Abstract
Liver damage induced by ovarian stimulation has been demonstrated in some cases reported in the literature. However, there has never been a fruitful debate on this topic. The present manuscript tried to fill this gap. We reported a case of a 35-year-old nulliparous woman admitted to our obstetric emergency room for severe pre-eclampsia. She had been subjected to four cycles of controlled ovarian stimulation for intrauterine insemination. At 32 weeks of gestation, she developed severe pre-eclampsia, which led to HELLP syndrome complicated by fatal liver failure. The etiological link between ovarian stimulation and HELLP syndrome is intriguing. Further investigations are needed to understand whether repeated ovarian stimulation may represent a risk factor in pre-eclamptic patients.
Keywords: Controlled ovarian stimulation, Endothelial dysfunction, HELLP syndrome, Intrauterine insemination, Liver rupture, Ovarian hyperstimulation syndrome
Introduction
The correlation between liver damage, ovarian stimulation, and pre-eclampsia is not well known. Pre-eclampsia is a multisystem disorder affecting 2% to 8% of pregnancies [1]. Hepatic function may be significantly altered in women with severe pre-eclampsia, in turn causing HELLP syndrome in approximately 20% of such cases [2]. This is due to its pathophysiology characterized mainly by liver endothelial dysfunction. On the other hand, ovarian hyperstimulation syndrome (OHSS) may induce microvascular thrombosis and liver tissue ischemia resulting in hepatic dysfunction [3]. This issue has not been well elucidated; therefore, we present here an interesting case of severe pre-eclampsia complicated by fatal spontaneous hepatic rupture in a pregnancy obtained by IUI after repeated cycles of controlled ovarian stimulation (COS). Our discussion is focused on analyzing this rare correlation (liver failure-ovarian stimulation) and proposing a causal link.
Case report
A 35-year-old nulliparous woman was admitted at 32 weeks of gestation to our obstetric emergency room for hypertensive crisis (240/140 mm Hg). She had been subjected to four cycles of COS for IUI. She had not any risk factors for pre-eclampsia. The first cycle of 150 IU/day recombinant FSH (Fostimon, IBSA Institut Biochimique SA, Lugano, Switzerland) was interrupted by the onset of moderate OHSS. The next two cycles with 75 IU/day recombinant FSH were not successful. During the fourth COS the patient received a total dose of 750 IU of r-FSH; the triggering of ovulation was performed by 10,000 IU of HCG (Gonasi HP, IBSA Institut Biochimique SA) and after 36 hours IUI was performed. After this cycle, a pregnancy began and its course was regular until the third trimester (last visit at the 27th week of gestation). At admission, she reported general malaise and non-specific abdominal pain. She was immediately treated with 10 mg of nifedipine sublingually; a reduction in blood pressure was achieved even though it remained high (180/100 mm Hg). Blood samples showed hyperuricemia (uric acid, 5.8 mg/dL) and hypertransaminasemia (alanine transaminase [ALT], 137 IU/L, lactate dehydrogenase [LDH], 870 IU/L). A urine sample showed proteinuria. An obstetric ultrasound examination showed oligohydramnios, severe foetal growth restriction, and flowmetry changes (absent umbilical artery end-diastolic flow). The cardiotocographic control revealed reduced short-term variability and absence of episodes of high variation. Based on the clinical symptoms of the patient, we decided to perform emergency Caesarean section (CS) without waiting for respiratory distress syndrome prophylaxis. The female newborn (1,050 g) had an Apgar score of 7 at the first minute and 9 at the fifth minute. Immediately after the CS, the patient was considerably relieved and the blood pressure stabilized (140/85 mm Hg). After about 3 hours, the patient complained of acute right upper quadrant pain. Pressure values decreased rapidly. Blood tests revealed severe anaemia and disseminated intravascular coagulation (DIC). An explorative laparotomy was immediately performed and hemoperitoneum was noted (about 1,050 mL of fresh blood were aspirated). The uterus was flabby, purple, and bleeding; thus we decided to perform a total hysterectomy. A mid-line xifo-pubic abdominal incision was performed to explore the superior abdomen and the surgeon identified a large laceration of Glisson's capsule causing an active and massive haemorrhage. Good haemostasis was not achievable because the liver was oedematous. Thus packing was performed by three large gauze packs to compress the bleeding lesions and two supra and underhepatic drains were left in the abdomen. Post surgery, the clinical situation worsened despite the efforts to stabilize the blood coagulation parameters. The day after the first surgical operation, laboratory findings showed an increase in liver enzymes (aspartate aminotransferase, 3,045 U/L; ALT, 2,532 U/L; LDH, 5,814 U/LE), low levels of haemoglobin (5.5 g/dL), haematocrit (16%), and platelets (35×103/µL), and a low glomerular filtration rate (18 mL/mL). A second laparotomy was necessary. This second attempt at haemostasis was successful, but liver function was already impaired. Therefore, the patient was placed on a waiting list for liver transplant; about 24 hours later an appropriate organ became available and a liver transplantation was carried out. The patient's condition seemed to improve, but in the following day a complication occurred that caused death from sepsis.
