Abstract
We aimed to investigate the determinants of optimal highly active antiretroviral therapy (HAART) adherence and time interval between successive clinic visits, as well as the association between these two processes. This was done by reviewing routinely collected patient information in the Centre for the AIDS Programme of Research in South Africa (CAPRISA). Records of 688 patients enrolled in the CAPRISA AIDS treatment (CAT) programme between 2004 and 2006 were analysed. Patients were considered adherent if they had taken at least 95% of their prescribed drugs. The adherence has been measured using the pill counts data. A multivariate generalized mixed random effects approach was used to jointly analyse optimal HAART adherence and time interval between successive visits. The results showed that on the overall, the association between optimal HAART adherence and time interval between successive visits was negative. The results further showed that the interaction between time and treatment site had a significant joint effect on optimal HAART adherence and time interval between successive visits. The interaction revealed that as the number of follow-up visits increased, the interval between successive visits also increased while at the same time high levels of optimal adherence were maintained in the rural treatment site. Moreover, after accounting for the time interval between successive visits, the results showed that optimal HAART adherence was significantly associated with having a cell phone, living with a partner as well as interactions that include time and gender, time and treatment site, age and gender and age and education. The findings provide evidence of a negative association between optimal HAART adherence and the time interval between successive clinic visits on the overall, which therefore indicates that longer time interval between successive clinic visits is undesirable if optimal HAART adherence is to be maintained. This notwithstanding, rural patients were able to maintain HAART adherence for longer time interval between successive clinic visits. Furthermore, the findings indicated that optimal HAART adherence was low for some sub-populations, such as the urban and male populations, thus vigorous ongoing adherence counseling is required.
Keywords: adherence rate, adjusted odds ratio (aOR), joint modeling, generalized mixed model, pill counts method
Introduction
The introduction of highly active antiretroviral therapy (HAART) has produced a remarkable decrease in HIV morbidity and mortality (Mocroft et al., 2003). The success of HAART relies on high levels of sustained medication adherence in order to maximize its clinical effectiveness (Bangsberg et al., 2001). Strict adherence is fundamental to achieving viral suppression, avoiding viral rebound, increasing levels of CD4 cell counts and minimizing both the potential risks associated with the development of drug resistance and the risk of death (Bangsberg et al., 2003). Patients who take 95% or more of their prescribed medication benefit more from treatment than those who take less than 95% (Paterson et al., 2000). As HIV cannot be eradicated, it is likely that people on antiretroviral therapy (ART) will need to take antiretroviral drugs for the foreseeable future (Cambiano et al., 2010). This coupled with rapidly expanding access to antiretroviral drugs in resource-poor settings, it is vital to closely monitor whether patients are able to maintain HAART adherence over time and identify predictors of long-term adherence in order to develop interventions that can encourage sustained high levels of adherence.
A related process to strict adherence to medication is regular adherence to clinic attendance. Most patients on ART are scheduled to attend a health facility monthly for a clinical examination, laboratory examination if needed and to refill their ARV drugs (Seguy et al., 2007). It has been shown that inconsistency in clinic attendance is a potential risk for poor adherence to medication (Ross-Degnan et al., 2010). Studies have revealed that a greater number of total elapsed days between missed visit and the next visit, as well as a greater number of missed clinic appointments were risk factors for virological failure in HIV-infected patients receiving HAART (Lucas, Chaisson & Moore, 1999). Thus, jointly modeling HAART adherence and time that elapse between successive visits can reveal a great deal of insight about factors that influence behaviour change of patients.
