Summary
Debate on appropriate triggers for transfusion of allogeneic blood products and their effects on short- and long-term survival in surgical and critically ill patients continue with no definitive evidence or decisive resolution. Although transfusion-related immune modulation (TRIM) is well established, its influence on immune competence in the recipient and its effects on cancer recurrence after a curative resection remains controversial. An association between perioperative transfusion of allogeneic blood products and risk for recurrence has been shown in colorectal cancer in randomized trials; whether the same is true for other types of cancer remains to be determined. This article focuses on the laboratory, animal, and clinical evidence to date on the mechanistic understanding of inflammatory and immune-modulatory effects of blood products and their significance for recurrence in the cancer surgical patient.
Keywords: cancer, transfusion
Editor's key points.
There is a known association between perioperative blood transfusion and cancer recurrence in colorectal cancer.
The authors reviewed laboratory and clinical evidence of this association for all types of cancer.
There is laboratory and animal experiments evidence that stored blood and old erythrocytes may have tumour-promoting effects.
Awareness of these issues is important in making individualized decisions on blood transfusion in patients undergoing cancer surgery.
In evaluating clinical studies relating blood transfusion to outcomes, it is worth remembering that the circumstances under which patients are given blood products perioperatively are likely to influence cancer recurrence. Recurrence depends on preoperative nutrition and functional status, the presence of preoperative anaemia, tumour type and stage, degree of resectability, duration and type of anaesthesia, amount of blood loss, perioperative stress response, and the presence of postoperative complications.1–10 The potential impacts of many of these important confounding factors on cancer recurrence are complex and difficult to understand from retrospective studies. There is recent interest in the perioperative management strategies and protocols including regional anaesthesia, avoidance of inhalatory anaesthetics and opioids, and blood transfusion goals that may influence control of minimal residual disease and metastatic spread of tumour.11–13 First, tumour manipulation during surgical resection increases the load of circulating malignant cells.14,15 Secondly, volatile anaesthetics and opioids depress the function of host cellular defences, especially NK cells, NKT cells, and cytotoxic lymphocytes. Thirdly, perioperative factors such as inflammatory response to injury, physiological stress response to surgery, hyperglycaemia, and hypothermia cause a significant imbalance between Th1 and Th2 responses in favour of the latter, which constitutes a pro-tumour environment. The perioperative period, therefore, can foster a potentially pro-tumour environment that may facilitate distal seeding of circulating cells, the growth of micrometastases into established clinical metastases, or both. The combination of surgery-induced and anaesthetic-contributed immunosuppression may be further aggravated by administration of blood products that themselves have inflammatory and immunosuppressive effects. The clinical question, then, is whether transfusion augments the risk of cancer recurrence after potentially curative surgery.4,16
While blood transfusion is life saving in many circumstances and is safer than it has ever been, it still poses significant risks, including incompatibility, transmission of infectious agents, coagulopathy, and allergic reactions. It is an accepted fact that the administration of blood products also causes profound negative effects on the human immune system, a condition termed transfusion-related immune modulation (TRIM; Table 1). Mechanisms for TRIM include suppression of cytotoxic cell and monocyte activity, release of immunosuppressive prostaglandins, inhibition of interleukin-2 (IL-2) production, and increase in suppressor T-cell activity.17–20 The immunosuppressive effects of allogeneic blood transfusion were even used therapeutically to reduce renal allograft rejection before effective immunosuppressant drugs became available.21
Table 1.
Abbreviations
| AP-1 | Activator protein 1 |
| BDNF | Brain-derived growth factor |
| CTL | Cytolytic T lymphocytes |
| CXCL-7 | Chemokine ligand 7 |
| COX | Cyclooxygenase |
| EGF | Epidermal growth factor |
| FGF-2 | Fibroblast growth factor-2 |
| FFP | Fresh-frozen plasma |
| HLA | Human leucocyte antigen |
| IFN | Interferon |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| Lyso-PC | Lysophosphatidylcholine |
| MDC | Myeloid dendritic cells |
| MIP-1α | Macrophage inflammatory protein |
| NK | Natural killer cell |
| NF-kB | Nuclear factor-kappa beta |
| NKT | Natural killer T cell |
| OR | Odds ratio |
| PDGF | Platelet-derived growth factor |
| PG | Prostaglandin |
| pRBC | Packed red blood cells |
| RCT | Randomized controlled trial |
| RBC | Red blood cell |
| CCL5 (RANTES) | Chemokine ligand 5 |
| STAT-3 | Signal transducer and activator of transcription-3 |
| Tc | T cytotoxic |
| TF | Tissue factor |
| Th | T helper |
| TGF-β1 | Transforming growth factor-beta 1 |
| Tregs | T regulatory cell |
| TLR | Toll-like receptor |
| TXA | Thromboxane |
| TRIM | Transfusion-related immune suppression |
| VEGF | Vascular endothelial growth factor |
In this article, we will discuss the current understanding of the mechanisms by which transfusion of blood components affects the inflammatory response and immune function; the linkage between inflammation, immunity, and cancer progression; and finally, the clinical data on the effects of blood transfusion on recurrence in the cancer patient.
Inflammatory burden and immune modulation from administration of blood products
Pre-storage leucoreduction of red cell units is now routine because of the perceived benefits of reducing post-transfusion infections, preventing febrile transfusion reactions, and decreasing the likelihood of HLA alloimmunization and platelet refractoriness in the recipient. One of the major advantages of leucoreduction is to avoid the accumulation of bioactive substances released from white cells implicated in transfusion-related immune suppression (Table 2).22,23 Leucocyte numbers are reduced 3 log (99.9) after leucocyte reduction with the use of the most recent third- and fourth-generation screen filters; however, a few leucocytes remain and may still modulate immune responses in the recipient.22 The concentrations of Th1 and predominantly Th2 cytokines are increased in non-leucoreduced pRBC units.24–26 Cytokine concentrations remain non-trivial in aged leucoreduced units.27 Furthermore, exposure of leucoreduced stored RBC supernatant to whole blood triggers release of IL-6, IL-10, and TNF-α,28 reduces lipopolysaccharide-induced release of TNF-α,29 and induces regulatory T-cell (Treg) activation.30 In humans, Treg cells, comprise ∼1–2% of circulating CD4+ T-helper cells that coexpress a very high density of the IL-2 receptor-alpha (CD25hi), inhibit IL-2 production and suppress the functions of Th1 responses by CD4+ and CD8+ T cells.31–33 The activation of Treg cells is antigen non-specific as they can be activated by LPS and through the Toll-like receptor-4 pathway to become immune suppressive.34 These findings may explain why inflammation and immunosuppression may still be encountered after administration of stored RBC products regardless of whether they are leucoreduced.
Table 2.
