Abstract
Optical diagnosis of polyp histology can potentially result in enormous cost savings by way of the "resect and discard" strategy for diminutive polyps and the "do not resect" strategy for diminutive hyperplastic polyps in the distal colon. Narrow Band Imaging (NBI) highlights the surface mucosal and vascular pattern on polyps and has been shown to accurately characterize adenomatous and hyperplastic polyps by experts. However, the results have been a little discouraging amongst lesser experienced endoscopists. Studies have also shown that using the NBI diagnosis of diminutive polyp histology, experts can accurately define the future surveillance colonoscopy intervals. However nonexperts in academic or community setting have as yet failed to achieve the recommended thresholds. The subjectivity in assessment by endoscopists leads to the variable accuracy rates and can be circumvented by computer based automated tools. Although initial experience with a few computer based algorithms have shown accuracies comparable to experts, further refinement and validation will be required before these can be implemented in clinical practice. Incorporation of optical diagnosis of diminutive polyps into clinical practice is bound to face several hurdles. But the potential for enormous cost saving makes it an attractive strategy that can make colonoscopy more cost effective.
Keywords: Narrow band imaging, Optical diagnosis, Diminutive polyps
INTRODUCTION
Colonoscopy continues to be at the forefront of prevention and detection of colorectal cancer. Detection of polyps and their removal from the basic premise by colonoscopy prevents the development of colorectal cancer. Adenomatous polyps are considered the precursor lesions for colon cancer, and therefore adenoma detection rate has been widely considered as an important quality indicator of colonoscopy.1 The other major category of polyps is the hyperplastic polyp which is general not considered premalignant. Therefore removal of these polyps is a somewhat redundant practice especially if they are diminutive (≤5 mm) and present in the distal colon. However, as polyp histology cannot be reliably differentiated with white light colonoscopy,2 the current standard of care dictates removal of all polyps detected during colonoscopy with the exception of multiple pale hyperplastic appearing polyps in the rectosigmoid region. This practice has several pitfalls. The major one is the unnecessary cost involved with removal of small hyperplastic polyps and the evaluation of diminutive colorectal polyps by pathologists. The other is the risk of complications involved with the unnecessary or avoidable polypectomies of distal hyperplastic polyps. This can be clinically significant given the large number of colonoscopies performed annually and that polypectomy has been shown to be a major independent factor for colonoscopy related complications like bleeding and perforation.3 Recently the practice of sending all diminutive polyps to pathology has come under scrutiny. Majority of the polyps detected during colonoscopy are diminutive, i.e, ≤5 mm.4,5 Multiple studies have shown that the prevalence of advanced histology (villous features, high grade dysplasia, or cancer) in these polyps is very low.4,5 Therefore sending these diminutive polyps for histopathological evaluation primarily serves the purpose of knowing whether they are adenomas or not as this will then guide the postpolypectomy surveillance interval recommendations. This practice results in huge financial drain on the health care system with limited clinical benefit in return. If the histology of diminutive polyps can be characterized by the endoscopist during colonoscopy, then this cost burden can potentially be alleviated if the resected polyps are not sent for histopathology? "resect and discard" strategy. The diminutive polyps in the distal colon that are characterized as hyperplastic could be left behind? "do not resect" strategy.
Chromoendoscopy is a dye spraying technique that has been around for more than two decades and has been shown to accurately differentiate between adenomas and hyperplastic polyps. Kudo et al.6 described pit patterns (type 1 to V) on the surface of polyps following spraying of indigo carmine or methylene blue, that correlate well with the histology. However, chromoendoscopy has not been adopted in routine clinical practice especially in the west as it is perceived to be cumbersome and impractical, requires extra time (for dye spraying), and increases cost of the procedure (dye, spray catheter).