Under the assumption that the time interval between one clinic visit to the next is the same across all follow-up visits, it was established that social, demographic, behavioural, economic and clinical factors affect optimal HAART adherence over time (Maqutu, Zewotir, North, Naidoo & Grobler, 2010a). The assumption of equal time interval between successive visits to the clinic is a commonly made assumption in longitudinal studies exploring factors associated with long-term adherence in Sub-Saharan Africa (see for example (Byakika-Tusiime et al., 2005; Etard et al., 2007; Maqutu et al., 2010a)). In reality, however, this assumption of equal interval between successive visits might not necessarily hold because at times patients are unable to keep their scheduled clinic appointments for various reasons; they either make an early or a late visit to the clinic. Studies that evaluate factors influencing HAART adherence taking into account the time interval between successive visits are limited in the literature, hence the motivation for this study. To this end, there are no studies that assess and provide an empirical evidence of the association between optimal HAART adherence over time and time interval between successive visits. We therefore aim to investigate social, demographic, behavioural, economic and clinical factors that jointly affect both optimal HAART adherence status of patients and time interval between successive visits. And in the process, we seek to assess whether the explanatory variables that were found to be significantly related with optimal HAART adherence in the previous study (Maqutu et al., 2010a) would still have a significant effect on long-term optimal HAART adherence even when time interval between successive clinic visits was accounted for. Also evaluating the association between the optimal HAART adherence and time interval between successive clinic visits was of interest.
Methods
Study design
The data used in this study are secondary data obtained from the Centre for the AIDS Programme of Research in South Africa (CAPRISA). CAPRISA started a HAART rollout program in 2004. The CAPRISA AIDS Treatment (CAT) Programme offers HIV care services at two sites in KwaZulu-Natal, South Africa, namely the eThekwini Clinical Research site (urban site), and the Vulindlela Clinical Research site (rural site). The programme started providing free HAART through a President’s Emergency Plan for AIDS Relief (PEPfAR) grant at a time when access to HAART in the public sector was limited. Adult patients with a CD4+ count below 200 cells/μL, or patients with World Health Organisation (WHO) stage 4 of the HIV disease, were eligible for HAART initiation. Prior to HAART initiation, all patients received three sessions of adherence education, motivation and preparedness training.
Patients visited the treatment sites monthly to collect their treatment and to undergo a clinical examination. All patients were on regimens containing two nucleoside reverse transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitor. Patient information was recorded on data collection sheets at the clinics; it underwent two levels of quality control, and was faxed to a central data management centre. The secondary data analysed in this study consisted of a retrospective review of patients’ records in the CAT programme between June 2004 and September 2006. Only patients with pill count data for the initial visit, and at least one other clinic visit for the defined study period, were included in the analysis. The number of follow-up visits differed per patient, as some patients started treatment earlier and therefore had more visits, while some patients dropped out of the treatment programme prematurely. Approval for the data collection and analysis was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee
Variables of interest
Outcome variables
The first outcome variable was optimal adherence to HAART, which has been measured using the pill counts data. Pill counts data were recorded by the pharmacist at every patient’s visit to the clinic, thus enabling the measurement of adherence at every visit. Patients were classified as optimally adherent if they took at least 95% of the prescribed drugs in a given regimen (Paterson et al., 2000) otherwise, they were considered non-adherent. The second outcome variable was time interval which was measured as the number of days between successive visits for each patient.
Independent covariates
Baseline demographic and socio-economic variables included age (in years), gender, educational status, treatment site, whether or not a patient lived with a partner, whether or not the patient was a source of household income, access to tap water and electricity, and whether a patient owned a cell phone. Other variables recorded at baseline and included were World Health Organization (WHO) HIV stages, CD4 cell count (cells/μL), weight (kilograms), whether or not a patient was optimally adherent at baseline. Patients were asked why they did an HIV test and their responses included being unwell, testing for no specific reason, testing because a partner died of HIV, being ill and unfaithfulness. Reason for testing was therefore classified as possible exposure to HIV, no specific reason and unwell.
Time was measured as a continuous variable representing monthly follow-up visits to the treatment site. The variable time starts with the value 1 for the first follow-up visit, 2 for the second visit, up to 17 for the seventeenth follow-up visit. Weight was measured at every follow-up visit and was modeled as a time-varying covariate. Additional details of the study design and some of the variables can be obtained from Maqutu et al. (2010a; 2010b).
Statistical analysis
Socio-demographic and clinical characteristics of the study population were summarized using the mean and standard deviation (SD) as well as the median and inter-quartile range (IQR) while for categorical variables proportions were used.
