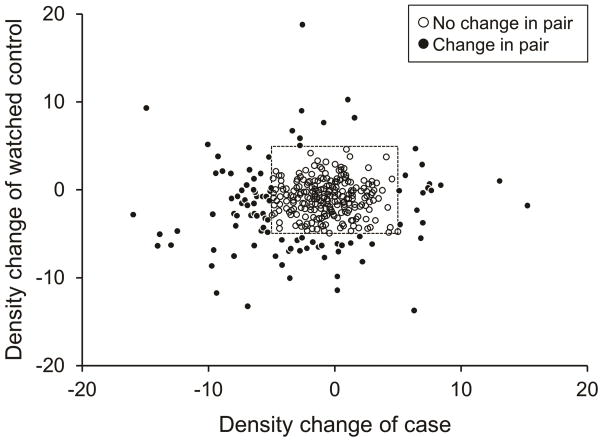
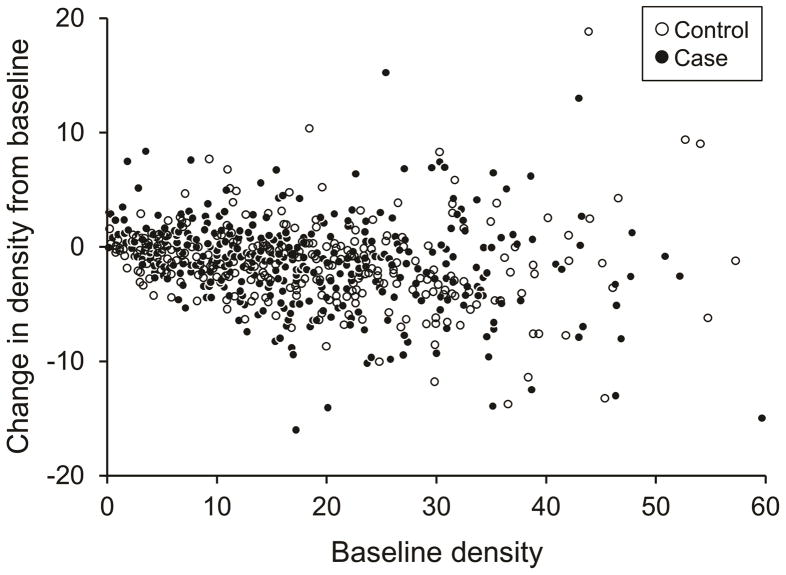
The distribution of change in MBD between baseline and one-year for the 369 cases and their matched controls is shown in Table 2 and Figure 1. The median change in density at one year among cases was −1.0% (Interquartile range [IR]: −3.2 to 0.8%) compared to −0.8% (IR: −2.8 to 0.4%) among their matched controls. Moreover, 82% of the cases and 88% of their controls remained within 5% of their baseline MBD, as shown by the box in Figure 1. Figure 2 shows the change in MBD by baseline mammographic density.
Figure 1.
Distribution of change in breast density for pairs of cases on AI therapy and matched controls.
Figure 2.
Change in breast density with aromatase inhibitor therapy for cases and matched controls by the pre-treatment (baseline) density.
The primary endpoint was the difference in the change in MBD over one year between the case and her matched control. A difference value of 0 indicates that the case and her control had an identical change in breast density while a negative value indicates that the case had a larger decrease in density than her control. The median difference for all 369 pairs was −0.1% (with 10th percentile of −5.9% and 90th percentile of 5.2%). Thus, there was no evidence to conclude that the change in MBD over one year differs between a case and her matched control whether considering all matched pairs (n=369; p=0.51); the subset of matched pairs where both the case and her matched control had a baseline MBD greater than 10% (n=222; p=0.55); those cases on MA27 randomized to either AI therapy (n=178; p=0.41; n=164; p=0.56) or among those concordant on prior HT status (n=240; p=0.85). Among the 90 matched pairs where the case had taken aromatase inhibitors for at least 12 months, though, there was some suggestion of a greater difference between case-control pairs (median difference for pairs=−0.90% (95% CI: −1.62 to −0.10) p=0.08), but these results only approached statistical significance.