Discussion
The HELLP syndrome is a pregnancy-speci.c disorder characterized by haemolysis, elevated liver enzymes and low platelet count that can affect 4% to 20% of pregnancies complicated by pre-eclampsia [2]. Hepatic manifestations of HELLP syndrome range from mild hepatocellular necrosis to subcapsular hematoma and hepatic rupture. The aetiology and pathogenesis of hepatic injury in the setting of HELLP syndrome are unknown; there is evidence of a vascular origin (endothelial dysfunction), which would produce DIC, hypervolemia, liver ischemia, and haemorrhage with the development of hematoma [4]. It is difficult to demonstrate a correlation between ovarian stimulation and hepatic rupture because it is extremely rare. However, some pathophysiological explanations are conceivable. Some authors have reported cases of liver injury after ovarian stimulation [5-7]. Autopsies performed on patients who died from OHSS showed interesting hepatic histological lesions. Ryley et al. [8] showed the presence of macrovesicular steatosis involving the periportal areas with an inflammatory infiltrate composed mainly of mononuclear cells and marked Kupffer cell hyperplasia. Electron microscopic ultrastructural examination of hepatocytes disclosed the presence of mitochondrial crystalline inclusions and dilatation of the rough endoplasmic reticulum, similar to that observed occasionally in pregnancy or during administration of oral contraceptives or anabolic steroids [9,10]. These observations and the supraphysiological level of estradiol observed during ovarian stimulation might suggest a role for oestrogens as pathogenic factors, with a compensatory structural rearrangement of enzyme protein subunits to enhance the metabolic degradation of these increased oestrogens [11]. Alternatively, the hypothesis of a circulatory dysfunction has also been proposed. The increase in different mediators observed in OHSS (e.g., renin-angiotensin or interleukin 6), may induce microvascular thrombosis and liver tissue ischemia, resulting in hepatic dysfunction [12-15]. Endothelial dysfunction is also the most common clinical manifestation in preeclampsia, including enhanced endothelial-cell permeability and platelet aggregation [16]. Furthermore, recent studies have demonstrated a significant association between IVF and risk of pre-eclampsia. For instance, Chen et al. [17] conducted a retrospective cohort study of 1,357 pregnancies conceived by assisted reproductive technology demonstrating a higher incidence of pre-eclampsia. Carbone et al. [18] prospectively analysed 426 pregnancies conceived by IVF; he showed an increase in risk for early pre-eclampsia (before 34th week of gestation) hypothesizing an impairment in placental perfusion. Imudia et al. [19] found that an elevated peak serum estradiol level on the day of hCG administration was associated with greater odds of developing pre-eclampsia and delivery of a small for gestational age infant.
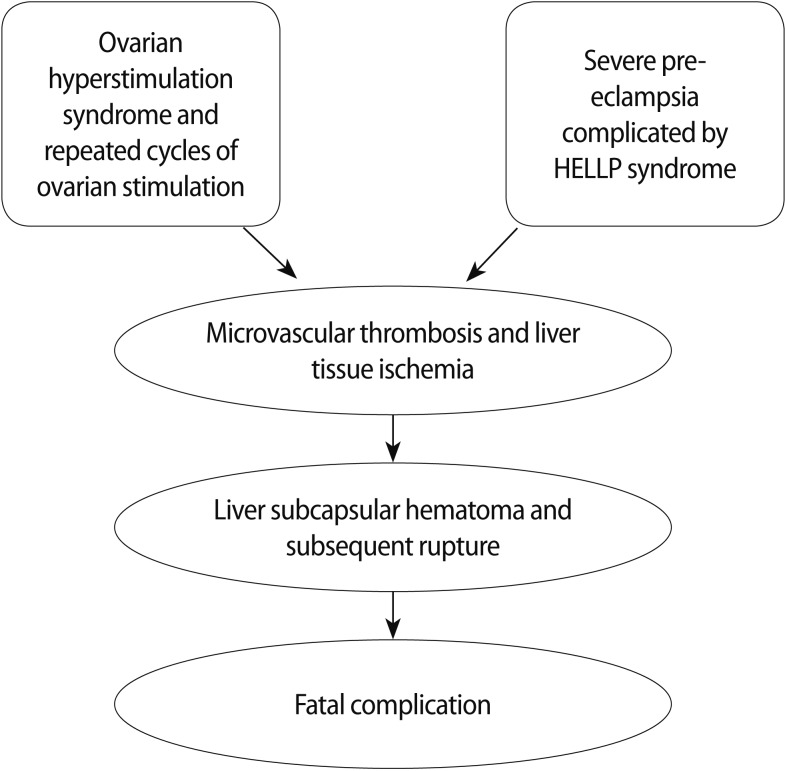
Considering such assumptions, it is conceivable that the relationship between the previous episode of OHSS and multiple consecutive cycles of COS could have existed, favouring hepatic endothelial damage through increased levels of estradiol and inflammatory mediators [3] that would have triggered progressive microvascular thrombosis and liver tissue ischemia. This might have led to a silent liver dysfunction worsened by endothelial failure induced by severe preeclampsia complicated by HELLP syndrome. Afterward, it had a devastating impact on an already damaged liver leading to a rare and fatal complication (Figure 1). These hypotheses are based on pathophysiological considerations but have a scientific rationale as well; therefore, they merit further study. The clinical relevance of this case is extraordinary. In the literature, no study has reported any similar case linking ovarian stimulation and hepatic failure. In conclusion, the present study raises the opportunity of stimulating a wider clinical investigation on the relationship between ovarian stimulation and liver damage.
