Abstract
Objective
We investigated the norepinephrine transporter (NET) expression in normal and pre-eclamptic placentas and analyzed the invasion activity of trophoblastic cells based on norepinephrine (NE)-NET regulation.
Methods
NET and NE expression levels were examined by western blot and enzyme-linked immunosorbent assay, respectively. Trophoblast invasion activity, depending on NE-NET regulation, was determined by NET-small interfering RNA (siRNA) and NET transfection into the human extravillous trophoblast cells with or without NE treatment and invasion rates were analyzed by zymography and an invasion assay.
Results
NET mRNA was expressed at a low level in pre-eclamptic placentas compared with normal placentas and NE concentration in maternal plasma increased significantly in pre-eclamptic women compared to normal pregnant women (p<0.05). NET gene upregulation and NE treatment stimulated trophoblast cell invasion up to 2.5-fold (p<0.05) by stimulating matrix metalloproteinase-9 activity via the phosphoinositol-3-kinase/AKT signaling pathway, whereas NET-siRNA with NE treatment reduced invasion rates.
Conclusion
NET expression is reduced by inadequate regulation of NE levels during placental development. This suggests that a complementary balance between NET and NE regulates trophoblast cell invasion activities during placental development.
Keywords: Placenta, Pre-eclampsia, Norepinephrine transporter, Enzyme-linked immunosorbent assay, Small interfering RNA
Introduction
Pre-eclampsia (PE), which is one of the most common causes of perinatal and maternal morbidity and mortality from obstetric diseases, is a multisystem disorder diagnosed by accompanying hypertension, proteinuria, and edema during the second half of pregnancy, and its frequency is approximately 5% of all pregnancies [1]. The only curative therapy for PE is termination of the pregnancy, which generally leads to symptom resolution [2]. Divergent opinions exist on the precise mechanism for the primary pathophysiological process of PE, but the root causes start at the early stage of implantation. In particular, PE has the characteristic of a lack of trophoblast invasion into the maternal arteries. The lack of successful invasion by extravillous cytotrophoblasts inadequate to meet the endovascular remodeling of spiral arterioles in the maternal myometrium affects the physiological modification of the spiral arteries [3]. Therefore, failure of invasion results in low fetal perfusion [4], nutrients, and gas exchange toward the fetal side of the placenta and causes PE, with its hallmark generalized vascular endothelial dysfunction [5]. Hypertension in women with PE is due to peripheral vasoconstriction and increased blood flow resistance. During normotensive and pre-eclamptic pregnancies several monoamine neurotransmitters are secreted from the sympathetic nervous system and are important factors in the pathophysiology of hypertension [6,7].
Norepinephrine (NE) is one of the dominant monoamine neurotransmitters released during pregnancy, and higher blood levels of NE occur in gynecological diseases, including PE [6]. The norepinephrine transporter (NET) plays a major role as a specific uptake system for NE in synaptic membranes of neurons and is responsible for fast termination of NE after neuronal release [8]. The human placenta expresses NET [9] and other neurotransmitter transporters [10]. Moreover, catecholamine clearance normally maintains the fetal circulation and placental metabolic functions via re-uptake of NE in the extracellular space [11]. In pre-eclampsia, a decrease in NET expression [12] or NET blockade (e.g., use of drugs) is postulated to increase NE levels. In placental defects, NET's function is physiologically valuable because NE is mainly regulated by NET and is associated with vasoconstriction [12,13].
Decreased NET expression during early gestation contributes to a high NE concentration, which causes vasoconstriction of the uterine spiral arteries and induces an inflammatory response on the maternal side [14]. These conditions suggest failure of ordinary invasion percolation by extravillous trophoblasts, leading to incomplete spiral artery vascular remodeling [15]. However, the invasion activity of trophoblasts through alterations in NE and NET expression and their associations during placental development and PE are still unclear. Recent studies have shown that neurotransmitters are involved in the regulation of cancer cell invasion (e.g., ovarian, nasopharyngeal, and pancreatic cancer cells) via β-adrenoceptors [16,17]. The increase in interstitial NE concentration is expected to lower NET function and is associated with the downregulation of myocardial β-adrenoceptors [18].
The purposes of this study were to determine NET and NE expression patterns in normal placentas compared to pre-eclamptic placentas and to examine the invasive potential of a first-trimester extravillous trophoblast cell line (i.e., HTR-8/SVneo) using cDNA-NET upregulation and small interfering RNA (siRNA)-based NET downregulation. Furthermore, we analyzed the expression pattern of matrix metalloproteinases (MMP-2 and MMP-9) involved in the invasion ability of extravillous cytotrophoblasts.
Methods
1. Collection of placental tissues
The collection and use of samples for the study was approved by the institutional review board of CHA General Hospital, and informed consent was obtained from all patients prior to sample collection. All women included in our sample underwent elective cesarean sections without labor to avoid the effect of labor. Normal placenta and blood were collected from normal pregnant women who had uncomplicated pregnancies (≥37 gestational weeks). PE was diagnosed based on hypertension, proteinuria, and edema (Table 1). Hypertension was defined as a systolic blood pressure of 140 mm Hg or a diastolic blood pressure of 90 mm Hg on at least two occasions. Proteinuria was defined as 2+ protein by urine dipstick or higher than 300 mg on a 24-hour urine collection. Placental tissues were obtained from the central area of the placentas from normal pregnant women including at term (n=15, 38±2.5 weeks), with 3rd trimester PE (n=15, 37±3.4 weeks), and with 2nd trimester PE (n=11, 33±3.3 weeks). The tissues were snap-frozen in liquid nitrogen and stored at -80℃ until use. Additionally, blood samples were collected before delivery and separated for the plasma and red blood cell layers by centrifugation; the plasma was transferred to a new tube and stored at -70℃ until use.
Table 1.