NARROW BAND IMAGING
About a decade ago, a new technology called narrow band imaging (NBI) was developed.7 This is a filter based technology that accentuates the contrast between tissue vasculature and the surrounding tissue. The working principle of NBI is based on the fact that hemoglobin is the major tissue chromophore and the peak absorption of oxyhemoglobin is at 415 nm (blue light). There is a secondary peak of absorption at 540 nm (green light). These optical characteristics are exploited in NBI wherein a special filter is placed in the light source that allows only a narrow band of blue light with wavelength centered around 415 nm and a very narrow band of green light with wavelength centered around 540 nm to pass through. As a result the vasculature of the mucosa appears bluish green and the surface vascular pattern as well as the morphology of the tissue is enhanced. NBI is activated by merely pushing a button on the handle of the endoscope and therefore is an easy to use, practical and hassle free technology. NBI is also referred to as electronic or digital chromoendoscopy.
NBI FOR POLYP HISTOLOGY CHARACTERIZATION
Since its development and commercial availability there has been a deluge of studies evaluating NBI for polyp histology characterization. The earlier studies extrapolated the use of Kudo's pit patterns (described with chromoendoscopy) to NBI.8,9 Although these studies showed reasonably good accuracies in differentiating between adenomatous and hyperplastic polyps with NBI, it became clear to other investigators that the Kudo's pit pattern could not be reliably applied to NBI.10 Moreover, the Kudo's pit patterns had been described with magnification or zoom colonoscopes that are not used in routine clinical practice, thereby limiting their applicability. In the study by East et al.10 images of 30 polyps ≤6 mm under chromoendoscopy and NBI (with optical magnification) were recorded. These were then evaluated by an experienced European and Japanese-trained endoscopist for the Kudo's pit patterns. The European and Japanese endoscopist scored the pit patterns between chromoendoscopy and NBI differently in 20 and 12 of 33 polyps, respectively. The combined agreement for the chromoendoscopic and NBI pit patterns was unimpressive with a kappa of only 0.23. The authors concluded that the Kudo's pit patterns were not always identical with chromoendoscopy and NBI and that the Kudo's classification may need to be modified and revalidated before it can be used with NBI. They also evaluated the NBI images for vascular pattern intensity, i.e., the color intensity of the lines surrounding the mucosal pits on a 3 point scale: 1, weaker (paler) than the surrounding mucosa; 2, same as the surrounding mucosa; 3, stronger (darker) than the surrounding mucosa. Polyps with strong vascular pattern intensity (Fig. 1A) were considered neoplastic (adenoma) while those with normal or weak vascular pattern intensity (Fig. 1B) were considered nonneoplastic (hyperplastic). The performance characteristics of this simple vascular pattern intensity classification was similar to that of chromoendoscopic and NBI pit patterns and offered a simpler and faster method for polyp classification with NBI. In a prospective study by the same group11 the vascular pattern intensity showed a sensitivity, specificity, and accuracy of 94%, 89%, and 91.4%, respectively, in characterizing 116 polyps <10 mm in size. Similar results were seen for the subgroup analysis of diminutive (≤5 mm) polyps. Furthermore, as there was no difference seen in the performance characteristics in the first half versus the second half of the trial, the authors concluded that vascular pattern intensity was very easy to learn without a significant learning curve. Some other studies used another simple criteria of a brown vascular pattern visible on the polyp with NBI as a distinguishing feature for adenomatous histology.12,13
Fig. 1.
A) A 3-mm polyp with strong vascular pattern intensity. Histopathology showed it was an adenoma (courtesy Dr. James East, MD). (B) A 3-mm hyperplastic polyp showing weak pattern intensity (courtesy Dr. James East, MD).