Summary of the biological mediators involved in transfusion immune modulation and tumour growth after blood transfusion
| Biological modulator | Activity on tumours | References |
|---|---|---|
| Cytokines | ||
| TH1 (IL-2, IFN-γ) | Antitumoral | Yu and colleagues,68 Smyth and colleagues,78 Swann and Smyth,79 Palucka and colleagues,77 |
| TH2 (IL-4, IL-5, and IL-10) | Pro-tumoral | Cognasse and colleagues,80 Picker and colleagues,81 Yu and colleagues,68 Swann and Smyth,79 Palucka and colleagues77 |
| Eicosanoids | ||
| Thromboxane A2 | Pro-tumoral | Gately and colleagues44 |
| PGE2, PGI2 | Pro-tumoral | Baratelli and colleagues,42 Soontrapa and colleagues,41 Mulligan and colleagues,43 Gately and colleagues44 |
| Lysophosphatidycholines | Pro-tumoral | |
| Growth factors | ||
| TGF-β | Pro-tumoral | Apelseth and colleagues,60 |
| VEGF | Pro-tumoral | Kanter and colleagues,59 |
| PDGF-D | Pro-tumoral | Apelseth and collegues,60 |
| IGF | Pro-tumoral | Hansen-Pupp and colleagues64 |
| Other modifiers | ||
| Fas ligand | Pro-tumoral | Ghio and colleagues,23 Hashimoto and colleagues82 |
| HLA class 1 molecules | Pro-tumoral | Ghio and colleagues23 |
| Ubiquitin | Pro-tumoral | Patel and colleagues,29 |
| Tissue plasminogen activator | Pro-tumoral | Holmes and colleagues61 |
In addition to residual leucocytes and biologically active cytokines, pRBC units also contain non-polar lipids and a mixture of pro-inflammatory lysophosphatidylcholines (lyso-PCs).35 Lyso-PC modulates the activity of NKT and T cells,36 acts as an NK cell chemoattractant,37 induces dendritic cell maturation,38 and stimulates the production of pro-inflammatory cytokines.39 Ecosanoids (prostaglandins and thromboxans) can also accumulate in pRBCs.40 The overall effects of these biological substances are immunosuppression and tumour-promoting action.41–44
Atzil and and colleagues have demonstrated the role of donor erythrocytes and their storage periods on tumour progression in two non-immunogenic (MADB106 mammary adenocarcinoma and CRNK-16 leukaemia) animal models. Leucocytes were separated before a 14-day storage period and then transfused; their effects on tumour progression were then compared with those for similarly stored packed cells and saline in the two models. Fluorescence-activated cell-sorting analysis indicated that the transfused leucocytes contained all their subpopulations. The results indicated that stored leucocyte transfusion did not cause any increase in tumour retention, whereas packed cells caused an ∼3-fold increase in tumour size. These findings raise questions and challenge the role of stored leucocytes (or factors they secrete) in mediating metastasis-promoting effects. In addition, both the leucodepleted erythrocytes and the packed cells resulted in a 3-fold increase in tumour retention compared with that for saline. The investigators also reported that both autologous and allogeneic blood transfusions increased cancer progression when they had been stored for >9 days, whereas fresh blood, whether allogeneic or syngeneic, had no deleterious effects. Proposed hypotheses explaining these observations are immunologic responses (the innate cellular immunity-NK cell activity), the cytokine response to release of degraded lipid membrane-derived factors, and other non-immunogenic mechanisms (hypoxia-induced angiogenic factors). In a hamster model, Tsai and colleagues have shown that exchange transfusion with stored erythrocytes reduced microvascular flow and functional capillary density by >50% of the level achieved with fresh erythrocytes. Moreover, tissue oxygen levels were significantly reduced for stored erythrocytes compared with fresh cells. It is, thus, conceivable that the transfusion of stored erythrocytes may induce a favourable environment for tumour progression through both immunologic and non-immune signalling pathways triggered by tissue hypoxia.
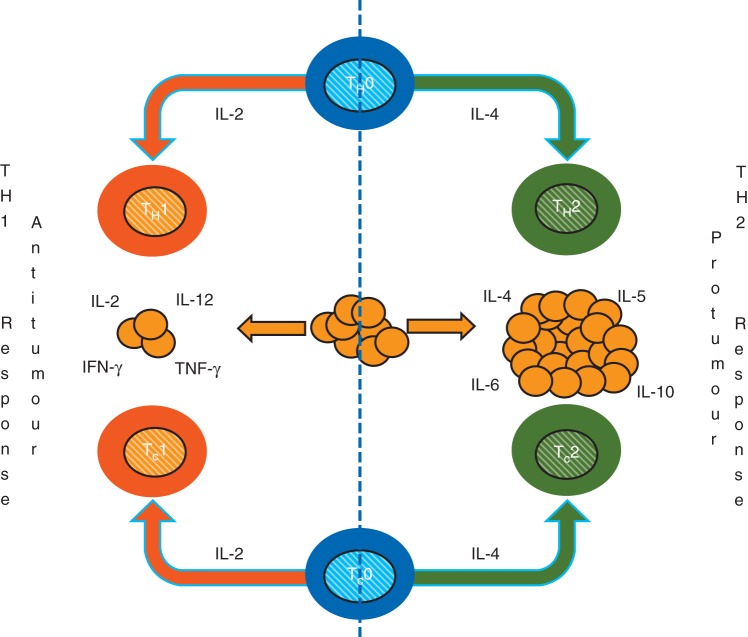
Platelet concentrates are usually collected by apheresis, leucoreduced, and then stored at 20–24°C for up to 5 days.45 Stored platelets remain partially activated and, upon transfusion, result in the immediate release of biologically active lipids, growth factors, chemokines, cytokines, and accumulated microparticles, that have significant effects on immune function and tumour growth in the recipients.46,47 The CD40 ligand is an important multifunctional protein that is released after platelet transfusion. This ligand plays multiple roles in the immune system, including stimulation of proliferation of B-lymphocytes and induction of cytokines with important functions in the inflammatory cascade.48–50 Microparticles, another component of platelet concentrates, also have significant effects on the immune system through maturation of antigen-presenting cells such as dendritic cells.51 There are two subsets of dendritic cells, myeloid-derived dendritic cells and plasmacytoid dendritic cells. Plasmacytoid dendritic cells are the most potent producers of IFN-α.52,53 They greatly influence the differentiation of CD4+ T cells towards Th1 responses by producing IL-10 and inhibiting the production of IL-12 by myeloid-derived dendritic cells, or enhancing the development of Treg cells, which, in turn, suppresses antigen-specific immune responses.54,55 On the other hand, IL-12 production by MDC enhances the production of IFN-γ by T cells and NK cells and favours a Th1 immune response (Fig. 1).56
Fig 1.
The figure illustrates the interaction between cellular and humoral responses against cancer cells. The cellular immune response is composed of Th1 and Tc1 (anti-tumour) and Th2 and Tc2 (pro-tumoral) lymphocytes. Similarly, the cytokine immune response is accomplished by Th1 (IL-2, IL-12, IFN-γ, and TNF-γ) predominantly antitumour cytokines; in contrast, the Th2 cytokines (IL-4, IL-5, IL-6, and IL-10) have mainly pro-tumour actions.
Growth factors, including VEGF, platelet-derived growth factor, fibroblast growth factor-2, brain-derived neurotropic factor, epidermal growth factor (EGF), and transforming growth factor-β1 (TGF-β1), are present in leucoreduced platelet concentrates after storage for 6 days;57–59 washing substantially reduces the concentration of most of these substances.59 Remarkably, circulating concentrations of TGF-β1 increase within an hour of transfusion.60 Furthermore, it has been reported that the addition of supernatant from platelet concentrates induces tumour growth and invasion.61 As with growth factors, cytokine, and chemokine plasma concentrations in the recipient are elevated within an hour after transfusion of platelet concentrates.60
Fresh-frozen plasma is a cell-free blood product that is rich in fibrinogen, D-dimer, factor XIII, and von Willebrand factor, along with predominantly Th2 cytokines.62 Addition of fresh-frozen plasma to peripheral blood obtained from healthy volunteers induces spontaneous and dose-dependent release of TNF-α and IL-10.63 Growth factors are also present in plasma preparations; for example, insulin-like growth factor is measurable in plasma obtained from healthy donors.64
In summary, transfusion of allogeneic blood products (red cells, platelets, and fresh-frozen plasma) is associated with a pro-inflammatory burden (bioactive products) in the recipient. The extent of this pro-inflammatory load in the recipient seems to be proportional to the stored age of the blood products. Many of these biological factors have the potential to directly and indirectly affect the innate immune function (NK-cell activity) in the recipient—a key protective mechanism for local tumour control and against metastatic spread in the surgical patient. In addition, other non-immunogenic mechanisms (hypoxia-induced signalling pathways) secondary to pre-existing patient conditions or transfusion of stored cold blood may play an equally important role in promoting a pro-angiogenic environment favouring tumour growth.