Maternal age and severity in normal and preeclamptic pregnancies
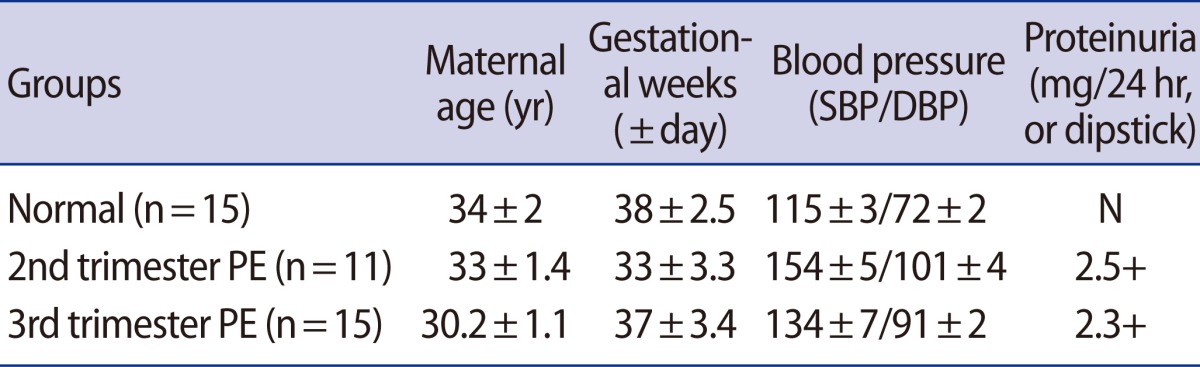
SBP, systolic blood pressure; DBP, diastolic blood pressure; N, negative; PE, pre-eclampsia.
2. Cell culture
The human extravillous trophoblast cell line HTR-8/SVneo was provided by Dr. Charles H. Graham and cultured as previously described [19]. Cells were cultured in RPMI-1640 medium supplemented with 5% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco-BRL, Grand Island, NY, USA) at 37℃ in a humidified 5% CO2 , 95% air atmosphere.
3. Cell viability assay post NE treatment
For all experiments, up to 70% confluent cells were incubated for 24 hours prior to drug administration. NE (Sigma, St. Louis, MO, USA) was stored in serum-free medium at -20℃. After NET upregulation or downregulation for 24 hours, cells (NE-HTR8) were exposed to NE for 24 hours. HTR8 cells' viability was measured using 0.4% trypan blue (Gibco-BRL) for 3 minutes. Blue-stained cells were considered nonviable, whereas unstained cells were considered viable. Treated cells were counted separately on a hemocytometer under an inverted microscope.
4. NET expression in HTR-8/SVneo cells
Full-length NET cDNA was inserted into the pcDNA3.1/V5 expression vector (Invitrogen, Carlsbad, CA, USA). This expression vector (NET-pcDNA) was constructed as previous reports [20]. The NET plasmid was transfected into HTR-8/SVneo cells using Lipofectamine 2000, according to the manufacturer's instructions (Invitrogen). To analyze the loss of NET function, HTR-8/SVneo cells were cultured at 50% to 60% confluence and transfected with the NET-siRNA duplex (NET-siRNA) or a negative control (Bioneer, Daejeon, Korea). The NET-specific target sequences used in the study were 5'-GAUUUCGUGACUGUAGUUU-3' (sense) and 5'-AAACUACAGUCACGAAAUC-3' (antisense). Otherwise, the negative control-specific target sequences were 5'-CCUACGCCACCAAUUUCGU-3' (sense) and 5'-ACGAAAUUGGUGGCGUAGG-3' (antisense). Cells were transfected with NET-siRNA or a negative control using Lipofectamine 2000, according to the manufacturer's instructions. Cells or medium were harvested and analyzed 24 hours after transfection.
5. Cell invasion assay
Cell invasion was performed in a 24-well cell culture insert system (8-µm pore size, Becton Dickinson Labware, Bedford, MA, USA). Cells (2.5×104) were seeded into the upper chamber. After a 24-hour incubation, the remaining cells in the upper chamber were completely removed with a cotton swab, and the invading cells on the bottom surface were fixed with absolute methanol and stained with Mayer's hematoxylin (Dako, Produktionsnej, Glostrup, Denmark). The number of stained cells on the membranes was counted in five randomly non-overlapping fields at a magnification of ×100.
6. Semiquantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from the cells and frozen tissue using TRIzol reagent (Invitrogen). The cDNA synthesis was performed using SuperScript III Reverse Transcriptase and total RNA (1 µg), according to the manufacturer's instructions (Invitrogen). Polymerase chain reaction (PCR) reactions were conducted in a T3000 Thermocycler (Biometra, Goettingen, Germany) under the following conditions: denaturation at 95℃ for 15 minutes, amplification at 95℃ for 20 seconds, annealing at the appropriate temperature for each set of primers for 40 seconds, extension at 72℃ for 40 cycles at 1 minute each, and termination at 72℃ for 5 minutes. The PCR primers used in the amplifications were as follows: hNET, 5'-TGAAGACATCAGGAAAGGTGGTG-3' (sense) and 5'-CAATCAATACTCCAAATCCAGCCC-3' (antisense); 28S rRNA, 5'-TTGAAAATCCGGGGGAGAG-3' (sense) and 5'-ACATTGTTCCAACATGCCAG-3' (antisense). The PCR products were electrophoresed on 1.5% agarose gels. A semiquantitative evaluation was visualized using a Gel DOC XR Molecular Imager (Bio-Rad, Hercules, CA, USA), and the density value of the PCR products was measured using Quantity One software ver. 4.6 (Bio-Rad). All experiments were performed in triplicate.
7. Western blot analysis
Cell and placental tissues were homogenized and lysed with cold ProteoJET Mammalian Cell Lysis Reagent (Fermentas, Burlington, ON, Canada) supplemented with a complete protease inhibitor cocktail (Roche, Grenzacherstrasse, Basel, Switzerland) according to the manufacturers' instructions. Total protein was extracted using two sonication cycles (Sonics & Materials, Newtown, CT, USA) at an amplitude of 50 for 15 seconds and then cleared by centrifugation at 15,000 rpm at 4℃ for 15 minutes. The total protein (20-30 µg) was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane (Bio-Rad). After blocking with 7% skim milk solution, the membranes were incubated with one of the following primary antibodies: rabbit anti-NET (1:2,000, Chemicon, Temecula, CA, USA), rabbit anti-phosphorylated AKT (ser473) (1:1,000, Cell Signaling Technology, Danvers, MA, USA), mouse anti-phosphorylated ERK 1/2 (1:2,000, Cell Signaling Technology), rabbit anti-phosphorylated Stat3 (Try-705) (1:500, Cell Signaling Technology), and mouse anti-actin (1:5,000, Santa-Cruz Biotechnology, Santa Cruz, CA, USA) at 4℃ overnight, followed by an incubation with horseradish peroxidase-conjugated goat anti-rabbit (1:3,000, Bio-Rad) or goat anti-mouse (1:10,000, Bio-Rad) secondary antibody. After five washings, the bands were detected using chemiluminescence reagent (Amersham Bioscience, Little Chalfont, Buckinghamshire, UK). The experiments were repeated at least twice.