Discussion
Within this large study of aromatase inhibitors and breast density, we found that 14% of cases initiating AI therapy had a MBD reduction of at least 5% at an average of 10 months of therapy. Cases that experienced a reduction of 5% or more in MBD with AI therapy were more likely to have a higher baseline MBD, to have used AI therapy for more than 12 months and used postmenopausal hormones prior to AI therapy. However, when comparing the cases on AI therapy to matched controls of the same age, BMI, and baseline MBD, there was no evidence of an association of change in breast density with AI therapy use. Results were similar for exemestane and anastrozole, when restricting to pairs who were concordant on postmenopausal hormone status, and those with at least 10% MBD at baseline.
Our investigations of factors associated with MBD reductions among the AI cases identified higher baseline MBD, longer duration of endocrine treatment and prior HT use. Kim et al. (14) also found higher baseline MBD and longer duration associated with MBD reductions, but they did not evaluate prior HT use. In previous work, we examined factors that predict changes in MBD among healthy women, and found prior HT use and higher baseline density were associated with greater reductions in MBD in women of mammogram age (38).
Our finding of no overall association between AI therapy and MBD is consistent with five of the previous studies that have examined the influence of AIs on breast density among postmenopausal women (Table 1). These previous studies were comprised of breast cancer cases on five years tamoxifen, randomized to letrozole or placebo (22); cases randomized to letrozole, anastrozole or placebo as adjuvant therapy (24, 26); healthy women with some visible density randomized to exemestane or placebo (25); and healthy women on postmenopausal hormone therapy who took letrozole (23, 27). Surprisingly, results did not vary for the studies of AI therapy that required visible density (25) or at least 25% density (24) on the baseline mammogram, which is consistent with our findings of no difference between cases and matched controls who had at least 10% baseline density.
Four studies did find significant reductions in women on AI therapy. These included 40 postmenopausal women on combination hormone therapy who experienced a statistically significant reduction in percent density when taking letrozole for a median of 24 months (n=18) compared to women who did not take letrozole over this same time period (n=22) (6.8% vs. 1.4% reduction, respectively) (27) and studies comprised of small numbers of breast cancer cases (26) and high risk women (28), that examined changes in MBD at 6 and 12 months of therapy and found evidence of reduction in MBD at 12 months (Table 1). However, for the latter studies, there was no comparison group evaluated, so these changes are difficult to interpret since density is expected to decrease with age (38), as seen in the controls in the current study. The recent study by Kim et al. (14) also examined change in MBD at an average 13 months for 175 women on AI therapy and 890 women on TAM therapy, finding an overall average reduction of 5.9% (range −17.2% to 36.9%) with either therapy and a smaller mean reduction (−3.1±6.3%) at one year with AI therapy compared to TAM (−6.5±7.1%). Also, women on AI therapy who experienced a reduction in MBD of less than 5% over the first 13 months of therapy had a 7 times greater risk of recurrence at 68.8 months of follow-up than women who had a 5% or greater reduction (14), although this was not statistically significant (95% CI: 0.90–56.37) and the confidence intervals were wide.
Regarding the studies noted above, there were some inconsistencies in eligibility, populations and analyses (Table 1). Kim et al. (14) was conducted in a Korean population, and some Asian populations have been shown to have smaller or similar dense area but higher percent density than Caucasian women because of their smaller breast size (39, 40). In Kim et al., 16% of women had a baseline MBD above 50% and 76% of women above 25% MBD; on the other hand, only 2% had a baseline MBD of 10% or less. Thus, these women who were younger (median of 49 years compared to 63 in our study) and started with much higher baseline MBD compared to our postmenopausal population that had 29%–37% of women under 10% baseline MBD. This could have resulted in differing ability to detect changes, as 34% of the Korean cases on AI experienced a MBD decrease of 5% or greater compared to 14% in our population. Another major difference in the Kim et al. study was the high proportion of patients who received adjuvant chemotherapy (77%), which might influence MBD reduction through ablative effects on ovarian function in the premenopausal women since the menopausal transition is consistently associated with decreases in density (38, 41). Our study population was postmenopausal at the time of baseline mammogram and the small proportion of chemotherapy use (31%) in cases after the baseline mammogram would not have affected their ovarian function. Other differences in the Kim et al. (vs. current) study included the use of digital instead of film mammography to assess changes in density, and the inability to account for BMI in their analyses.