Immunity, inflammation, and cancer
An inflammatory microenvironment is an essential component of most tumours.Inflammatory responses in the body play decisive roles in tumorigenesis and metastasis. Metastatic potential of tumours is dependent on the complex and dynamic interplay between cancer cells, immune and inflammatory cells, and stromal elements in the tissue of origin. The process of metastasis can be divided into four major steps. The first step is epithelial-mesenchymal transition. This is the phase in which cancer cells acquire fibroblastoid characteristics that increase their motility and allow them to invade epithelial linings/basal membranes and reach efferent blood vessels or lymphatics.65 In the second step, cancer cells intravasate into blood vessels and lymphatics. Inflammation may promote this process through the production of mediators that increase vascular permeability. This is followed by the third step, in which metastasis-initiating cells survive and travel throughout the circulation. It has been estimated that only ∼0.01% of cancer cells that enter the circulation eventually survive and give rise to micrometastases.66 In the final step, single metastatic progenitors interact with immune, inflammatory, and stromal cells and start to proliferate.67 Cancer cell invasion requires extensive proteolysis of the extracellular matrix at the invasive front. Inflammatory cells are important sources of proteases that degrade the extracellular matrix. Th2 cytokines promote matrix metalloproteinase expression, invasiveness, and metastasis, while other cytokines, part of the Th1 response, suppress tumour growth.68
Once free tumour cells enter the circulation, they must survive in suspension and resist detachment-induced cell death or anoikis. The survival of circulating cancer cells is affected by inflammatory mediators released by immune cells in response to cancer-derived or pathogen-derived stimuli.69,70 Some of these effects depend on activation of NF-kB in either inflammatory cells or cancer cells. A variety of cytokines present in the tumour microenvironment, including TNF-α, IL-6, and epiregulin, can promote the survival of circulating metastatic seeds.71 In addition to activating NF-kB and STAT3, some of these transcription factors can physically link cancer cells to tumour-associated macrophages, allowing them to travel together throughout the circulation.72 On the other hand, single metastatic cells, which are no longer present within an immunosuppressive environment, may be targeted again by immunosurveillance. It is likely that immunosurveillance and tumour-promoting inflammation can coexist even in the same tumour.73 NK cells and CTLs engage in tumour killing, whereas Th1 cells boost cytotoxic immunity.74–76 On the other hand, Tregs suppress anti-tumour immune responses and are therefore pro-tumorigenic.74 Other critical components of this system are dendritic cells and macrophages, which present antigens and respond to danger and stress signals, and immunoregulatory and cytotoxic cytokines (Th1 cytokines) (Table 2).77–79
As is seen from the foregoing discussion, the complex interplay between the inflammatory cells, immune function, and the tumour cells determines tumour progression and establishment of distant metastasis. It is not clear whether acute and chronic inflammatory conditions have similar consequences and whether the same holds true for different types of acute inflammation. Furthermore, is the inflammatory burden from transfusion of stored blood products the same as an inflammatory burden from an acute infectious stimulus? The question still remains whether an acute inflammatory burden (perioperative blood transfusion) in an immunosuppressive environment (perioperative stress) compounds the problem and creates a pro-tumour environment for the establishment of distant metastasis.
Clinical studies linking perioperative blood transfusions and tumour progression
Allogeneic or autologous red cell transfusions
In evaluating clinical studies relating blood transfusion to outcomes, it is worth remembering that the circumstances under which patients are given blood products perioperatively are likely to influence cancer recurrence. Recurrence depends on preoperative nutrition and functional status, the presence of preoperative anaemia, tumour type and stage, degree of resectability, duration and type of anaesthesia, amount of blood loss, perioperative stress response, and the presence of postoperative complications.1–10 The potential impacts of many of these important confounding factors on cancer recurrence are complex and difficult to understand from retrospective studies. There are relatively few randomized trials related to transfusions and cancer recurrence.
Early studies, mostly retrospective, suggested that allogeneic perioperative blood transfusions increased the risk of cancer recurrence and mortality after oncologic surgery.83–92 Two initial meta-analyses concluded that perioperative blood transfusions were associated with poor outcomes after surgeries for colorectal cancer and cancer of the ampulla of Vater.93,94 This finding was corroborated by other retrospective studies that included patients with colorectal, prostate, pancreatic-duodenal, hepatic, and head and neck cancers.95–99 Specifically, in patients with head and neck cancer and hepatocellular carcinoma, blood transfusion appears to be an independent predictor of both recurrence [odds ratio (OR) 1.6 for both cancer types] and survival (hazard ratios 1.5 and 2.0). These results have been disputed by investigators who did not identify an association between blood transfusion and cancer recurrence.100–106
Perhaps the best current evidence comes from a large meta-analysis conducted by the Cochrane group that included randomized control trials (Table 3), along with prospective and retrospective observational studies. Pooled estimates of the effect of perioperative blood transfusions on recurrence in randomized studies yielded an OR of 1.42 (95% confidence interval, 1.20–1.67, P<0.0001) against transfused patients. Although heterogeneity was detected, stratified meta-analyses confirmed these findings by site and stage of disease, timing of administration of blood products, type of products administered, and volume of transfused products. However, given the heterogeneity and the inability to assess the effect of the surgical technique, the authors have not been able to attribute a definite causal relationship.107
Table 3.
Summary of randomized controlled trials evaluating cancer recurrence in patients who underwent colon cancer surgery with or without perioperative transfusions. CI, confidence interval
| Year | First author | Sample size | Odds ratio | [95% CI] | Outcome |
|---|---|---|---|---|---|
| 1985 | Frankish | 174 | 1.02 | [0.5, 2.06] | Recurrence in 24% of both transfused and non-transfused patients |
| 1990 | Cheslyn-Curtis | 961 | 1.16 | [0.88, 1.52] | Local recurrence or distal metastasis in 33 vs 36% of transfused and non-transfused patients, respectively |
| 1990 | Harder | 266 | 1.7 | [0.93, 3.10] | Recurrence in 40 vs 28% of transfused and non-transfused patients, respectively |
| 1992 | Tartter | 339 | 2.39 | [1.46, 3.91] | Recurrence in 40 vs 21% of transfused and non-transfused patients, respectively |
| 1994 | Heiss | 100 | 2.1 | [0.83, 5.32] | Recurrence in 33 vs 19% of transfused and non-transfused patients, respectively |
| 1994 | Houbiers | 697 | 1.23 | [0.87, 174] | Recurrence in 30 vs 26% of transfused and non-transfused patients, respectively |
| 1995 | Busch | 420 | 1.9 | [1.22, 296] | Recurrence in 41 vs 27% of transfused and non-transfused patients, respectively |
Less clear is whether administration of autologous blood modifies the risk of cancer recurrence in comparison with that for allogeneic transfusions.108,109 An early observational study suggested that, compared with autologous transfusions, allogeneic blood transfusions in patients undergoing head and neck cancer surgery were associated with a 40% increase in cancer recurrence.110 However, this result contrasts with that of a randomized controlled trial that allocated colorectal cancer patients undergoing surgical tumour resection to allogeneic vs autologous blood transfusion.111
Preoperative autologous blood collection and its transfusion intraoperatively appear to be safe in patients with hepatic cellular carcinoma, although the data remain limited.112,113 In fact, a small retrospective study suggests that the long-term cancer-free survival is longer in patients given autologous blood than in patients given allogeneic blood.113 Intraoperative cell salvage techniques are sometimes used in major surgeries and patients who refuse allogeneic blood products. A concern for the use of cell salvage techniques during oncologic surgery is the potential for re-infusion of malignant cells collected from the surgical site.114,115 For instance, malignant cells have been observed in surgically collected and double-filtrated blood samples of patients with hepatocellular carcinoma undergoing liver transplantation.116 That said, at least one study demonstrated lack of cell staining for markers of malignancy after filtration.117 More importantly, intraoperative cell salvage techniques and autologous blood transfusion do not seem to have a demonstrable effect on the rate of recurrence in patients undergoing oncologic surgery.118–120
In summary, the administration of perioperative blood transfusions in patients with colorectal cancer seems to be associated with increased risk of cancer recurrence.121–126 This phenomenon is less understood with other cancers. Intraoperative autologous transfusion appears to be safe, but it remains unclear whether it offers any advantages over administration of allogeneic blood transfusions in reference to cancer recurrence or overall mortality.