8. Enzyme-linked immunosorbent assay
The concentrations of total NE, total NE in maternal blood plasma and conditioned medium, and placental total protein were determined using a commercial ELISA (Labor Diagnostika Nord GmbH, Nordhorn, Germany) according to the manufacturer's instructions. Conditioned medium from HTR8 cells was collected at 24 hours following NET gene upregulation or downregulation to determine the endogenous NE concentration. The supernatants were centrifuged at 12,000 rpm for 3 minutes at 4℃ to remove debris, and the cleared supernatants were stored (-80℃). All experiments were performed in triplicate.
9. Gelatin zymography
NET-siRNA and NE treatments of HTR8 cells were assayed by zymography to determine the level of MMP activity in NET-pcDNA conditioned medium. The cells (2.0×105) were seeded into 6-well culture dishes, and the conditioned medium was collected at 24 hours. For each aliquot, 30 µL was separated on 12% SDS-PAGE containing 1 mg/mL gelatin (Sigma). After electrophoresis, the gels were washed twice for 15 minutes in a renaturation buffer (Bio-Rad), rinsed briefly with water, and incubated overnight at 37℃ in a development buffer (Bio-Rad). Subsequently, the gels were stained for 1 hour at room temperature with Coomassie Brilliant Blue R-250 in 10% acetic acid/40% methanol, destained in 10% acetic acid/40% methanol, and then dried between cellophane sheets. MMP activities were detected as the density of the unstained band.
10. Statistics
We used SAS software (SAS Institute Inc., Cary, NC, USA) for statistical analyses. The results are presented as means±SE. All of the experiments were analyzed using analysis of variance followed by Tukey's multiple comparison test or Scheffe's multiple comparison test with p<0.05 for significance.
Results
1. Analysis of NE and NET expression in maternal blood and placental tissues
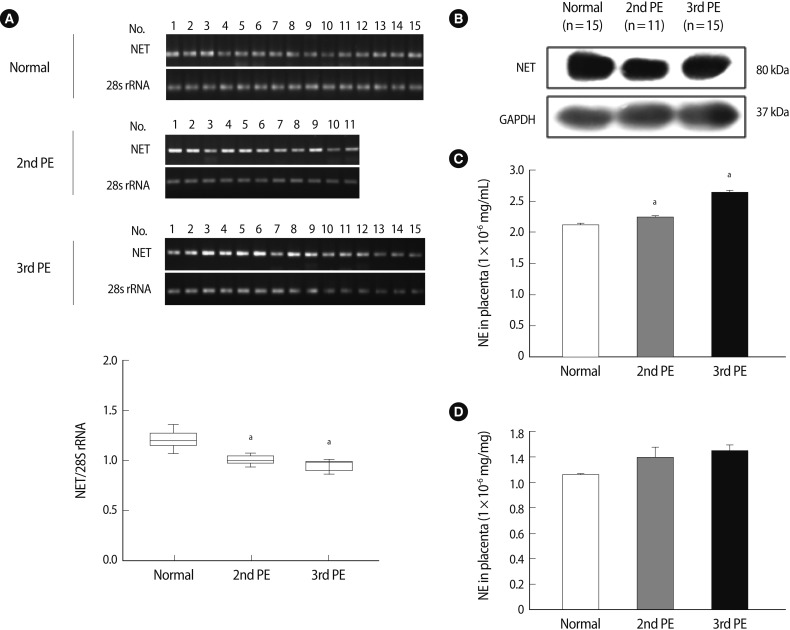
We conducted reverse transcription-polymerase chain reaction (RT-PCR) and western blot analyses to analyze NET expression in placental tissues. The NET expression in 2nd-PE and 3rd-PE placental tissues decreased significantly compared to 3rd trimester normal placental tissues (p<0.001) (Figure 1A). Otherwise, no differences were observed between the 2nd PE and 3rd PE. Similar to RT-PCR results, NET expression decreased more in 2nd PE than normal placental tissues (Figure 1B). Also, NET expression was localized in cytotrophoblasts and chorionic villi syncytiotrophoblasts (data not shown). These findings indicate that cellular NET mRNA expression decreased in women with PE compared to normal women, but that the expression seemed likely to affect posttranslational regulation. Next, we determined NE expression in maternal blood plasma and placental tissue by ELISA. As shown in Figure 1C, plasma NE concentrations in the 3rd trimester were significantly higher in the PE group than in the normal group. Furthermore, plasma NE concentrations in the 3rd PE were significantly higher than those of the 2nd PE (p<0.05). Placental NE expressions were higher in the PE group than in the normal group, but the difference was not significant (Figure 1D).
Figure 1.
The expression of NET and NE in placental tissues and maternal blood. (A) NET mRNA expression (upper) and densitometric analysis (lower) in placental tissues using semi quantitative reverse transcription-polymerase chain reaction. NET mRNA expression was normalized to the amount of 28S rRNA and ispresented in a box plot. The expression levels of NET mRNA were more significantly reduced in a pre-eclamptic placenta than a normal placenta. Significant differences are indicated by ap<0.05 vs. normal. Data are expressed as the means±SE. of three separate experiments in duplicate. (B) NET expression in normal and pre-eclamptic placental tissues using western blot analysis. GAPDH was used as an internal control. (C) The NE concentration in maternal plasma between normal and pre-eclamptic pregnancies. The NE concentration increased significantly more in pre-eclamptic pregnancies than normal pregnancies. (D) The NE expression in placental tissues in normal and pre-eclamptic pregnancies. No difference was observed in each group. Significant differences are indicated by ap<0.05 vs. normal or 2nd PE. Columns, average value for triplicate experiments; NE, norepinephrine; NET, norepinephrine transporter; PE, pre-eclampsia; 2nd PE, second trimester pre-eclampsia; 3rd PE, third trimester pre-eclampsia.