An association of greater reductions in MBD with longer time on AI therapy was not seen consistently across studies. We and others (14) showed duration of therapy was a factor associated with MBD reduction among the cases. Also, results from our case-control comparisons among the 90 pairs who had used AIs at least 12 months showed evidence of a greater decrease among cases than controls, although this was not statistically significant (p=0.08). The studies by Prowell and Smith, which examined changes at 6 and 12 months of therapy in the same women (n=50 and 16, respectively), also found greater reduction with the longer duration of therapy. However, two of the three studies that examined the longest duration of AI therapy (24 months) found no association between AI use and density change (24, 25) (Table 1). Time on therapy has been shown to be relevant in studies of tamoxifen and MBD, where reductions in density were seen with 12–18 months of tamoxifen therapy vs. placebo, but were even stronger with 54 months of therapy (10).
Even if there is a subgroup of women who experiences changes in density with AI use that potentially translates to reduced risk or recurrence, it will be difficult to identify these women in the clinical setting. Postmenopausal women for whom AIs are currently indicated generally have lower MBD, and reductions with AI therapy are often small (<5%) and within the noise of the mammographic density measure. In fact, in our study, there were only 2% of women on AIs who experienced reductions in density of at least 10% (Table 2). Even in the study by Smith et al. (28), which saw decreases among 11 of 16 patients on letrozole at 12 months (Table 1), only 2 out of 16 patients experienced a decrease of at least 10% at 12 months (28). However, other imaging modalities, such as magnetic resonance imaging or MRI, may be able to better characterize small changes in breast density with AI therapy use, as has been seen with studies of tamoxifen. Even with the ability to detect changes, it is not clear how a clinically meaningful reduction in density will be defined, since the majority of women experience a decline in density as they age, with the greatest changes during perimenopause (38, 42).
The lack of association in our study between AI therapy and density change was surprising in light of the numerous studies that have shown mammographic density reductions resulting from tamoxifen therapy in Caucasians (9, 10) and the recent study of reductions in MBD with AI use among younger Korean women (14). Cuzick et al (10) in the largest study of tamoxifen and density to date (818 women total) showed a decrease in breast density with tamoxifen use compared to placebo among both premenopausal and postmenopausal women, but the reduction was much greater among those under 45 years compared to those over 55 years (mean change of −13.4% (95% CI = −8.6% to −18.1% vs. −1.1% (95% CI = −3.0% to 5.1%, respectively). This is similar to the magnitude of change in density at 1-year among cases on AI therapy over age 55 in the current study (mean change of −1.04% (95% CI: −1.4%, −0.63%)). Importantly, the Cuzick study required a baseline MBD of at least 10% to allow the possibility of a density reduction, and 98% of the population in Kim et al. (14) also had MBD above 10%, which may have been responsible for detection of the relatively small change among the postmenopausal AI users. However, even in our examination of the 90 case and control pairs with increased baseline densities (above 10%), we did not find evidence of a statistically significant association between AIs and density reduction. The study by Cuzick et al. (noted above) required 12 to 18 months of therapy and Kim et al. had an average of 13 months of therapy. Our findings that women who had taken at least 12 months of AI therapy had a difference that approached statistical significance raises the possibility that longer follow-up of patients would have revealed a difference. However, the difference even in these patients was small and, for MBD to be a useful predictive biomarker of AI effect, changes would need to be seen in a reasonably short period of time.