The optimal adherence outcome was modeled as a binary variable that follows Bernoulli distribution while time interval between successive visits outcome was modeled as a non-negative variable that follows a Gamma distribution. We formulated a joint model for both outcomes through a multivariate generalized mixed model approach. In this case, the bivariate joint distribution of both outcomes is specified by assuming a general form of a mixed model where the residual error structure and the link function are allowed to change with the nature of the various outcomes (Molenberghs & Verbeke, 2005). Because our primary interest was in assessing the joint effect of the predictor variables at the population level, a joint marginal model was fitted where the association between the two outcomes at each time point as well as the association emerging from the longitudinal structure of the data is treated as a nuisance parameter that had to be accounted for.
To evaluate the association between the optimal HAART adherence and time interval between successive visits, a conditional random-intercepts model was fitted. In this model, the correlation between the two outcomes as well as the correlation coming from the longitudinal structure of the data is specified through the random effects structure (Gueorguieva, 2001). In our case, it was done by assuming separate random intercepts for each outcome variable and then combining them by imposing a joint multivariate distribution on the random intercepts.
All statistical tests were conducted at a 5% level of significance and analyses were done using SAS (version 9.1.3).
Results
Baseline socio-demographic and clinical characteristics of patients
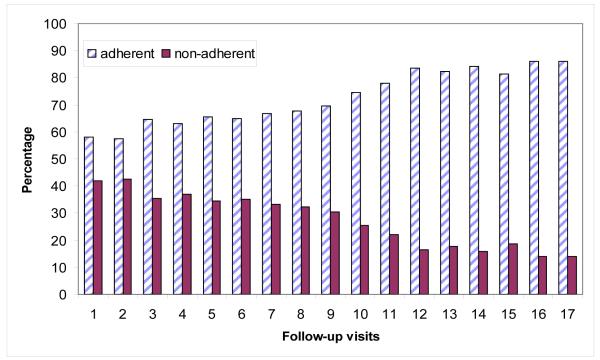
The baseline socio-demographic and clinical characteristics of patients included in the analysis are presented in Table 1. A total of 688 patients, 369 (54%) from the urban site and 319 (46%) from the rural site were included in the analysis. The mean age of patients was 34.1 years (standard deviation (SD) = 8.2 years), 70% were male and 75% were not living with a partner. Most of the patients had attained secondary or higher level of education (69%), and 28% of patients were classified as sources of their household income. Over 90% of the patients stayed in households that had access to tap water and electricity, while 42% of the households had cell phones. The average baseline weight was 61.4kg (SD = 13.0kg), mean CD4+ cell count of 107.5 cells/μL (SD=64.2 cells/ μL) and 64% of patients were classified as WHO stage 3. Over half of the patients (56%) reported to have taken an HIV test as they were not well, while 26% reported no specific reason for testing and 18% took an HIV test as they were concerned that they had been exposed to HIV. In the initial month of treatment, 79% of the patients were at least 95% adherent to HAART. The number of follow-up visits ranged between 2 and 17 per patient, with the median of 8 (IQR: 5-12). The median of time interval between successive visits is 28 days (IQR: 26-30). Optimal adherence has been increasing over the follow-up visits (Figure 1). The proportion of patients who were at least 95% adherent (optimally adherent) to HAART increased from 58% at the first follow-up visit to 86% at the last follow-up visit. Detailed discussions of the baseline characteristics can also be found from Maqutu et al., (2010a).
Table 1.