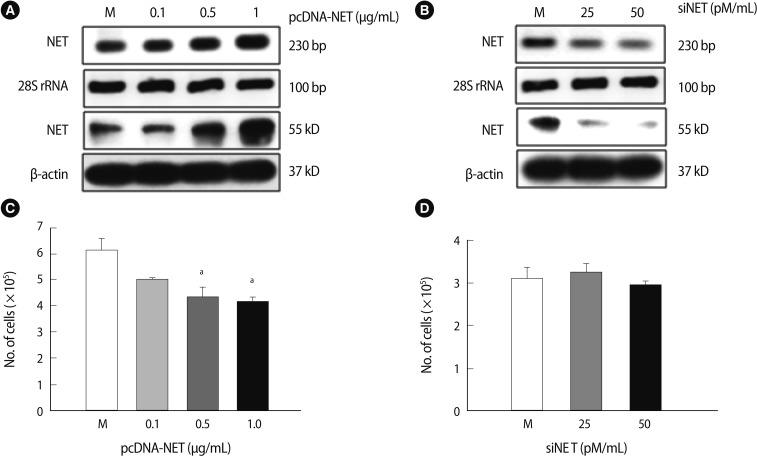
2. NET expression depends on HTR-8/SVneo cell gene-regulation
pcDNA-NET or siRNA-NET was transfected into HTR-8/SVneo cells and analyzed by RT-PCR and western blot analyses to investigate the NET functions dependent on gene regulation. As shown in Figure 2A, NET expression (RNA and protein levels) increased compared to the mock group when 1 µg/mL of pcDNA-NET was transfected into HTR-8/SVneo cells (Figure 2A). Moreover, both NET RNA and protein levels decreased when 50 pmole of siRNA-NET were transfected into HTR-8/SVneo cells (Figure 2B). Furthermore, the viability of HTR-8 cells was significantly higher in 0.5 and 1 µg/mL of pcDNA-NET compared to the mock group (p<0.05) (Figure 2C). However, no difference in the viability of HTR-8/SVneo cells was observed following the knockdown of NET by siRNA-NET (Figure 2D). Therefore, the optimal concentrations of pcDNA-NET and siRNA-NET that did not affect cell viability were 0.1 µg/mL and 50 pM/mL, respectively. These findings suggest that NET gene overexpression, but not NET downregulation, decreases viability of the HTR-8/SVneo trophoblast cell line.
Figure 2.
NET expression affects the viability of HTR-8/SVneo cells. (A) NET expression in HTR-8/SVneo trophoblast cells following transfection of a vector containing NET cDNA or an empty vector, using reverse transcription-polymerase chain reaction (RT-PCR) (upper) and western blot analysis (lower). (B) NET expression in HTR-8/SVneo trophoblast cells by siRNA targeting NET or a scrambled sequence using RT-PCR (upper) and western blot (lower). 28S rRNA and β-actin were used as internal controls for RT-PCR and western blot, respectively. (C) The viability of HTR-8/SVneo cells after transfection with NET-pcDNA in a dose-dependent manner for 24 hours. The cell viability was significantly reduced at 0.5 and 1.0 µg/mL pcDNA-NET. (D) The viability of HTR-8/SVneo cells after transfection with siNET. Significant differences are indicated by ap<0.05 vs. Mock. NET, norepinephrine transporter; Columns, average value for three independent experiments; Bar, SE; M, mock control.
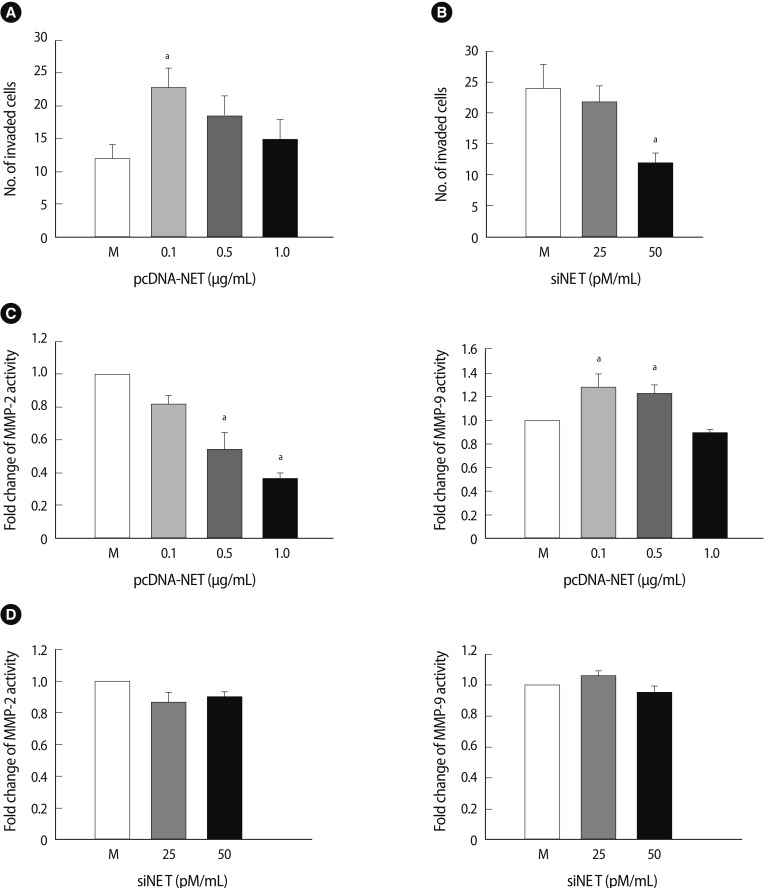
3. Invasion and MMP expression in HTR-8/SVneo cells depends on NET expression
We conducted an invasion assay with HTR-8/SVneo cells to explore whether NET expression is associated with invasion activity of HTR-8/SVneo cells under gene regulation. The number of invading HTR-8/SVneo cells transfected with pcDNA-NET increased by 1.8-fold at 0.1 µg/mL of pcDNA-NET compared to the mock group (p<0.05) (Figure 3A). In contrast, the number of invading cells transfected with siRNA-NET decreased by 50% at 50 pmole of siRNA-NET compared to the mock group (p<0.05) (Figure 3B).