Leucocyte-reduced vs non-reduced packed RBCs
The presence of leucocytes and their products in allogeneic blood units may be responsible for some of the immunological derangements observed after red cell transfusions. It has, thus, been supposed that leucodepleted pRBCs would induce less immune suppression and possibly have a beneficial effect by leading to fewer recurrences after oncologic surgery. However, there is still no convincing proof in human trials that an amelioration of the immunomodulatory effect of blood transfusion results from leucoreduction.
A recent animal study found that erythrocytes rather than leucocytes are implicated in cancer-promoting effects of both autologous and allogeneic blood transfusions.127 van de Watering and colleagues128 found no effect of leucocyte depletion status of RBCs on overall 5-year survival or cancer recurrence in colorectal cancer. Finally, two other randomized controlled trials did not support improvement in disease-free survival after leucoreduced vs non-leucoreduced pRBC transfusions in patients with gastrointestinal cancer.129,130
Thus, the literature suggests that the leucoreduced status of RBC units transfused during the perioperative period may not decrease cancer recurrence after oncologic surgery (Table 4).
Table 4.
The table summarizes according to the type of study the effect of different blood product transfusions on cancer recurrence and overall survival after oncological surgery. BT, blood transfusion; LD, leucodepleted; 84, overall effect no association between BT and cancer recurrence, however, there was an association in patients with intrahepatic metastasis; 92, BT had effect only in local recurrence, not in metastasis; 120, no difference in RFS; however, the number of distant metastasis was statistically significant larger in the transfused group of patients; 123, patients who received autologous BT had better RFS for local recurrence but not distant metastasis; 128, an association for poor RFS and OS was found for transfusions of >3 units; 135, an association for poor OS was found for transfusions of >2 units. OS, overall survival; RFS, recurrence-free survival; DFS, disease-free survival
| Impact on RFS or DFS |
Impact on OS |
|||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| RBCs | ||||
| BT vs no BT | ||||
| Meta-analysis | 118, 128 | 104 | 7 | 91 |
| RCT | 119, 120 | 92, 108, 127 | 1, 3, 80, 86, 99, 100, 101, 135 | 108, 118, 125, 127 |
| Observational | 4, 9, 84, 86, 97, 98, 99, 100, 101, 117 | 7 | 10, 120 | |
| Retrospective | 1, 2, 81, 85, 87, 89, 93, 94, 95, 96, 102, 122, 128 | 2, 4, 81, 82, 83, 85, 91, 93, 94, 95, 96, 128, 129, 130, 135, 136 | ||
| Autolog vs Allog | ||||
| RCT | 92, 108, 123 | |||
| Retrospective | 97, 101, 105 | 107 | ||
| LD vs non-LD | ||||
| RCT | 125, 127 | 118 | ||
| Retrospective | 129 | |||
| Duration of storage | ||||
| Retrospective | 16, 108, 123 | |||
| Fresh-frozen plasma | ||||
| Retrospective | 3 | 3 | ||
| Platelets | ||||
| Retrospective | 138 | |||
Transfusion volume, blood product storage duration, and timing of transfusions
Several observational reports (Table 4) indicate that large-volume perioperative transfusions (generally >3 units) are associated with a greater chance of recurrence.94,131 In fact, intraoperative administration of ≥3 units has been associated with a relative risk of 2.1 [1.048, 4.135; P=0.036] of having shorter survival after surgery for ampulla of Vater cancer, and administration of ≥2 units has been associated with a hazard ratio of 1.6 [1.1, 2.3; P=0.015] after surgery for oesophageal cancer.94,132 The strongest evidence comes from Amato and Pescatori's meta-analysis, which reports that the risk of cancer recurrence increases by 40, 69, and 102% after 1-2 [OR, 1.40 (1.18, 1.67; P<0.0001)], 3–4 [OR, 1.69 (1.4, 2.03; P<0.00001)], and >5 [OR, 2.02 (1.65, 2.48, P<0.00001)] units of packed RBC transfusion, respectively.107 In contrast, the amount of blood transfused (≥8 units) in the perioperative period is associated with shorter overall survival after oesophageal cancer surgery [OR: 2.14 (1.14, 4.01, P=0.01)], but not with higher risk of local or distant recurrence.6,133 It is plausible to speculate that the number of units transfused may depend on the complexity of surgery and tumour size or invasiveness to adjacent structures, thus reflecting only disease stage which would make patients with higher tumour stages more likely to have a poor prognosis regardless the number of units transfused. Interestingly, Swisher and colleagues133 found that the those patients receiving 8 units or more still had a poorer survival after adjusting for tumour grade, lymph nodes, metastasis, and infectious complications.
One of the consequences of multiple blood transfusions is the risk of coagulopathy. This per se is associated to poor prognosis in non-operable cancer patients and the tissue factor (TF) pathway has been the focus of attention of several investigators.134,135 Thus, it is plausible to speculate that perioperative coagulation disorders associated with blood transfusion, mainly after massive blood transfusion, may also have a negative impact on cancer recurrence. Unfortunately, there are no clinical studies to support this hypothesis.
Storage duration of the transfused blood units has also been considered as a potential deleterious factor in the context of cancer recurrence.136 In an animal model, for instance, prolonged storage of transfused RBCs has been shown to enhance tumour progression.127 In contrast, in a post hoc analysis of a randomized controlled trial, Mynster and Nielsen137 did not find increased cancer recurrence after transfusion of older vs younger blood in patients who had colorectal surgery—a finding consistent with those of two other retrospective studies (Table 4).16,138
The timing of transfusions has also been considered. Briefly, pre-, intra-, and postoperative administration of blood increases the likelihood of cancer recurrence by 50, 74, and 36%, respectively.107 However, in a retrospective analysis of patients who underwent pancreatic surgery for exocrine tumours, postoperative blood transfusion was associated with higher mortality.139
In summary, perioperative administration of blood is associated with greater risk of cancer recurrence in colorectal cancers, and this association is stronger when larger volumes of blood are administered. However, clinical studies thus far have suggested that duration of storage contributes little, if any, additional risk of cancer recurrence.