Figure 1.
Synthesis of the possible pathophysiological mechanisms arranged by repeated ovarian stimulation.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 2.Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) Am J Obstet Gynecol. 1993;169:1000–1006. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- 3.Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS) Hum Reprod Update. 2003;9:77–96. doi: 10.1093/humupd/dmg005. [DOI] [PubMed] [Google Scholar]
- 4.Araujo AC, Leao MD, Nobrega MH, Bezerra PF, Pereira FV, Dantas EM, et al. Characteristics and treatment of hepatic rupture caused by HELLP syndrome. Am J Obstet Gynecol. 2006;195:129–133. doi: 10.1016/j.ajog.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Marsepoil T, Cordesse A, Varguy P, Dauptain G, Levesque P. Hepatic involvement in the course of ovarian hyperstimulation syndrome. Rev Fr Gynecol Obstet. 1992;87:148–149. [PubMed] [Google Scholar]
- 6.Mitchell C, Gottlieb L. Transaminitis after treatment with clomiphene citrate: a case report. J Reprod Med. 2007;52:437–438. [PubMed] [Google Scholar]
- 7.Obrzut B, Kuczynski W, Grygoruk C, Putowski L, Kluz S, Skret A. Liver dysfunction in severe ovarian hyperstimulation syndrome. Gynecol Endocrinol. 2005;21:45–49. doi: 10.1080/09513590500099511. [DOI] [PubMed] [Google Scholar]
- 8.Ryley NG, Forman R, Barlow D, Fleming KA, Trowell JM. Liver abnormality in ovarian hyperstimulation syndrome. Hum Reprod. 1990;5:938–943. doi: 10.1093/oxfordjournals.humrep.a137224. [DOI] [PubMed] [Google Scholar]
- 9.Perez V, Gorosdisch S, De Martire J, Nicholson R, Di Paola G. Oral contraceptives: long-term use produces fine structural changes in liver mitochondria. Science. 1969;165:805–807. doi: 10.1126/science.165.3895.805. [DOI] [PubMed] [Google Scholar]
- 10.Adlercreutz H, Tenhunen R. Some aspects of the interaction between natural and synthetic female sex hormones and the liver. Am J Med. 1970;49:630–648. doi: 10.1016/s0002-9343(70)80130-9. [DOI] [PubMed] [Google Scholar]
- 11.Sueldo CE. Transient liver function tests abnormalities in OHSS. Fertil Steril. 1988;50:995–996. doi: 10.1016/s0015-0282(16)60392-8. [DOI] [PubMed] [Google Scholar]
- 12.Balasch J, Arroyo V, Carmona F, Llach J, Jimenez W, Pare JC, et al. Severe ovarian hyperstimulation syndrome: role of peripheral vasodilation. Fertil Steril. 1991;56:1077–1083. doi: 10.1016/s0015-0282(16)54720-7. [DOI] [PubMed] [Google Scholar]
- 13.Barak V, Elchalal U, Edelstein M, Kalickman I, Lewin A, Abramov Y. Interleukin-18 levels correlate with severe ovarian hyperstimulation syndrome. Fertil Steril. 2004;82:415–420. doi: 10.1016/j.fertnstert.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Borgaonkar MR, Marshall JK. Marked elevation of serum transaminases may be associated with ovarian hyperstimulation syndrome. Am J Gastroenterol. 1999;94:3373. doi: 10.1111/j.1572-0241.1999.03373.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen CD, Wu MY, Chen HF, Chen SU, Ho HN, Yang YS. Relationships of serum pro-inflammatory cytokines and vascular endothelial growth factor with liver dysfunction in severe ovarian hyperstimulation syndrome. Hum Reprod. 2000;15:66–71. doi: 10.1093/humrep/15.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A. New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thromb Res. 2011;127(Suppl 3):S72–S75. doi: 10.1016/S0049-3848(11)70020-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen XK, Wen SW, Bottomley J, Smith GN, Leader A, Walker MC. In vitro fertilization is associated with an increased risk for preeclampsia. Hypertens Pregnancy. 2009;28:1–12. doi: 10.1080/10641950802001859. [DOI] [PubMed] [Google Scholar]
- 18.Carbone IF, Cruz JJ, Sarquis R, Akolekar R, Nicolaides KH. Assisted conception and placental perfusion assessed by uterine artery Doppler at 11-13 weeks' gestation. Hum Reprod. 2011;26:1659–1664. doi: 10.1093/humrep/der117. [DOI] [PubMed] [Google Scholar]
- 19.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]