Figure 3.
Invasion analysis and enzyme activity of HTR-8/SVneo cells affected by NET expression. (A) The pcDNA-NET concentration-dependent invasion activity of HTR-8/SVneo cells. (B) The siNET concentration-dependent invasion activity of HTR-8/SVneo cells. (C) The enzyme activity of MMP-2 (left) and MMP-9 (right) in conditioned medium due to pcDNA-NET modulation. (D) The enzyme activity of MMP-2 (left) and MMP-9 (right) in conditioned medium due to siRNA-NET modulation. The columns in the graph represent the intensity of MMP-2 and MMP-9 measured in triplicate. Significant difference are indicated by ap<0.05 vs. Mock. The values are expressed as the means±SE of three separate experiments. NET, norepinephrine transporter; M, mock control; MMPs, matrix metalloproteinase.
We collected HTR-8/SVneo supernatants from cells transfected with pcDNA-NET or siRNA-NET and analyzed their MMP activity using zymography to investigate whether the altered invasiveness in accordance with NET regulation could affect MMP expression. As shown in Figure 3C, MMP activities in the transfected HTR-8/SVneo cells with pcDNA-NET increased the activity of MMP-9 significantly at 0.1-0.5 µg/mL of pcDNA-NET compared to the mock group. Otherwise, increasing concentrations of pcDNA-NET showed a tendency to decrease MMP-2 activity (p<0.05) (Figure 3C). However, no differences in MMP-2 or MMP-9 activity occurred following NET down-regulation in HTR-8/SVneo cells (Figure 3D). These findings indicate that these alterations in NET gene expression could be causing regulation of trophoblast invasion through MMP-2 and -9 activities in trophoblast cells although their regulation mechanism is not clear.
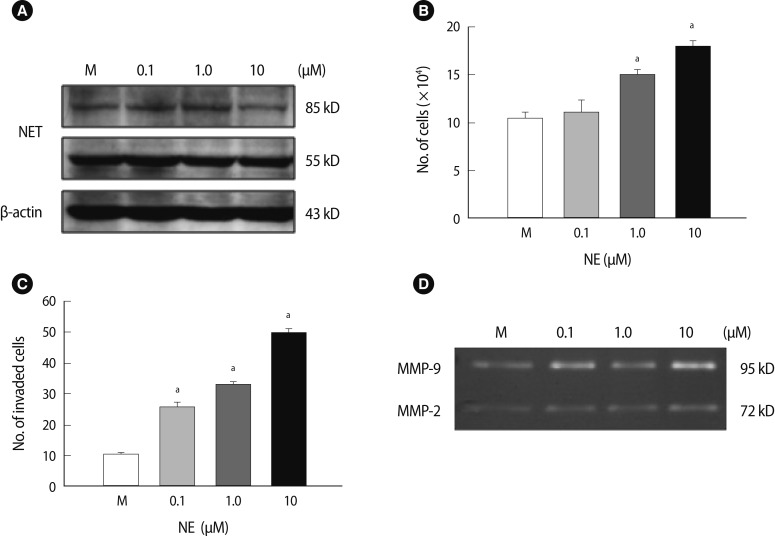
4. Invasion activity and NET expression of HTR8 cells following NE treatment
We demonstrated that an alteration in NET expression in HTR-8/SVneo trophoblast cells regulated trophoblast cell invasion activities via control of MMP expression. NET expression and function was correlated with NE concentration. Therefore, we investigated whether an exogenous NE treatment would affect the viability and invasion of HTR-8/SVneo cells and whether an association exists between the NE-NET balance and trophoblast cell invasion. Interestingly, there were no differences in the expression of the 55 kDa subunit of NET following NE treatment. In contrast, the expression of the 85 kDa subunit of NET was increased with 0.1 and 1 µM NE treatments but decreased with 10 µM NE (Figure 4A). Furthermore, increased exogenous NE significantly enhanced trophoblast cell viability at concentrations up to 1 µM (p<0.05) (Figure 4B). However, higher doses of NE (>20 µM) resulted in trophoblast cell death (data not shown). Moreover, a higher concentration of exogenous NE significantly increased the trophoblast cell invasion rate at 1 to 10 µM. Cell invasiveness increased to a maximum of 5.2-fold when compared to the mock group (p<0.05) (Figure 4C). MMP-2 and MMP-9 activity increased in HTR-8/SVneo cells treated with NE using zymography; in particular, it induced a 2.5-fold peak at 10 µM NE (Figure 4D). These results suggest that exogenous NE increased the proliferation of HTR-8 cells and invasion activity of trophoblast cells by inducing MMP activity through the regulation of NET expression.
Figure 4.
Proliferation activity of HTR-8/SVneo cells and expression of the NET based on NE treatment. (A) The NET expression in HTR-8/SVneo cells after a 24-hour NE treatment. β-actin was used as an internal control. (B) The viability in HTR-8/SVneo cells after a 24-hour NE treatment. NE significantly induced an increase in cell proliferation. (C) The invasion activity of HTR-8/SVneo cells after NE treatment. (D) MMP-2 and MMP-9 activity in HTR-8/SVneo cell medium after 24-hour incubation with NE using zymography. The columns in the graph represent the intensity of MMP-2 and MMP-9 measured in triplicate. Significant difference are indicated by ap<0.05 vs. Mock. The values are expressed as the means±SE. M, mock control; MMP, matrix metalloproteinase; NE, norepinephrine; NET, norepinephrine transporter.