Platelet concentrates and fresh-frozen plasma
As we have seen, administration of whole blood or pRBCs containing platelets and plasma may substantially impair anti-cancer immunity. But whether platelet concentrates and fresh-frozen plasma administration increases the risk of cancer recurrence after potentially curative cancer surgery remains unclear (Table 4). Experimental studies have indicated that the plasma fraction recovered from packed erythrocyte units stimulate tumour growth. Furthermore, enhancement of tumour growth was greatest with plasma from units that were stored longer suggesting storage-dependent growth promotion.140 In contrast, Tomimaru and colleagues3 have reported that administration of fresh-frozen plasma to patients undergoing resection of hepatocellular cancers did not worsen disease-free survival duration, although it did worsen overall survival duration.
Limited information exists regarding the administration of platelet concentrates and cancer recurrence after oncologic surgery. The only data available are those from a small retrospective study in which patients given platelet concentrates during surgery for hepatic adenocarcinoma had a higher risk of recurrence.141
Currently, there is no conclusive evidence that transfusion of fresh-frozen plasma or platelet concentrates to patients undergoing cancer surgery accelerates cancer recurrence.
Conclusion
Perioperative blood product transfusions cause substantial alterations to the anti-inflammatory/pro-inflammatory milieu in the recipient. Administration of stored blood products may be more deleterious than fresh products from the inflammatory-immunomodulatory perspective. Recent data in animal models suggest that old red cells, rather than leucocytes or soluble fractions, may be responsible for tumour-promoting effects. The net result of the transfusion of blood products in the recipient is immunodepression, although the extent and clinical significance of this phenomenon are not yet certain. Because of the growing evidence indicating that the presence of circulating tumour cells are linked to prognosis, studies showing no association between cell saver utilization and cancer recurrence should be accepted with caution.142
The best clinical evidence to date associating perioperative blood transfusions with cancer recurrence is in colorectal cancer. Whether blood transfusion also increases recurrence risk for other cancers is unclear because of the paucity of well-conducted randomized controlled trials that have addressed this question.
Future directions
Blood transfusion is an inherently hazardous and costly therapy that should be prescribed only when definite evidence for patient benefit outweighs the potential for harm. Accumulating evidence for potentially deleterious effects of blood transfusions in the cancer patient (i.e. recurrence) demands a more deliberate and cautionary approach in deciding the need for perioperative blood transfusions.
It is desirable that we institute a multidisciplinary team to develop patient-specific blood management protocols for the perioperative period. The aim of this approach should be to manage patients safely and effectively through their perioperative care continuum by utilizing all available measures to optimize patient erythrocyte mass and function and limit the need for transfusion of blood products. Patient blood management protocols should comprise three main components: (i) evaluating high-risk patients and optimizing erythrocyte mass and function for such patients, (ii) minimizing perioperative erythrocyte loss through blood-sparing surgical techniques, maintenance of normothermia, intraoperative cell salvage techniques when appropriate, use of antifibrinolytics when indicated, and optimized fluid therapy and haemodynamic control, and (iii) using patient-specific transfusion triggers to decide when administration of blood products is warranted.
It is necessary to highlight the fact that cancer progression and postoperative recurrence is affected by factors other than blood transfusions including stage of disease at the time of surgery, age, preoperative Karnofsky status, the presence of residual disease on postoperative margins, regional lymph nodes, or perioperative adjuvant therapy.143–145 Thus, we need to study whether comprehensive perioperative regimens aimed at preserving the immune function have a long-term effect on recurrence-free and overall survival in the cancer patient. There is an immediate need for well-designed clinical trials to study the effects of allogeneic blood transfusion on recurrence-free and overall survival in the cancer patient population.
Declaration of interest
D.I.S.'s department is funded by more than a dozen companies including Covidien, Arizant, Hospira, LMA North America, Drager, and Hutchinson. He has no personal financial interest in any of them. J.P.C.'s department is currently funded by Hospira.
Funding
This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Centre Support (grant, CA016672).
References
- 1.Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–80. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- 2.Shiba H, Ishida Y, Wakiyama S, et al. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg. 2009;13:1636–42. doi: 10.1007/s11605-009-0963-y. [DOI] [PubMed] [Google Scholar]
- 3.Tomimaru Y, Wada H, Marubashi S, et al. Fresh frozen plasma transfusion does not affect outcomes following hepatic resection for hepatocellular carcinoma. World J Gastroenterol. 2010;16:5603–10. doi: 10.3748/wjg.v16.i44.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugita S, Sasaki A, Iwaki K, et al. Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intraoperative allogenic blood transfusion: a retrospective study. Eur J Surg Oncol. 2008;34:339–45. doi: 10.1016/j.ejso.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 5.de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259–64. doi: 10.1159/000103656. [DOI] [PubMed] [Google Scholar]
- 6.Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–23. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 7.Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg. 2000;87:1553–62. doi: 10.1046/j.1365-2168.2000.01570.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagai S, Fujii T, Kodera Y, et al. Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas. 2011;40:3–9. doi: 10.1097/MPA.0b013e3181f7147a. [DOI] [PubMed] [Google Scholar]
- 9.Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg. 2011;15:120–9. doi: 10.1007/s11605-010-1379-4. [DOI] [PubMed] [Google Scholar]
- 10.Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol) 2006;18:60–6. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Colvin LA, Fallon MT, Buggy DJ. Cancer biology, analgesics, and anaesthetics: is there a link? Br J Anaesth. 2012;109:140–3. doi: 10.1093/bja/aes255. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh T, Buggy DJ. Can anaesthetic technique effect postoperative outcome? Curr Opin Anaesthesiol. 2012;25:185–98. doi: 10.1097/ACO.0b013e32834f6c4c. [DOI] [PubMed] [Google Scholar]
- 13.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch B, Guller U, Schnider A, et al. Perioperative detection of disseminated tumour cells is an independent prognostic factor in patients with colorectal cancer. Br J Surg. 2003;90:882–8. doi: 10.1002/bjs.4129. [DOI] [PubMed] [Google Scholar]
- 16.Cata JP, Klein EA, Hoeltge GA, et al. Blood storage duration and biochemical recurrence of cancer after radical prostatectomy. Mayo Clin Proc. 2011;86:120–7. doi: 10.4065/mcp.2010.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Twuyver E, Mooijaart RJ, ten Berge IJ, et al. Pretransplantation blood transfusion revisited. N Engl J Med. 1991;325:1210–3. doi: 10.1056/NEJM199110243251704. [DOI] [PubMed] [Google Scholar]