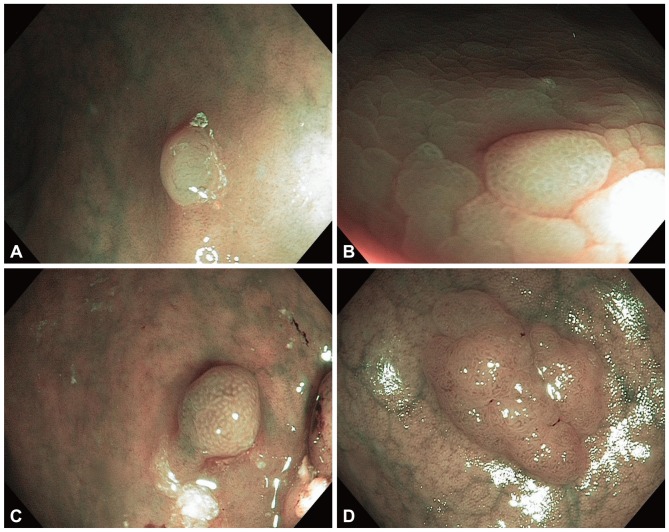
Following these early reports several investigators described different NBI pattern classifications for characterizing the histology of polyps. Rastogi et al.14 in a pilot study described two patterns each for hyperplastic and adenomatous polyps with NBI without magnification. The pattern for hyperplastic polyps were: 1) fine capillary network but absent mucosal pattern-the polyp has a rather bland appearance with fine lacy vessels coursing the surface of the polyp (Fig. 2A) and 2) circular pattern with dots-there are dark dots seen on polyp surface surrounded by clear white areas (Fig. 2B). The patterns for adenomas were: 1) round or oval pattern-dark brown oval or circular lines surrounding clear white areas (Fig. 2C) and 2) tubulogyrus pattern-dark brown linear or convoluted tubular structures (Fig. 2D). These patterns showed an accuracy of 92% in differentiating between hyperplastic and adenomatous polyps on review of images. These patterns were then validated in a prospective study on 100 patients in whom 236 polyps were detected and characterized with NBI that showed a sensitivity, specificity and accuracy of 96%, 89%, and 93%, respectively, in predicting adenomas.2 The sensitivity and accuracy of NBI was significantly superior to high-definition white light. In another study by the same group15 four endoscopists were educated about these patterns and then assessed images of 65 polyps under NBI. The kappa value for the interobserver agreement for polyp surface pattern was 0.57 (moderate) and for prediction of polyp type was 0.63 (substantial). Rex et al.16 described similar endoscopic features with NBI without magnification that were predictive of adenomatous and hyperplastic histology. In a prospective evaluation on 451 polyps the sensitivity, specificity and accuracy for the identification of adenomas was 92%, 87%, and 89%, respectively.
Fig. 2.
(A) A 2-mm hyperplastic polyp showing fine capillary network but absent mucosal pattern (bland pattern). (B) A 4-mm hyperplastic polyp showing the circular pattern with dots. (C) A 4-mm adenoma showing the round or oval pattern. (D) A 6-mm adenoma showing the tubulogyrus pattern.
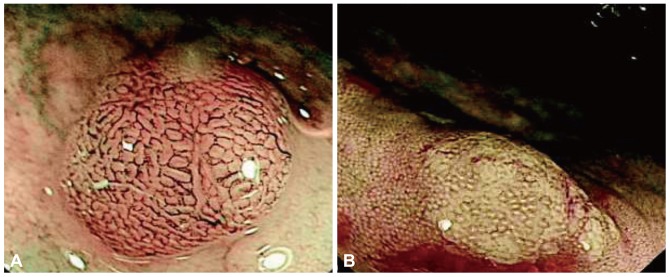
Sano and coworkers17 described the meshed capillary vessels by magnification NBI in which the vessels are arranged in a honeycomb pattern around the mucosal glands as the hallmark for adenomas (Fig. 3). Subsequently they proposed the meshed capillary pattern (CP) classification for distinguishing the polyp histology:18 CP I, microvascular architecture arranged in a regular honeycomb pattern that was faintly visible or invisible by NBI (Fig. 4A); CP II, clearly visible microvascular architecture arranged in a round, oval, honeycomb-like pattern (Fig. 4B); CP III, microvascular architecture that was not arranged regularly in a honeycomb-like pattern and exhibited at least one feature among irregularity of size, complex branching, disruption, or irregular winding (Fig. 4C). CP I was suggestive of hyperplastic polyps while CP II and CP III were diagnostic of adenomas. In a prospective study with 92 patients, 150 polyps were evaluated with NBI and magnification using the meshed capillary vessel classification. The sensitivity, specificity, and accuracy for polyp histology characterization were 96.4%, 92.3%, and 95.3%, respectively. This meshed capillary vessel classification was subsequently tested with NBI without optical magnification and showed a diagnostic accuracy of 91% with a sensitivity and negative predictive value (NPV) of 93% and 91%, respectively.19 For lesions ≤5 mm, the sensitivity, NPV and accuracy were 87%, 91%, and 90%, respectively. On similar lines, Tischendorf et al.20 described a vascular classification with respect to the intensity and shape of the small blood vessels on polyps. A fine CP with normal size and distribution of vessels was characteristic of hyperplastic polyps while increased density, tortuous, cork-screw type and branching vascularization was seen on adenomas (Fig. 5A, B). Using this classification on 200 polyps from 131 patients, NBI with and without magnification showed an accuracy of 91% and 89%, respectively, in differentiating neoplastic from nonneoplastic polyps.