5. Invasion activity of HTR-8/SVneo cells depends on a balance between NE and NET expression
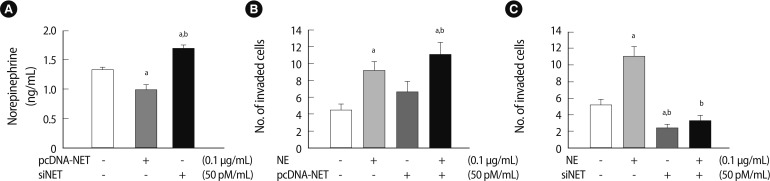
Next, we analyzed the endogenous NE clearance in conditioned medium via NET gene-regulation (pcDNA-NET and siRNA-NET). NE expression in conditioned medium by NET overexpression decreased significantly compared to the mock group (p<0.05). Nevertheless, NE expression increased significantly in the conditioned medium following NET knockdown (p<0.05) (Figure 5A). To determine whether the balance between NE and NET expression altered the invasion activity of HTR-8/SVneo cells, we performed an invasion assay to quantify HTR-8/SVneo cell invasion with NE treatment and with or without NET expression. As shown in Figure 5, the invasion activity of HTR-8/SVneo cells following NE treatment stimulated the invasiveness of HTR-8/SVneo cells 2-fold (p<0.05). NET overexpression tended to increase the invasion activity of HTR-8/SVneo cells. Furthermore, NE treatment and NET upregulation stimulated the invasion ability of HTR-8/SVneo cells more than other groups including the mock group (p<0.05) (Figure 5B). NET downregulation by siRNA-NET significantly inhibited the invasion activity of HTR-8/SVneo cells compared to the control (p<0.05). Furthermore, downregulating NET completely inhibited the increased NE-induced invasion activity of HTR-8/SVneo cells, which was decreased by 70% compared to the NE-only treatment group (p<0.05) (Figure 5C). From these results, we confirmed that NE induced HTR-8/SVneo cells to increase invasiveness because the cells exposed to NE secreted much more MMP-2 than MMP-9. Additionally, the excessive concentration of exogenous NE may have regulated NET function and invasion activity and aided clearance of NE via NET. Taken together, the results suggest that the balance between NE and NET expression levels plays an important role in regulating HTR-8/SVneo cell invasion activity.
Figure 5.
Invasion activity of HTR-8/SVneo cells depends on the balance between NE and NET expression. (A) The concentration of endogenous NE in the medium of HTR-8/SVneo cells transfected with pcDNA-NET or siNET. The NE concentration in the medium decreased following NET overexpression (0.1 µg/mL); otherwise, it increased following NET downregulation (50 pM/mL) compared with the mock group. Significant differences are indicated by ap<0.05 vs. Mock and bp<0.05 NET vs. siNET. (B) The invasion activity of HTR-8/SVneo cells depended on a balance between NE and NET overexpression. Significant differences are indicated by ap<0.05 vs. Mock and bp<0.05 NE vs. NET. (C) The invasion activity in HTR-8/SVneo cells depended on a balance between NE and NET downregulation. The experiments were performed in triplicate. Significant difference are indicated by ap<0.05 vs. Mock and bp<0.05 NE vs. siNET. The values are expressed as the means±SE. NE, norepinephrine; NET, norepinephrine transporter.
6. MMP activity via the AKT signal pathway regulate invasion of HTR-8/SVneo cells
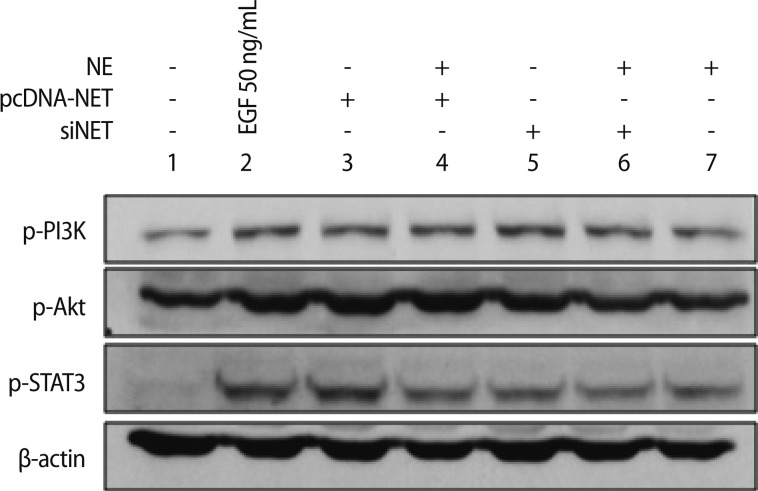
We demonstrated that alteration of NET expression in HTR-8/SVneo cells affected MMP-2 and MMP-9 activities using zymography. Therefore, we investigated whether NE and NET expression could be activated by MMP-2 and MMP-9 and sought to determine which signaling pathways are involved. Generally, phosphoinositol-3-kinase (PI3K)/AKT and the extracellular signal-regulated kinase (ERK) signaling pathways are involved in the migration and invasion of cancer cells [21,22]. Thus, we analyzed the phosphorylated PI3K/AKT and STAT3 pathways and MMP-2 and MMP-9 activity in HTR-8/SVneo cells based on the alteration of NE-NET expression. As shown in Figure 6, the PI3K/AKT and STAT3 signaling pathways were phosphorylated in HTR-8/SVneo cells exposed to 50 ng/mL epidermal growth factor (EGF). NET overexpression induced PI3K/AKT and STAT3 phosphorylation similar to that of EGF treatment; otherwise, NE treatment reduced this phosphorylation. Moreover, downregulated NET and the combination of NE and NET showed a tendency to drop compared to the NET-overexpressing group (Figure 6). Therefore, NET upregulation in HTR-8/SVneo trophoblastic cells could be induced effectively by PI3K/AKT and STAT3 phosphorylation. These results suggest that a balance of NE-NET expression is involved in trophoblastic cell invasion. These results further support the idea that PI3K/AKT and STAT3 phosphorylation via NET alterations play an important role in HTR-8/SVneo trophoblastic cell invasion.
Figure 6.
PI3K/AKT and STAT3 signaling pathways in HTR-8/SVneo cells exposed to 50 ng/mL EGF, NE, and NET alterations. PI3K/AKT and STAT3 signaling pathways were analyzed by the combination with NET, NE, and siNET in HTR-8/SVneo cells. β-actin was used as an internal control. NE, norepinephrine; NET, norepinephrine transporter; EGF, epidermal growth factor.