To date, then, studies would indicate that tamoxifen is associated with reductions in MBD, even among postmenopausal women, while AI’s show either a lesser or no effect on MBD. However, this differential influence of endocrine therapies on MBD does not directly translate to therapeutic efficacy. In fact, AIs have been found to be better than tamoxifen in reducing recurrence (15, 43) and contralateral breast cancer (44) in postmenopausal women, despite the lack of their effect on MBD. Importantly, we do not understand the molecular mechanism underlying the positive association of MBD and breast cancer risk, nor how tamoxifen influences MBD. Tamoxifen competitively binds to estrogen receptors on breast tumors and other tissue targets, producing a nuclear complex that decreases DNA synthesis and inhibits estrogen effects. Tamoxifen function can be regulated by a number of different variables including growth factors. AIs, however, have only one function, which is the blockade of the aromatase enzyme and thus the conversion of androstenedioneto estrone and testosterone to estradiol. Further, tamoxifen and AIs have different effects on systemic levels of hormones and gene expression at the tissue level (20, 26, 45–48). Difference in mechanisms of action between AIs (decrease ligand, i.e. estrogen) and tamoxifen (blocks the estrogen receptor), therefore, may be an explanation for the difference in impact on MBD.
Thus, increases in MBD with tamoxifen, but to a lesser extent with AIs, would suggest that MBD is influenced by regulation of the estrogen receptor, but not necessarily the concentration of estrogens in the breast tissue. In fact, studies of postmenopausal breast cancer patients on AIs (anastrozole and letrozole) showed suppression of estrone, estradiol and estrone-sulfate levels from pre-treatment levels (20, 26, 48). However, tamoxifen did not result in similar suppression of estrogens, and even showed an increase in estrone-sulfate (48). We would have expected a greater effect of AIs than tamoxifen on MBD if mediated solely through estrogens. We hypothesize, then, that reduction in estrogens alone is sufficient to reduce breast cancer risk in postmenopausal women but is not sufficient to impact MBD. This is consistent with the mixed findings from studies that have examined blood estrogen levels with MBD (49–52) and data from Prowell et al that showed suppression of estrone-sulfate with 1-year of AI therapy was uncorrelated with changes in MBD over the same time period (26). On the other hand, studies of ERα expression in the breast epithelial tissue and MBD (53–55), which would support a mechanism by which tamoxifen reduces MBD, have shown no evidence of an association. Thus, at present, the mechanistic basis for differential effects of tamoxifen and AIs on density are unknown and the subject of future research. Our current efforts to identify genes involved in involution and breast density may shed light on the response of MBD to these hormonally-related therapies.
There were several strengths to our study, including the large sample size, knowledge of actual start date of AI therapy for the trials, ability to examine two types of AI therapy, validated measure of breast density, and the closely matched control group. We also recognize limitations of the observational design, our inability to match on prior postmenopausal hormone therapy due to the prioritization of the other matching variables, and the analysis of mammograms taken within 1-year prior to the study instead of at the time of randomization. However, our closely matched control group will help minimize bias in comparisons of AI therapy vs. controls; the distribution of prior hormone use was equal in the cases and controls and separate analyses within the pairs concordant on PMH group were similar to the overall results; and finally, analyses showed that interval between mammograms was not associated with having a change in breast density.
In summary, we present results from the largest study of AIs in postmenopausal white women examining the influence of AIs on breast density. We found several factors associated with reduction of density among cases on AI, including higher baseline density, prior HT use and longer time on therapy. However, we found no evidence of an association of change in breast density with AI therapy use among the cases compared to controls closely matched on baseline breast density, age, baseline BMI, and interval between mammograms. Our findings may be a consequence of the lack of influence of the drug on the overall dense tissue and/or the limitations of mammography in detecting small changes that may occur among postmenopausal white women with already low breast densities. These findings indicate that MBD is not likely to be a clinically useful biomarker, at least in the short term necessary for clinical utilization, for predicting the value of AI adjuvant therapy in white women.
Statement of Translational Relevance.
Mammographic breast density is one of strongest risk factors for breast cancer and provides information not only for risk assessment, but also potentially for determining response to therapies. Tamoxifen is consistently associated with reductions in breast density and these reductions have been shown to translate to decreased breast cancer risk and recurrence. Whether similar associations hold for aromatase inhibitors is not clear. Our data on 387 postmenopausal and predominately white women with early stage breast cancer showed that only 14% had a reduction in density of 5% or more at an average 10 months of aromatase inhibitor therapy. In addition, comparisons to a closely matched control group showed no association between density change and aromatase inhibitor therapy. These findings suggest that breast density, as currently assessed, is not likely to be a clinically useful biomarker for predicting the value of aromatase inhibitor therapy in white women.