Baseline socio-demographic and clinical characteristics of the HAART patients (n = 688)
| Characteristic | Mean (SD) | n (%) |
|---|---|---|
| Age (years) | 34.1 (8.2) | |
| Gender: | ||
| Men | 206 (30%) | |
| Women | 482 (70%) | |
| Education: | ||
| No schooling | 74 (12%) | |
| Primary school | 116 (19%) | |
| Secondary school or higher | 429 (69%) | |
| Treatment site: | ||
| Urban | 369 (54%) | |
| Rural | 319 (46%) | |
| Living with or without a partner: | ||
| Living with a partner | 168 (25%) | |
| Living without a partner | 510 (75%) | |
| Contribution to household income: | ||
| Source of income | 186 (28%) | |
| Not source of income | 489 (72%) | |
| WHO stage of HIV disease: | ||
| Stage 1 | 71 (10%) | |
| Stage 2 | 121 (16%) | |
| Stage 3 | 438 (64%) | |
| Stage 4 | 58 (8%) | |
| Baseline CD4+ count (cells/μL) | 107.5 (64.2) | |
| Baseline weight (kg) | 61.4 (13.0) | |
| Reason for taking HIV test: | ||
| Unwell | 374 (56%) | |
| No specific reason | 170 (26%) | |
| Possible exposure to HIV | 121 (18%) | |
| Household access to tap water: | ||
| Yes | 611 (91%) | |
| No | 59 (9%) | |
| Household access to electricity: | ||
| Yes | 607 (91%) | |
| No | 63 (9%) | |
| Cell phone ownership: | ||
| Yes | 281 (42%) | |
| No | 389 (58%) | |
| First-month optimal HAART adherence: | ||
| Optimally adherent | 546 (79%) | |
| Not optimally adherent | 142 (21%) |
Figure 1.
Optimal HAART adherence and non-adherence rates over the follow-up visits
Marginal model results
The results showed that the interaction of time and treatment site had a significant joint effect on both optimal HAART adherence and time interval between successive visits (Table 2). Both outcomes increased as the number of follow-up visits increased, however, the rate at which they increased differed depending on whether a patient attended a rural or urban treatment site. The rate at which optimal adherence increased over time in the rural site was higher compared to the urban site [aOR = 1.06, P=0.0002] while at the same time as the number of follow-up visits increased, the expected time interval between successive visits was likely to increase in the rural site compared to the urban treatment site [aOR=1.007, P=0.0003].
Table 2.
Adjusted odds ratio (aOR) of a joint marginal model for adherence and time interval outcomes with AR(1) working covariance structure
| Adherence | Time interval | |||
|---|---|---|---|---|
|
|
||||
| Parameter | aOR | Pvalue | aOR | Pvalue |
| Intercept | 2.517 | 0.019 | 23.831 | <.001 |
| Age | 0.989 | 0.161 | 1.001 | 0.466 |
| Gender (ref=male) | ||||
| Female | 0.312 | <.001* | 1.020 | 0.649 |
| Education (ref=sec+) | ||||
| No schooling | 5.629 | 0.001* | 1.085 | 0.251 |
| Primary | 1.113 | 0.810 | 0.987 | 0.845 |
| Site (ref=rural) | ||||
| Urban site | 3.320 | <.001* | 0.996 | 0.817 |
| Income (ref=source) | ||||
| Not source | 0.992 | 0.921 | 1.013 | 0.209 |
| Access to tap water (ref=no) | ||||
| Yes | 1.127 | 0.309 | 1.016 | 0.948 |
| Hhd with electricity (ref=no) | ||||
| Yes | 0.993 | 0.948 | 1.020 | 0.238 |
| Cell phone ownership (ref=no) | ||||
| Yes | 1.269 | 0.001* | 1.005 | 0.585 |
| Reason for HIV test (ref=unwell) | ||||
| No specific reason | 0.961 | 0.796 | 1.004 | 0.852 |
| Possible exposure to HIV | 0.641 | 0.011* | 0.984 | 0.396 |
| Staying with a partner (ref=no) | ||||
| Yes | 1.347 | 0.002* | 1.0003 | 0.975 |
| WHO staging of HIV (ref=stage4) | ||||
| Stage 1 | 0.715 | 0.061 | 1.025 | 0.310 |
| Stage 2 | 0.781 | 0.125 | 1.030 | 0.174 |
| Stage 3 | 0.890 | 0.426 | 1.023 | 0.278 |
| Baseline CD4+ cell count | 0.999 | 0.438 | 1.00004 | 0.595 |
| Baseline adh (ref=not adherent) | ||||
| Adherent | 0.858 | 0.070 | 0.011 | 0.251 |
| Time (follow-up visit) | 1.107 | <.001* | 1.013 | <.001* |
| Baseline weight | 0.999 | 0.795 | 0.001 | 0.089 |
| Weight at follow-up visits | 0.998 | 0.665 | 1.002 | 0.450 |
| Time*gender (ref=male) | ||||
| Female | 1.074 | <.0001* | 1.001 | 0.760 |
| Time*site (ref=rural site) | ||||
| Urban site | 0.941 | 0.0002* | 0.993 | 0.003* |
| Time*reason for test (ref=unwell) | ||||
| No specific reason | 0.989 | 0.560 | 1.0001 | 0.965 |
| Possible exposure to HIV | 1.046 | 0.032* | 1.001 | 0.632 |
| Age*gender (ref=male) | ||||
| Female | 1.023 | 0.008* | 0.9999 | 0.914 |
| Age*education (ref=secondary+) | ||||
| No schooling | 0.965 | 0.014* | 0.998 | 0.209 |