Discussion
The human placenta, the most important fetal-development organ during pregnancy, regulates dynamic gene expression, which is relevant to placental and fetal development. The balanced expression of various genes in the placenta acts to maintain pregnancy including fetal development [23]. Nevertheless, aberrant gene expression in the placenta induces obstetric diseases (e.g., PE or an intrauterine growth restriction [IUGR]) [24]. Trophoblastic cells, which are one of the major placental cell types, regulate early implantation and placental development through proliferation, differentiation, cell-cell fusion, and apoptosis [25]. It was reported that trophoblast cell growth and survival was regulated by several neurotrophic factors such as brain-derived neurotrophic factor and neurotransmitter [12-26]. In particular, inadequate invasiveness of the trophoblast results in infertility and obstetric diseases (e.g., PE, IUGR). The invasion and apoptosis of trophoblastic cells is regulated by various secreting factors under hypoxic conditions during early pregnancy; however, the precise mechanism of invasion is poorly understood [27].
NE clearance into the cytosol occurs by uptake, transport, and degradation of NET. The NE concentration in the extracellular environment is controlled by the degree of NET expression. Therefore, the balance between NE and NET has been implicated in the pathogenesis of various diseases, including hypertension, PE, and depression [12-28]. NET selectively regulates extracellular catecholamine concentrations as a way of maintaining homeostasis in the placental circulation. The pathological and physiological reasons for the association of PE with NET in the placenta are still unclear [6-29]. Bzoskie et al. [11] showed that the ovine placenta contributes to 40% of the uterine NE clearance.
We confirmed that NET mRNA expression levels were significantly higher in the placental tissue from normotensive pregnancies than pre-eclamptic pregnancies, and that NE levels were significantly lower in the maternal blood of normal pregnancies than pre-eclamptic pregnancies. Although no difference was observed in the placental tissues, these results suggest that secreted NE levels were significantly higher during a pre-eclamptic pregnancy than a normal pregnancy. However, the NET alteration in trophoblastic cells could be due to regulated synthesis and secretion of NE. These findings are well matched with previous reports. In an in vitro system using HTR-8/SVneo trophoblastic cells, we also demonstrated that reduced NET expression during PE increased the NE concentration in placental circulation, whereas NET upregulation in HTR8 cells presented an inverse result. Furthermore, we found that altering NET expression regulated trophoblastic cell invasion and NE clearance. Therefore, it might be assumed that to avoid constriction of uterine spiral arteries and maintain circulatory homeostasis, trophoblastic cells cleared excessive NE from the maternal blood. Thus, early gestational trophoblast cells lack the ability to clear NE in the pre-eclamptic environment.
This is the first report to substantiate a correlation between the NET level, NE concentration, invasion activity in first-trimester trophoblast cells, and MMP-2 and MMP-9 activity. We found that transfecting HTR8 cell lines with pcDNA-NET or siRNA-NET resulted in increased and decreased invasion activity, respectively. Placental trophoblast cells have the capacity to synthesize NE [6], and an uptake system for NE has been described on the brush border membranes of placental cells [30]. Therefore, when the NET gene is downregulated, HTR-8/SVneo trophoblastic cells lack regulation of endogenous NE, which may be related to insufficient invasion of the trophoblast to spiral arteries and affect inadequate uterine spiral artery remodeling. pcDNA-NET or siRNA-NET could be regulating MMP-2 and MMP-9 activities. Although MMP-2 and -9 are derived from different genes, they have similar structures and substrate specificities. These proteins are expressed both in invasive extravillous trophoblasts and in the placenta during pregnancy in humans. Because NET-downregulated HTR-8/SVneo trophoblastic cells result in the poor clearance of endogenous NE, increased NE concentrations in the extracellular environment affected the altered MMP expression level in trophoblastic cells, leading to decreased invasion activity.
Invasion is a key step in physiological placentation, but little is known about how catecholamines affect the invasive potential of trophoblastic cells. NE is related to cellular invasion. In particular, various tumor cells expressing high amounts of NE increase invasion activity and angiogenesis, which affects tumor cell metastasis [16]. We found that HTR-8/SVneo trophoblastic cells exposed to exogenous NE were more viable and showed increased invasion activity, a greater production of factors responsible for invasion, and decreased NET protein (approximately 85 kDa) at a high dose. NE decreases glycosylated NET expression via oxidative stress in a PC12 cell line [31]. Although we did not investigate the mechanism of NET downregulation, human trophoblastic cells reduced glycosylated NET protein at high doses of NE. Because of reduced expression of the NET protein due to excessive NE concentrations, we suggest that a variety of symptoms, including a decrease in NE uptake, invasion activity, and a change in immune response, may occur during placentation, leading to the development of PE. NE in HTR-8/SVneo trophoblastic cells robustly increased invasion activity at a relevant dose. Furthermore, an alteration in MMP expression was observed at a relevant dose. MMP-2 and MMP-9 expression and activity were promoted by phosphorylation of the PI3K/AKT signal pathway [21-32]. Trophoblast cells are highly invasive due to the secretion of extracellular proteases, such as MMPs, which mediate extracellular matrix degradation, and balance tissue inhibitors of metalloproteinases [33]. Decreased NET protein, due to a failure to regulate the NE concentration, causes excessive NE levels in the uterus with subsequent contraction of spiral arteries and insufficient invasion activity, which reduces feto-placental circulation and promotes PE. Our results suggest that a balance between NE and NET plays a role in invasion by modulating the expression of MMPs in trophoblastic cells. However, the correlation between spiral transformation in the maternal myometrium via trophoblast invasion and NET function should be studied in the future.
Taken together, the results suggest that a balance between NE and NET may contribute to invasion of trophoblast cells through MMP expression via the PI3K/AKT and STAT3 signaling pathway. These findings will provide useful guidelines for understanding the mechanisms of trophoblast invasion as well as for developing a basic understanding of the function of NE and NET in gynecological diseases, including PE.