Acknowledgments
Financial Support: Supported, in part, by grants from the National Institutes of Health Specialized Program of Research Excellence (SPORE) in Breast Cancer, P50 CA116201 and Mayo Pharmacogenomics Research Network (PGRN), U01 GM61388. PG is supported by the Avon Foundation New York
We acknowledge the women on trials MA27, N063I and MC0532 who consented to this research.
Grant Support
Supported, in part, by grants from the National Institutes of Health, Specialized Program of Research Excellence (SPORE) in Breast Cancer, P50 CA116201, R01 CA97396, R01 CA140286, and Mayo Pharmacogenomics Research Network (PGRN), U01 GM61388. PG is supported by the Avon Foundation, New York.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: PEG, VJS, JNI, CMV, KRB
Development of methodology: PEG, VJS, CMV, FFW, KRB
Acquisition of data (provided animals, acquired, and managed patients, provided facilities, etc.): PEG, CE, LS, JNI, RW, JEO
Analysis and interpretation of data (e.g., statistical analysis, biostatics, computational analysis): PEG, VJS, MK, JNI, CMV, GU, KRB
Writing, review, and/or revision of the manuscript: PEG, VJS, JEO, AB, RW, LS, CMV, GU, KRB
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): VJS, MLK, LF, CE, CMV
Study supervision: CMV, VJS, JNI, LF, LS, RW
Immunohistochemical evaluation of the target biomarker:
Patient accrual: PEG, JNI, RW
References
- 1.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 2.Cil T, Fishell E, Hanna W, Sun P, Rawlinson E, Narod SA, et al. Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer. 2009;115:5780–7. doi: 10.1002/cncr.24638. [DOI] [PubMed] [Google Scholar]
- 3.Park CC, Rembert J, Chew K, Moore D, Kerlikowske K. High mammographic breast density is independent predictor of local but not distant recurrence after lumpectomy and radiotherapy for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2009;73:75–9. doi: 10.1016/j.ijrobp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Habel LA, Dignam JJ, Land SR, Salane M, Capra AM, Julian TB. Mammographic density and breast cancer after ductal carcinoma in situ. J Natl Cancer Inst. 2004;96:1467–72. doi: 10.1093/jnci/djh260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habel LA, Capra AM, Achacoso NS, Janga A, Acton L, Puligandla B, et al. Mammographic density and risk of second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2010;19:2488–95. doi: 10.1158/1055-9965.EPI-10-0769. [DOI] [PubMed] [Google Scholar]
- 6.Greendale GA, Reboussin BA, Sie A, Singh HR, Olson LK, Gatewood O, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators. Ann Intern Med. 1999;130:262–9. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 7.Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285:171–6. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: women’s health initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–76. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8:863–6. [PubMed] [Google Scholar]
- 10.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 11.Son HJ, Oh KK. Significance of follow-up mammography in estimating the effect of tamoxifen in breast cancer patients who have undergone surgery. AJR Am J Roentgenol. 1999;173:905–9. doi: 10.2214/ajr.173.4.10511146. [DOI] [PubMed] [Google Scholar]
- 12.Brisson J, Brisson B, Cote G, Maunsell E, Berube S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911–5. [PubMed] [Google Scholar]
- 13.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Han W, Moon HG, Ahn SK, Shin HC, You JM, et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012;14:R102. doi: 10.1186/bcr3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 16.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 17.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–7. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 18.Dowsett M, Jones A, Johnston SR, Jacobs S, Trunet P, Smith IE. In vivo measurement of aromatase inhibition by letrozole (CGS 20267) in postmenopausal patients with breast cancer. Clin Cancer Res. 1995;1:1511–5. [PubMed] [Google Scholar]