| Primary | 0.997 | 0.792 | 0.9996 | 0.798 |
Significant at 5% level
The results further showed that after accounting for the time interval between successive visits, optimal HAART adherence was significantly higher when patients had cell phones [adjusted odds ratio (aOR) = 1.269, P = 0.001] and when they lived with a partner [aOR = 1.347, P = 0.002]. Moreover, the results showed that optimal HAART adherence increased on average over time, however, the rate of increase differed by gender [aOR = 1.074, P<0.0001] in favour of females and reason for taking an HIV test where the rate of increase in optimal adherence was higher for patients who tested due to possible exposure to HIV, than for patients who tested as they were unwell [aOR=1.046, 95% P=0.021]. Also the rate of increase in optimal adherence was higher for patients who tested due to possible exposure to HIV, than for those who reported no specific reason for taking an HIV test [aOR=1.058, P=0.0339). Age also interacted significantly with gender and education. As the age of patients increased, females tend to adhere better to HAART than males [aOR: 1.023; 95%, P=0.009]. Among older patients, those with no schooling were less likely to achieve optimal HAART adherence than those with secondary and higher education [OR=0.965; 95%, P=0.014]. As patients got older, those with primary education were more likely to achieve optimal adherence than those with no schooling [aOR=1.033, P=0.0432].
The conditional independence random-intercept results
The results of a joint conditional independence random-intercepts model showed that the two random intercepts are significantly negatively associated [ρ = −0.684, P=<0.0001] (Table 3). This translates into a negative correlation between optimal HAART adherence and time interval between successive visits, which means that on the overall increasing the number of days between clinic visits tended to decrease the chances of being optimally adherent.
Table 3.
Covariance Parameter estimates from the joint conditional independence random intercepts model of the optimal HAART adherence and time interval outcomes
| Variance Components | Estimate | Standard error | Pvalue |
|---|---|---|---|
| Var. R.I (adherence) | 0.261 | 0.062 | <0.001 |
| Var. R.I (time interval) | 0.005 | 0.001 | <0.001 |
| Correlation between the R.I. (ρ) | −0.684 | 0.139 | <0.001 |
Discussion
It is shown that on the overall, optimal HAART adherence is negatively associated with time interval between successive visits over time. That is, with shorter intervals between one visit to the next, the patients tend to maintain optimal adherence. Studies have shown that patients who regularly attend the clinic on scheduled day (or within 2 days) maintain a more continuous supply of medicines, thus likely to be optimally adherent (Ross-Degnan et al., 2010). Notwithstanding the negative association between optimal adherence and the time interval between successive visits, the results further revealed that for a patient with sustained optimal adherence, the gap between successive clinic visits might be lengthened. More specifically, the findings highlighted that in the rural treatment site, the patients have been able to maintain high levels of optimal adherence with longer intervals between successive visits. Because of staff shortages in the rural treatment site, the time interval between successive visits was lengthened for the CAT patients with good record of adherence and our results demonstrates that optimal adherence was not compromised due to longer intervals between visits. Thus, with the on-going shortage of medical doctors and other health workers trained to deliver HIV/AIDS care and treatment in Sub-Saharan Africa especially in the rural areas (Vasan et al., 2009), our findings provide evidence to suggest that an ART delivery approach of dispensing longer supplies of medications for patients who have demonstrated sustained levels of optimal HAART adherence could be adopted. This might partly help reduce the large numbers of patients that present at the clinics on a daily basis, especially in situations where health workers are in short supply. The other advantages of providing patients with longer refills (two or three months rather than the one month refills) would be the reduced recurrent costs (transport), mean time spent at the clinic (Hardon et al., 2007).