Acknowledgments
We thank Dr. Charles H. Graham (Queen's University, Canada), who provided the HTR-8/SVneo cell lines.
Footnotes
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded the by the Ministry of Education, Science and Technology (2012R1A1A3009776), Republic of Korea.
No potential conflict of interest relevant to this article was reported.
References
- 1.Maynard SE, Karumanchi SA, Thadhani R. Hypertension and kidney disease in pregnancy. In: Brenner BM, editor. Brenner & Rector's the kidney. 8th ed. Philadelphia: Saunders; 2008. pp. 1567–1595. [Google Scholar]
- 2.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 3.Benischke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer-Verlag; 2000. [Google Scholar]
- 4.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia: a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007;14:508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 6.Manyonda IT, Slater DM, Fenske C, Hole D, Choy MY, Wilson C. A role for noradrenaline in pre-eclampsia: towards a unifying hypothesis for the pathophysiology. Br J Obstet Gynaecol. 1998;105:641–648. doi: 10.1111/j.1471-0528.1998.tb10179.x. [DOI] [PubMed] [Google Scholar]
- 7.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia: a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 8.Mao W, Qin F, Iwai C, Vulapalli R, Keng PC, Liang CS. Extracellular norepinephrine reduces neuronal uptake of norepinephrine by oxidative stress in PC12 cells. Am J Physiol Heart Circ Physiol. 2004;287:H29–H39. doi: 10.1152/ajpheart.01168.2003. [DOI] [PubMed] [Google Scholar]
- 9.Ramamoorthy S, Prasad PD, Kulanthaivel P, Leibach FH, Blakely RD, Ganapathy V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncytiotrophoblast. Biochemistry. 1993;32:1346–1353. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
- 10.Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bzoskie L, Blount L, Kashiwai K, Tseng YT, Hay WW, Jr, Padbury JF. Placental norepinephrine clearance: in vivo measurement and physiological role. Am J Physiol. 1995;269:E145–E149. doi: 10.1152/ajpendo.1995.269.1.E145. [DOI] [PubMed] [Google Scholar]
- 12.Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslen B, Marsal K, et al. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25:518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Kaaja RJ, Leinonen A, Moore P, Yandle T, Frampton CM, Nicholls MG. Effect of changes in body posture on vasoactive hormones in pre-eclamptic women. J Hum Hypertens. 2004;18:789–794. doi: 10.1038/sj.jhh.1001743. [DOI] [PubMed] [Google Scholar]
- 14.Moisey DM, Tulenko T. Increased sensitivity to angiotensin in uterine arteries from pregnant rabbits. Am J Physiol. 1983;244:H335–H340. doi: 10.1152/ajpheart.1983.244.3.H335. [DOI] [PubMed] [Google Scholar]
- 15.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 16.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22:825–830. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- 18.Dong E, Yatani A, Mohan A, Liang CS. Myocardial beta-adrenoceptor down-regulation by norepinephrine is linked to reduced norepinephrine uptake activity. Eur J Pharmacol. 1999;384:17–24. doi: 10.1016/s0014-2999(99)00652-4. [DOI] [PubMed] [Google Scholar]
- 19.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Hwang DY, Park JJ, Kim KS. A proximal promoter domain containing a homeodomain-binding core motif interacts with multiple transcription factors, including HoxA5 and Phox2 proteins, and critically regulates cell type-specific transcription of the human norepinephrine transporter gene. J Neurosci. 2002;22:2579–2589. doi: 10.1523/JNEUROSCI.22-07-02579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang W, et al. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology. 2011;53:181–192. doi: 10.1002/hep.24015. [DOI] [PubMed] [Google Scholar]
- 22.Yu T, Wu Y, Helman JI, Wen Y, Wang C, Li L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Mol Cancer Res. 2011;9:161–172. doi: 10.1158/1541-7786.MCR-10-0386. [DOI] [PubMed] [Google Scholar]
- 23.Spessotto P, Bulla R, Danussi C, Radillo O, Cervi M, Monami G, et al. EMILIN1 represents a major stromal element determining human trophoblast invasion of the uterine wall. J Cell Sci. 2006;119:4574–4584. doi: 10.1242/jcs.03232. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM, Von Versen-Hoeynck F. Maternal fetal/placental interactions and abnormal pregnancy outcomes. Hypertension. 2007;49:15–16. doi: 10.1161/01.HYP.0000251523.44824.02. [DOI] [PubMed] [Google Scholar]
- 25.Moore KL, Persaud TV. The developing human-clinically orientated embryology. 8th ed. Potland: Elsvier Saunders; 2008. [Google Scholar]
- 26.Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T. Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology. 2009;150:3774–3782. doi: 10.1210/en.2009-0213. [DOI] [PubMed] [Google Scholar]
- 27.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 28.Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganapathy V, Ramamoorthy S, Leibach FH. Transport and metabolism of monoamines in the human placenta: a review. Placenta. 1993;14:35–51. [Google Scholar]
- 30.Jayanthi LD, Prasad PD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Sodium- and chloride-dependent, cocaine-sensitive, high-affinity binding of nisoxetine to the human placental norepinephrine transporter. Biochemistry. 1993;32:12178–12185. doi: 10.1021/bi00096a030. [DOI] [PubMed] [Google Scholar]
- 31.Mao W, Iwai C, Qin F, Liang CS. Norepinephrine induces endoplasmic reticulum stress and downregulation of norepinephrine transporter density in PC12 cells via oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H2381–H2389. doi: 10.1152/ajpheart.00904.2004. [DOI] [PubMed] [Google Scholar]
- 32.Yu S, Wong SL, Lau CW, Huang Y, Yu CM. Oxidized LDL at low concentration promotes in-vitro angiogenesis and activates nitric oxide synthase through PI3K/Akt/eNOS pathway in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2011;407:44–48. doi: 10.1016/j.bbrc.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 33.Fisher SJ, Leitch MS, Kantor MS, Basbaum CB, Kramer RH. Degradation of extracellular matrix by the trophoblastic cells of first-trimester human placentas. J Cell Biochem. 1985;27:31–41. doi: 10.1002/jcb.240270105. [DOI] [PubMed] [Google Scholar]