- 18.Jensen LS, Andersen AJ, Christiansen PM, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513–6. doi: 10.1002/bjs.1800790613. [DOI] [PubMed] [Google Scholar]
- 19.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 20.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–60. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 21.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 22.Blajchman MA. Immunomodulation and blood transfusion. Am J Ther. 2002;9:389–95. doi: 10.1097/00045391-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Ghio M, Contini P, Negrini S, Mazzei C, Zocchi MR, Poggi A. Down regulation of human natural killer cell-mediated cytolysis induced by blood transfusion: role of transforming growth factor-beta(1), soluble Fas ligand, and soluble Class I human leukocyte antigen. Transfusion. 2011;51:1567–73. doi: 10.1111/j.1537-2995.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 24.Weisbach V, Wanke C, Zingsem J, Zimmermann R, Eckstein R. Cytokine generation in whole blood, leukocyte-depleted and temporarily warmed red blood cell concentrates. Vox Sang. 1999;76:100–6. [PubMed] [Google Scholar]
- 25.Stack G, Baril L, Napychank P, Snyder EL. Cytokine generation in stored, white cell-reduced, and bacterially contaminated units of red cells. Transfusion. 1995;35:199–203. doi: 10.1046/j.1537-2995.1995.35395184274.x. [DOI] [PubMed] [Google Scholar]
- 26.Shanwell A, Kristiansson M, Remberger M, Ringden O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–84. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner JM, Nydam TL, Clarke JH, Banerjee A, Silliman CC, McCarter MD. Red blood cell supernatant potentiates LPS-induced proinflammatory cytokine response from peripheral blood mononuclear cells. J Interferon Cytokine Res. 2009;29:333–8. doi: 10.1089/jir.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karam O, Tucci M, Toledano BJ, et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion. 2009;49:2326–34. doi: 10.1111/j.1537-2995.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 29.Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res. 2006;135:226–32. doi: 10.1016/j.jss.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner JM, Silliman CC, Moore EE, Banerjee A, McCarter MD. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg. 2009;208:110–9. doi: 10.1016/j.jamcollsurg.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 32.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 34.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- 36.Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y, Damaj BB, Maghazachi AA. Human resting CD16−, CD16+ and IL-2-, IL-12-, IL-15- or IFN-alpha-activated natural killer cells differentially respond to sphingosylphosphorylcholine, lysophosphatidylcholine and platelet-activating factor. Eur J Immunol. 2005;35:2699–708. doi: 10.1002/eji.200526129. [DOI] [PubMed] [Google Scholar]
- 38.Coutant F, Perrin-Cocon L, Agaugue S, Delair T, Andre P, Lotteau V. Mature dendritic cell generation promoted by lysophosphatidylcholine. J Immunol. 2002;169:1688–95. doi: 10.4049/jimmunol.169.4.1688. [DOI] [PubMed] [Google Scholar]
- 39.Olofsson KE, Andersson L, Nilsson J, Bjorkbacka H. Nanomolar concentrations of lysophosphatidylcholine recruit monocytes and induce pro-inflammatory cytokine production in macrophages. Biochem Biophys Res Commun. 2008;370:348–52. doi: 10.1016/j.bbrc.2008.03.087. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi KE, Wanke C, Jacobi A, Weisbach V, Hemmerling TM. Determination of eicosanoid and cytokine production in salvaged blood, stored red blood cell concentrates, and whole blood. J Clin Anesth. 2000;12:94–9. doi: 10.1016/s0952-8180(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 41.Soontrapa K, Honda T, Sakata D, et al. Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc Natl Acad Sci U S A. 2011;108:6668–73. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baratelli F, Lee JM, Hazra S, et al. PGE(2) contributes to TGF-beta induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010;2:356–67. [PMC free article] [PubMed] [Google Scholar]
- 43.Mulligan JK, Rosenzweig SA, Young MR. Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2. J Immunother. 2010;33:126–35. doi: 10.1097/CJI.0b013e3181b91c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31:2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 45.AuBuchon JP, Herschel L, Roger J. Further evaluation of a new standard of efficacy for stored platelets. Transfusion. 2005;45:1143–50. doi: 10.1111/j.1537-2995.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 46.Vlaar AP, Hofstra JJ, Kulik W, et al. Supernatant of stored platelets causes lung inflammation and coagulopathy in a novel in vivo transfusion model. Blood. 2010;116:1360–8. doi: 10.1182/blood-2009-10-248732. [DOI] [PubMed] [Google Scholar]
- 47.Matsubayashi H, Weidner J, Miraglia CC, McIntyre JA. Platelet membrane early activation markers during prolonged storage. Thromb Res. 1999;93:151–60. doi: 10.1016/s0049-3848(98)00173-x. [DOI] [PubMed] [Google Scholar]
- 48.Spriggs MK, Armitage RJ, Strockbine L, et al. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543–50. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 50.Cognasse F, Osselaer JC, Payrat JM, Chavarin P, Corash L, Garraud O. Release of immune modulation factors from platelet concentrates during storage after photochemical pathogen inactivation treatment. Transfusion. 2008;48:809–13. doi: 10.1111/j.1537-2995.2008.01655.x. [DOI] [PubMed] [Google Scholar]
- 51.Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186:6543–52. doi: 10.4049/jimmunol.1002788. [DOI] [PubMed] [Google Scholar]
- 52.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 53.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 54.Chen XQ, Yang J, Hu SP, Nie HX, Mao GY, Chen HB. Increased expression of CD86 and reduced production of IL-12 and IL-10 by monocyte-derived dendritic cells from allergic asthmatics and their effects on Th1- and Th2-type cytokine balance. Respiration. 2006;73:34–40. doi: 10.1159/000087457. [DOI] [PubMed] [Google Scholar]
- 55.Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep. 2005;5:136–41. doi: 10.1007/s11882-005-0087-8. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida M, Watson RM, Rerecich T, O'Byrne PM. Different profiles of T-cell IFN-gamma and IL-12 in allergen-induced early and dual responders with asthma. J Allergy Clin Immunol. 2005;115:1004–9. doi: 10.1016/j.jaci.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Patscheke H, Stegmeier K. Investigation on a selective non-prostanoic thromboxane antagonist, BM 13.177, in human platelets. Thromb Res. 1984;33:277–88. doi: 10.1016/0049-3848(84)90163-4. [DOI] [PubMed] [Google Scholar]
- 58.Seghatchian J. Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apher Sci. 2006;34:125–30. doi: 10.1016/j.transci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Kanter J, Khan SY, Kelher M, Gore L, Silliman CC. Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin Cancer Res. 2008;14:3942–7. doi: 10.1158/1078-0432.CCR-07-4824. [DOI] [PubMed] [Google Scholar]
- 60.Apelseth TO, Hervig T, Wentzel-Larsen T, Petersen K, Reikvam H, Bruserud O. A prospective observational study of the effect of platelet transfusions on levels of platelet-derived cytokines, chemokines and interleukins in acute leukaemia patients with severe chemotherapy-induced cytopenia. Eur Cytokine Netw. 2011;22:52–62. doi: 10.1684/ecn.2011.0271. [DOI] [PubMed] [Google Scholar]
- 61.Holmes CE, Levis JE, Ornstein DL. Activated platelets enhance ovarian cancer cell invasion in a cellular model of metastasis. Clin Exp Metastasis. 2009;26:653–61. doi: 10.1007/s10585-009-9264-9. [DOI] [PubMed] [Google Scholar]
- 62.Theusinger OM, Baulig W, Seifert B, Emmert MY, Spahn DR, Asmis LM. Relative concentrations of haemostatic factors and cytokines in solvent/detergent-treated and fresh-frozen plasma. Br J Anaesth. 2011;106:505–11. doi: 10.1093/bja/aer003. [DOI] [PubMed] [Google Scholar]