The results of this study reaffirm the significant determinants of optimal adherence over time reported by Maqutu et al. (2010a). That is, after accounting for the time interval measured in days between successive visits, cell phone ownership, living with a partner and two-way interaction terms that involved time with gender, treatment site and reason for taking an HIV test, as well as age with gender and educational level were still associated with optimal adherence. Despite studies consistently showing that demographic characteristics are generally poor predictors of HAART adherence (Fong et al., 2003), the results confirm that demographic factors predict HAART adherence through interactions among themselves or with other variables.
Moreover, it is confirmed that on average, optimal HAART adherence has been increasing but the rate of increase is higher for females than for males, which could again be characterized by different social and behavioural factors associated with HAART adherence among males and females (Berg et al., 2004). In addition, the rate at which optimal adherence increased over time has been higher in the rural than the urban treatment site, which has also been observed elsewhere (Birbeck et al., 2009). Furthermore, the results prove that patients who lived with a partner adhered better to HAART. Lack of social support has been associated with a decrease in adherence and living with a partner has been associated with increased social support and optimal adherence (Williams & Friedland, 1997). Also, the results confirm that cell phone ownership enhanced long-term optimal HAART adherence. This reinforces proposed interventions of providing memory aids for dosing times that include the use of new technologies such as reminders through cell phones (Nachega et al., 2007).
This study has some limitations. Firstly, adherence was assessed only through pill counts. Pill counts method is attractive due to its simplicity and empirical nature; however, it has disadvantages that include patients not bringing in all their medications or some may empty (pill dump) their bottles prior to a clinic visit (Bangsberg et al., 2001). Nevertheless, pill counts have also been shown to be associated with viral load and CD4 count (Bangsberg et al., 2000). Secondly, interactions between variables were identified using the data and model fit techniques. The interactions were not pre-specified or expected during data collection. Detailed information on why these interactions influenced adherence was therefore not collected, and the reasons for some of these findings cannot be explained.
Our findings provide evidence of a negative association between optimal HAART adherence and the time interval between successive clinic visits, indicating that the longer the time interval between successive clinic visits, optimal HAART adherence is likely to be compromised on the overall. Furthermore, the results confirmed that though optimal HAART adherence increased over time on average, the rate of increase is low for some sub-populations, such as the urban and male populations, thus vigorous ongoing adherence counseling is required.
Acknowledgements
CAPRISA was established in 2002 through a Comprehensive International Program of Research on AIDS (CIPRA) grant (AI51794) from the US National Institutes of Health (NIH), as a multi-institutional collaboration, incorporated as an independent non-profit AIDS Research Organization. The NIH funded the development of the research infrastructure, including the data management, laboratory and pharmacy cores established through the CIPRA grant. The US President’s Emergency Plan for AIDS Relief (PEPfAR) grant (1U2GPS001350) funded the care of all the patients in the CAT Programme. Dikokole Maqutu was supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant # D43TW00231). We gratefully acknowledge the patients in the CAT Programme. We also thank all the staff who worked on the CAT Programme, treating patients and helped in the data collection. Special thanks to the pharmacists for collection of pill count data.