- 19.Miller WR, Dixon JM. Local endocrine effects of aromatase inhibitors within the breast. J Steroid Biochem Mol Biol. 2001;79:93–102. doi: 10.1016/s0960-0760(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 20.Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010;70:3278–86. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vachon CM, Sasano H, Ghosh K, Brandt KR, Watson DA, Reynolds C, et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res Treat. 2011;125:243–52. doi: 10.1007/s10549-010-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vachon CM, Ingle JN, Suman VJ, Scott CG, Gottardt H, Olson JE, et al. Pilot study of the impact of letrozole vs. placebo on breast density in women completing 5 years of tamoxifen. Breast. 2007;16:204–10. doi: 10.1016/j.breast.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Fabian CJ, Kimler BF, Zalles CM, Khan QJ, Mayo MS, Phillips TA, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. 2007;106:75–84. doi: 10.1007/s10549-006-9476-5. [DOI] [PubMed] [Google Scholar]
- 24.Cigler T, Tu D, Yaffe MJ, Findlay B, Verma S, Johnston D, et al. A randomized, placebo-controlled trial (NCIC CTG MAP1) examining the effects of letrozole on mammographic breast density and other end organs in postmenopausal women. Breast Cancer Res Treat. 2010;120:427–35. doi: 10.1007/s10549-009-0662-0. [DOI] [PubMed] [Google Scholar]
- 25.Cigler T, Richardson H, Yaffe MJ, Fabian CJ, Johnston D, Ingle JN, et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat. 2011;126:453–61. doi: 10.1007/s10549-010-1322-0. [DOI] [PubMed] [Google Scholar]
- 26.Prowell TM, Blackford AL, Byrne C, Khouri NF, Dowsett M, Folkerd E, et al. Changes in breast density and circulating estrogens in postmenopausal women receiving adjuvant anastrozole. Cancer Prev Res. 2011;4:1993–2001. doi: 10.1158/1940-6207.CAPR-11-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mousa NA, Crystal P, Wolfman WL, Bedaiwy MA, Casper RF. Aromatase inhibitors and mammographic breast density in postmenopausal women receiving hormone therapy. Menopause. 2008;15:875–84. doi: 10.1097/gme.0b013e31816956c3. [DOI] [PubMed] [Google Scholar]
- 28.Smith J, Dilawari A, Ursin G, Andreopoulou E, Checka C, Axelrod D, et al. A pilot study of letrozole for one year in women at enhanced risk of developing breast cancer: effects on mammographic density. Anticancer Res. 2012;32:1327–31. [PubMed] [Google Scholar]
- 29.Venta LA, Hendrick RE, Adler YT, DeLeon P, Mengoni PM, Scharl AM, et al. Rates and causes of disagreement in interpretation of full-field digital mammography and film-screen mammography in a diagnostic setting. AJR Am J Roentgenol. 2001;176:1241–8. doi: 10.2214/ajr.176.5.1761241. [DOI] [PubMed] [Google Scholar]
- 30.Olson JE, Sellers TA, Scott CG, Schueler BA, Brandt KR, Serie DJ, et al. The influence of mammogram acquisition on the mammographic density and breast cancer association in the Mayo Mammography Health Study Cohort. Breast Cancer Res. 2012;14:R147. doi: 10.1186/bcr3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum PR. Optimal matching for observational studies. JASA. 1989;84:1024–32. [Google Scholar]
- 32.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Jong RA, Fishell E, et al. Mammographic densities and risk of breast cancer among subjects with a family history of this disease. J Natl Cancer Inst. 1999;91:1404–8. doi: 10.1093/jnci/91.16.1404. [DOI] [PubMed] [Google Scholar]
- 33.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 34.Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:43–9. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 35.Stevens KN, Lindstrom S, Scott CG, Thompson D, Sellers TA, Wang X, et al. Identification of a novel percent mammographic density locus at 12q24. Hum Mol Genet. 2012;21:3299–305. doi: 10.1093/hmg/dds158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vachon CM, Pankratz VS, Scott CG, Maloney SD, Ghosh K, Brandt KR, et al. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:921–8. doi: 10.1158/1055-9965.EPI-06-1047. [DOI] [PubMed] [Google Scholar]