- 63.Schneider SO, Rensing H, Graber S, et al. Impact of platelets and fresh frozen plasma in contrast to red cell concentrate on unstimulated and stimulated cytokine release in an in vitro model of transfusion. Scand J Immunol. 2009;70:101–5. doi: 10.1111/j.1365-3083.2009.02278.x. [DOI] [PubMed] [Google Scholar]
- 64.Hansen-Pupp I, Engstrom E, Niklasson A, et al. Fresh-frozen plasma as a source of exogenous insulin-like growth factor-I in the extremely preterm infant. J Clin Endocrinol Metab. 2009;94:477–82. doi: 10.1210/jc.2008-1293. [DOI] [PubMed] [Google Scholar]
- 65.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 68.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 69.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 72.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–8. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 74.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 75.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 76.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–50. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 78.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 79.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–46. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cognasse F, Boussoulade F, Chavarin P, et al. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–9. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 81.Picker SM, Steisel A, Gathof BS. Evaluation of white blood cell- and platelet-derived cytokine accumulation in MIRASOL-PRT-treated platelets. Transfus Med Hemother. 2009;36:114–20. doi: 10.1159/000203359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashimoto MN, Kimura EY, Yamamoto M, Bordin JO. Expression of Fas and Fas ligand on spleen T cells of experimental animals after unmodified or leukoreduced allogeneic blood transfusions. Transfusion. 2004;44:158–63. doi: 10.1111/j.1537-2995.2004.00646.x. [DOI] [PubMed] [Google Scholar]
- 83.Eisenkop SM, Spirtos NM, Montag TW, Moossazadeh J, Warren P, Hendrickson M. The clinical significance of blood transfusion at the time of radical hysterectomy. Obstet Gynecol. 1990;76:110–3. [PubMed] [Google Scholar]
- 84.Moores DW, Piantadosi S, McKneally MF. Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg. 1989;47:346–51. doi: 10.1016/0003-4975(89)90371-8. [DOI] [PubMed] [Google Scholar]
- 85.Chesi R, Cazzola A, Bacci G, Borghi B, Balladelli A, Urso G. Effect of perioperative transfusions on survival in osteosarcoma treated by multimodal therapy. Cancer. 1989;64:1727–37. doi: 10.1002/1097-0142(19891015)64:8<1727::aid-cncr2820640829>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 86.Edna TH, Vada K, Hesselberg F, Mjolnerod OK. Blood transfusion and survival following surgery for renal carcinoma. Br J Urol. 1992;70:135–8. doi: 10.1111/j.1464-410x.1992.tb15690.x. [DOI] [PubMed] [Google Scholar]
- 87.Matsumata T, Ikeda Y, Hayashi H, Kamakura T, Taketomi A, Sugimachi K. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72:1866–71. doi: 10.1002/1097-0142(19930915)72:6<1866::aid-cncr2820720613>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 88.Parrott NR, Lennard TW, Taylor RM, Proud G, Shenton BK, Johnston ID. Effect of perioperative blood transfusion on recurrence of colorectal cancer. Br J Surg. 1986;73:970–3. doi: 10.1002/bjs.1800731208. [DOI] [PubMed] [Google Scholar]
- 89.Jakobsen EB, Eickhoff JH, Andersen J, Lundvall L, Stenderup JK. Perioperative blood transfusion and recurrence and death after resection for cancer of the colon and rectum. Scand J Gastroenterol. 1990;25:435–42. doi: 10.3109/00365529009095512. [DOI] [PubMed] [Google Scholar]
- 90.Creasy TS, Veitch PS, Bell PR. A relationship between perioperative blood transfusion and recurrence of carcinoma of the sigmoid colon following potentially curative surgery. Ann R Coll Surg Engl. 1987;69:100–3. [PMC free article] [PubMed] [Google Scholar]
- 91.Mecklin JP, Jarvinen HJ, Ovaska JT. Blood transfusion and prognosis in colorectal carcinoma. Scand J Gastroenterol. 1989;24:33–9. doi: 10.3109/00365528909092236. [DOI] [PubMed] [Google Scholar]
- 92.Jones KR, Weissler MC. Blood transfusion and other risk factors for recurrence of cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1990;116:304–9. doi: 10.1001/archotol.1990.01870030068011. [DOI] [PubMed] [Google Scholar]
- 93.Chung M, Steinmetz OK, Gordon PH. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg. 1993;80:427–32. doi: 10.1002/bjs.1800800407. [DOI] [PubMed] [Google Scholar]
- 94.Yao HS, Wang Q, Wang WJ, Hu ZQ. Intraoperative allogeneic red blood cell transfusion in ampullary cancer outcome after curative pancreatoduodenectomy: a clinical study and meta-analysis. World J Surg. 2008;32:2038–46. doi: 10.1007/s00268-008-9675-9. [DOI] [PubMed] [Google Scholar]
- 95.Busch OR, Hop WC, Marquet RL, Jeekel J. Blood transfusions and local tumor recurrence in colorectal cancer. Evidence of a noncausal relationship. Ann Surg. 1994;220:791–7. doi: 10.1097/00000658-199412000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heal JM, Chuang C, Blumberg N. Perioperative blood transfusions and prostate cancer recurrence and survival. Am J Surg. 1988;156:374–80. doi: 10.1016/s0002-9610(88)80190-9. [DOI] [PubMed] [Google Scholar]
- 97.Kneuertz PJ, Patel SH, Chu CK, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18:1327–34. doi: 10.1245/s10434-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 98.Chau JK, Harris JR, Seikaly HR. Transfusion as a predictor of recurrence and survival in head and neck cancer surgery patients. J Otolaryngol Head Neck Surg. 2010;39:516–22. [PubMed] [Google Scholar]
- 99.Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2009;16:1832–42. doi: 10.1245/s10434-009-0448-y. [DOI] [PubMed] [Google Scholar]
- 100.Ford BS, Sharma S, Rezaishiraz H, Huben RS, Mohler JL. Effect of perioperative blood transfusion on prostate cancer recurrence. Urol Oncol. 2008;26:364–7. doi: 10.1016/j.urolonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Gallina A, Briganti A, Chun FK, et al. Effect of autologous blood transfusion on the rate of biochemical recurrence after radical prostatectomy. BJU Int. 2007;100:1249–53. doi: 10.1111/j.1464-410X.2007.07147.x. [DOI] [PubMed] [Google Scholar]
- 102.Morris PC, Haugen J, Tomjack J, Anderson B, Buller RE. Blood transfusion and the risk of recurrence in stage IB cervical cancer. Gynecol Oncol. 1995;57:401–6. doi: 10.1006/gyno.1995.1162. [DOI] [PubMed] [Google Scholar]
- 103.Sene A, Jeacock J, Robinson C, Walsh S, Kingston RD. Blood transfusion does not have an adverse effect on survival after operation for colorectal cancer. Ann R Coll Surg Engl. 1993;75:261–6. discussion 266–267. [PMC free article] [PubMed] [Google Scholar]
- 104.Paul R, Schmid R, Busch R, et al. Influence of blood transfusions during radical retropubic prostatectomy on disease outcome. Urology. 2006;67:137–41. doi: 10.1016/j.urology.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 105.Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771–8. [PubMed] [Google Scholar]
- 106.Kaibori M, Ishizaki M, Matsui K, Kitade H, Matsui Y, Kwon AH. Evaluation of metabolic factors on the prognosis of patients undergoing resection of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:536–43. doi: 10.1111/j.1440-1746.2010.06439.x. [DOI] [PubMed] [Google Scholar]
- 107.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;Jan:CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oefelein MG, Colangelo LA, Rademaker AW, McVary KT. Intraoperative blood loss and prognosis in prostate cancer patients undergoing radical retropubic prostatectomy. J Urol. 1995;154:442–7. doi: 10.1097/00005392-199508000-00029. [DOI] [PubMed] [Google Scholar]