Reference
- Bangsberg DR, Hecht FM, Clague H, Charlebois ED, Ciccarone D, Chesney M, Moss A. Provider assessment of adherence to HIV antiretroviral therapy. Journal of Acquired Immune Deficiency Syndrome. 2001;26:435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, Moss A. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. Journal of General Internal Medicine. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbeck GL, Chomba E, Kvalsund M, Bradbury R, Mang’ombe C, Malama K, Orgnek N. Antiretroviral adherence in rural Zambia: the first year of treatment availability. American Journal of Tropical Medicine and Hygiene. 2009;80:669–674. [PubMed] [Google Scholar]
- Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, Bangsberg DR. Adherence to HIV antiretroviral therapy in HIV+ Ugandan patients purchasing therapy. International Journal of STD and AIDS. 2005;16:38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- Cambiano V, Lampe FC, Rodger AJ, Smith CJ, Geretti AM, Lodwick RK, Phillips AN. Long-term trends in adherence to antiretroviral therapy from start of HAART. AIDS. 2010;24:1153–1162. doi: 10.1097/QAD.0b013e32833847af. [DOI] [PubMed] [Google Scholar]
- Etard JF, Laniece I, Fall MB, Cilote V, Blazejewski L, Diop K, Delaporte E. A 84-month follow up of adherence to HAART in a cohort of adult Senegalese patients. Tropical Medicine & International Health. 2007;12:1191–1198. doi: 10.1111/j.1365-3156.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- Fong OW, Ho CF, Fung LY, Lee FK, Tse WH, Yuen CY, Wong KH. Determinants of adherence to highly active antiretroviral therapy (HAART) in Chinese HIV/AIDS patients. HIV Medicine. 2003;4:133–138. doi: 10.1046/j.1468-1293.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R. A multivariate generalized linear mixed model for joint modeling of clustered outcomes in the exponential family. Statistical Modelling. 2001;1:177–193. [Google Scholar]
- Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, Laing R. Psychological and socio-medical aspects of AIDS/HIV. AIDS Care. 2007;19:658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- Lucas MG, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Annals of Internal Medicine. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- Maqutu D, Zewotir T, North D, Naidoo K, Grobler A. Determinants of optimal adherence over time to antiretroviral therapy amongst HIV positive adults in South Africa: a longitudinal study. AIDS and Behavior. 2010a doi: 10.1007/s10461-010-9688-x. Advance online publication. doi: 10.1007/s10461-010-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqutu D, Zewotir T, North D, Naidoo K, Grobler A. Factors affecting early adherence to antiretroviral therapy amongst HIV positive adults in South Africa. African Journal of AIDS Research. 2010b;9:117–124. doi: 10.2989/16085906.2010.517478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Youle M, Moore A, Sabin CA, Madge S, Lepri AC, Phillips AN. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS. 2001;15:185–194. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. Springer-Verlag; New York: 2005. [Google Scholar]
- Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Annals of Internal Medicine. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Ross-Degnan D, Pierre-Jacques M, Zhang F, Tadeg H, Gitau L, Ntaganira J, Wagner AK. Measuring adherence to antiretroviral treatment in resource-poor settings: the clinical validity of key indicators. BMC Health Services Research. 10:42. doi: 10.1186/1472-6963-10-42. doi: 10.1186/1472-6963-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguy N, Diaz T, Campos DP, Veloso VG, Grinsztejin B, Teixeira L, Pillotto JH. Evaluation of the consistency of refills for antiretroviral medications in two hospitals in the state of Rio de Janeiro, Brazil. AIDS Care. 2007;19:617–625. doi: 10.1080/09540120600787356. [DOI] [PubMed] [Google Scholar]
- Vasan A, Kenya-Mugisha N, Seung KJ, Achieng M, Banura P, Lule F, Madraa E. Agreement between physicians and non-physician clinicians in starting antiretroviral therapy in rural Uganda. Human Resources for Health. 2009;7:75. doi: 10.1186/1478-4491-7-75. doi: 10.1186/1478-4491-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Friedland G. Adherence, compliance, and HAART. AIDS Clinical Care. 1997;9:51–54. [PubMed] [Google Scholar]