- 37.Heine JJ, Scott CG, Sellers TA, Brandt KR, Serie DJ, Wu FF, et al. A novel automated mammographic density measure and breast * cancer risk. J Natl Cancer Inst. 2012;104:1028–37. doi: 10.1093/jnci/djs254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelemen LE, Pankratz VS, Sellers TA, Brandt KR, Wang A, Janney C, et al. Age-specific trends in mammographic density: the Minnesota Breast Cancer Family Study. Am J Epidemiol. 2008;167:1027–36. doi: 10.1093/aje/kwn063. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004;159:140–7. doi: 10.1093/aje/kwh028. [DOI] [PubMed] [Google Scholar]
- 40.Nie K, Su MY, Chau MK, Chan S, Nguyen H, Tseng T, et al. Age- and race-dependence of the fibroglandular breast density analyzed on 3D MRI. Med Phys. 2010;37:2770–6. doi: 10.1118/1.3426317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048–53. [PubMed] [Google Scholar]
- 42.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:732–9. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 43.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 44.Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 45.Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer--a study from the IMPACT trialists. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:2477–92. doi: 10.1200/JCO.2005.07.559. [DOI] [PubMed] [Google Scholar]
- 46.Miller WR, Larionov AA, Renshaw L, Anderson TJ, White S, Murray J, et al. Changes in breast cancer transcriptional profiles after treatment with the aromatase inhibitor, letrozole. Pharmacogenet Genomics. 2007;17:813–26. doi: 10.1097/FPC.0b013e32820b853a. [DOI] [PubMed] [Google Scholar]
- 47.Miller WR. Clinical, pathological, proliferative and molecular responses associated with neoadjuvant aromatase inhibitor treatment in breast cancer. J Steroid Biochem Mol Biol. 2010;118:273–6. doi: 10.1016/j.jsbmb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Ingle JN, Suman VJ, Johnson PA, Krook JE, Mailliard JA, Wheeler RH, et al. Evaluation of tamoxifen plus letrozole with assessment of pharmacokinetic interaction in postmenopausal women with metastatic breast cancer. Clin Cancer Res. 1999;5:1642–9. [PubMed] [Google Scholar]
- 49.McCormack VA, Dowsett M, Folkerd E, Johnson N, Palles C, Coupland B, et al. Sex steroids, growth factors and mammographic density: a cross-sectional study of UK postmenopausal Caucasian and Afro-Caribbean women. Breast Cancer Res. 2009;11:R38. doi: 10.1186/bcr2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verheus M, Peeters PH, van Noord PA, van der Schouw YT, Grobbee DE, van Gils CH. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast Cancer Res. 2007;9:R53. doi: 10.1186/bcr1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varghese JS, Smith PL, Folkerd E, Brown J, Leyland J, Audley T, et al. The heritability of mammographic breast density and circulating sex-hormone levels: two independent breast cancer risk factors. Cancer Epidemiol Biomarkers Prev. 2012;21:2167–75. doi: 10.1158/1055-9965.EPI-12-0789. [DOI] [PubMed] [Google Scholar]
- 52.Haakensen VD, Bjoro T, Luders T, Riis M, Bukholm IK, Kristensen VN, et al. Serum estradiol levels associated with specific gene expression patterns in normal breast tissue and in breast carcinomas. BMC Cancer. 2011;11:332. doi: 10.1186/1471-2407-11-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verheus M, Maskarinec G, Erber E, Steude JS, Killeen J, Hernandez BY, et al. Mammographic density and epithelial histopathologic markers. BMC Cancer. 2009;9:182. doi: 10.1186/1471-2407-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harvey JA, Santen RJ, Petroni GR, Bovbjerg VE, Smolkin ME, Sheriff FS, et al. Histologic changes in the breast with menopausal hormone therapy use: correlation with breast density, estrogen receptor, progesterone receptor, and proliferation indices. Menopause. 2008;15:67–73. doi: 10.1097/gme.0b013e318054e29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh K, Brandt KR, Reynolds C, Scott CG, Pankratz VS, Riehle DL, et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res Treat. 2012;131:267–75. doi: 10.1007/s10549-011-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]