- 109.Ness PM, Bourke DL, Walsh PC. A randomized trial of perioperative hemodilution versus transfusion of preoperatively deposited autologous blood in elective surgery. Transfusion. 1992;32:226–30. doi: 10.1046/j.1537-2995.1992.32392213805.x. [DOI] [PubMed] [Google Scholar]
- 110.Moir MS, Samy RN, Hanasono MM, Terris DJ. Autologous and heterologous blood transfusion in head and neck cancer surgery. Arch Otolaryngol Head Neck Surg. 1999;125:864–8. doi: 10.1001/archotol.125.8.864. [DOI] [PubMed] [Google Scholar]
- 111.Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–6. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 112.Fujimoto J, Okamoto E, Yamanaka N, et al. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg. 1993;128:1065–9. doi: 10.1001/archsurg.1993.01420210129021. [DOI] [PubMed] [Google Scholar]
- 113.Hirano T, Yamanaka J, Iimuro Y, Fujimoto J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg Today. 2005;35:1042–6. doi: 10.1007/s00595-005-3082-8. [DOI] [PubMed] [Google Scholar]
- 114.Ereth MH, Oliver WC, Jr, Santrach PJ. Perioperative interventions to decrease transfusion of allogeneic blood products. Mayo Clin Proc. 1994;69:575–86. doi: 10.1016/s0025-6196(12)62250-2. [DOI] [PubMed] [Google Scholar]
- 115.Popovsky MA, Devine PA, Taswell HF. Intraoperative autologous transfusion. Mayo Clin Proc. 1985;60:125–34. doi: 10.1016/s0025-6196(12)60299-7. [DOI] [PubMed] [Google Scholar]
- 116.Liang TB, Li DL, Liang L, et al. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863–9. doi: 10.1097/TP.0b013e3181671f2e. [DOI] [PubMed] [Google Scholar]
- 117.Martin RC, Wellhausen SR, Moehle DA, Martin AW, McMasters KM. Evaluation of intraoperative autotransfusion filtration for hepatectomy and pancreatectomy. Ann Surg Oncol. 2005;12:1017–24. doi: 10.1245/ASO.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 118.Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol. 2011;18:166–73. doi: 10.1245/s10434-010-1228-4. [DOI] [PubMed] [Google Scholar]
- 119.Muscari F, Suc B, Vigouroux D, et al. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int. 2005;18:1236–9. doi: 10.1111/j.1432-2277.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 120.Mirhashemi R, Averette HE, Deepika K, et al. The impact of intraoperative autologous blood transfusion during type III radical hysterectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1999;181:1310–5. doi: 10.1016/s0002-9378(99)70369-8. discussion 1315–1316. [DOI] [PubMed] [Google Scholar]
- 121.Houbiers JG, Brand A, van de Watering LM, et al. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;344:573–8. doi: 10.1016/s0140-6736(94)91965-8. [DOI] [PubMed] [Google Scholar]
- 122.Frankish PD, McNee RK, Alley PG, Woodfield DG. Relation between cancer of the colon and blood transfusion. Br Med J (Clin Res Ed) 1985;290:1827. doi: 10.1136/bmj.290.6484.1827-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheslyn-Curtis S, Fielding LP, Hittinger R, Fry JS, Phillips RK. Large bowel cancer: the effect of perioperative blood transfusion on outcome. Ann R Coll Surg Engl. 1990;72:53–9. [PMC free article] [PubMed] [Google Scholar]
- 124.Harder F, Laffer U, Berres M, Jaggi P, Metzger U. Following curative resection of colorectal cancer, portal chemotherapy especially benefits non-transfused patients. Chirurg. 1990;61:280–5. [PubMed] [Google Scholar]
- 125.Tartter PI. The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg. 1992;216:633–8. doi: 10.1097/00000658-199212000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Busch OR, Hop WC, Marquet RL, Jeekel J. The effect of blood transfusions on survival after surgery for colorectal cancer. Eur J Cancer. 1995;31A:1226–8. doi: 10.1016/0959-8049(95)00174-h. [DOI] [PubMed] [Google Scholar]
- 127.Atzil S, Arad M, Glasner A, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008;109:989–97. doi: 10.1097/ALN.0b013e31818ddb72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van de Watering LM, Brand A, Houbiers JG, Klein Kranenbarg WM, Hermans J, van de Velde C. Perioperative blood transfusions, with or without allogeneic leucocytes, relate to survival, not to cancer recurrence. Br J Surg. 2001;88:267–72. doi: 10.1046/j.1365-2168.2001.01674.x. [DOI] [PubMed] [Google Scholar]
- 129.van Hilten JA, van de Watering LM, van Bockel JH, et al. Effects of transfusion with red cells filtered to remove leucocytes: randomised controlled trial in patients undergoing major surgery. Br Med J. 2004;328:1281. doi: 10.1136/bmj.38103.735266.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lange MM, van Hilten JA, van de Watering LM, et al. Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg. 2009;96:734–40. doi: 10.1002/bjs.6636. [DOI] [PubMed] [Google Scholar]
- 131.Zdravkovic D, Bilanovic D, Randjelovic T, et al. Allogeneic blood transfusion in patients in Dukes B stage of colorectal cancer. Med Oncol. 2011;28:170–4. doi: 10.1007/s12032-010-9441-3. [DOI] [PubMed] [Google Scholar]
- 132.Ling FC, Hoelscher AH, Vallbohmer D, et al. Leukocyte depletion in allogeneic blood transfusion does not change the negative influence on survival following transthoracic resection for esophageal cancer. J Gastrointest Surg. 2009;13:581–6. doi: 10.1007/s11605-008-0787-1. [DOI] [PubMed] [Google Scholar]
- 133.Swisher SG, Holmes EC, Hunt KK, Gornbein JA, Zinner MJ, McFadden DW. Perioperative blood transfusions and decreased long-term survival in esophageal cancer. J Thorac Cardiovasc Surg. 1996;112:341–8. doi: 10.1016/S0022-5223(96)70260-X. [DOI] [PubMed] [Google Scholar]
- 134.Rhee J, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010;25:1537–42. doi: 10.1111/j.1440-1746.2010.06289.x. [DOI] [PubMed] [Google Scholar]
- 135.Garnier D, Milsom C, Magnus N, et al. Role of the tissue factor pathway in the biology of tumor initiating cells. Thromb Res. 2010;125(Suppl. 2):S44–50. doi: 10.1016/S0049-3848(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 136.Eickhoff JH, Gote H, Baeck J. Peri-operative blood transfusion in relation to tumour recurrence and death after surgery for prostatic cancer. Br J Urol. 1991;68:608–11. doi: 10.1111/j.1464-410x.1991.tb15424.x. [DOI] [PubMed] [Google Scholar]
- 137.Mynster T, Nielsen HJ. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44:955–64. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 138.Edna TH, Bjerkeset T. Perioperative blood transfusions reduce long-term survival following surgery for colorectal cancer. Dis Colon Rectum. 1998;41:451–9. doi: 10.1007/BF02235758. [DOI] [PubMed] [Google Scholar]
- 139.Yeh JJ, Gonen M, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. Br J Surg. 2007;94:466–72. doi: 10.1002/bjs.5488. [DOI] [PubMed] [Google Scholar]
- 140.Barnett CC, Jr, Beck AW, Holloway SE, et al. Intravenous delivery of the plasma fraction of stored packed erythrocytes promotes pancreatic cancer growth in immunocompetent mice. Cancer. 2010;116:3862–74. doi: 10.1002/cncr.25140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaido T, Takada Y, Egawa H, Uemoto S. The influence of intraoperative homologous blood transfusion on prognosis after liver transplantation for hepatocellular carcinoma. Hepatogastroenterology. 2009;56:808–12. [PubMed] [Google Scholar]
- 142.Lucci A, Hall C, Lodhi A, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;7:688–95. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 143.Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev. 2012;5:CD007583. doi: 10.1002/14651858.CD007583.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann Surg Oncol. 2012;19:801–8. doi: 10.1245/s10434-011-2035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gerber B, Heintze K, Stubert J, et al. Axillary lymph node dissection in early-stage invasive breast cancer: is it still standard today? Breast Cancer Res Treat. 2011;128:613–24. doi: 10.1007/s10549-011-1532-0. [DOI] [PubMed] [Google Scholar]