Fig. 3.
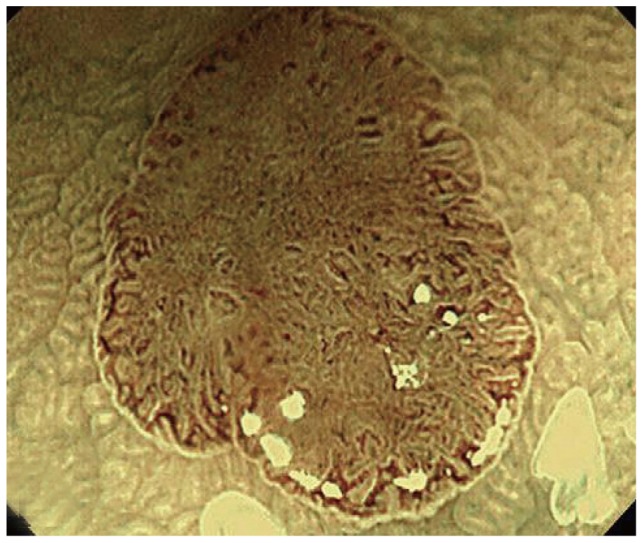
Lesion with clearly visible meshed capillary vessels, histologically diagnosed as an adenoma (courtesy Dr. Yasushi Sano, MD).
Fig. 4.
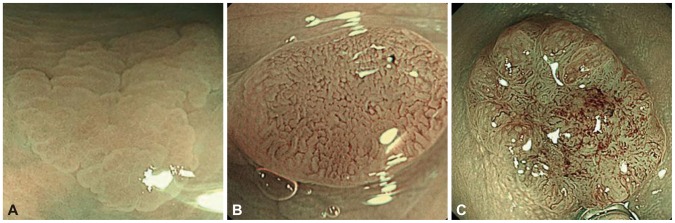
(A) Capillary pattern I; hyperplastic polyp (courtesy Dr. KI Fu, MD). (B) Capillary pattern II, adenoma with low-grade dysplasia (courtesy Dr. KI Fu, MD). (C) Capillary pattern III, adenoma with high-grade dysplasia (courtesy Dr. KI Fu, MD).
Fig. 5.
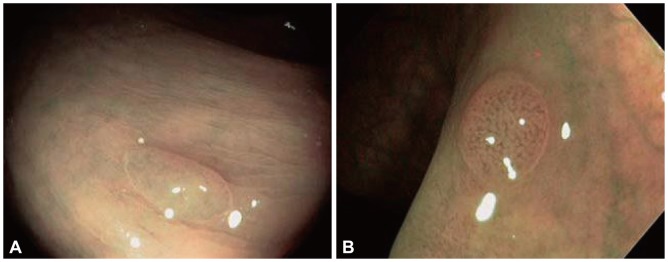
(A) Hyperplastic polyp. Only very few vessels are visualized on the surface and do not show increased branching (courtesy Dr. J Tischendorf, MD). (B) Polyp showing increased density of irregular, curved and dilated blood vessels. Histologic examination showed adenoma (courtesy Dr. J Tischendorf, MD).
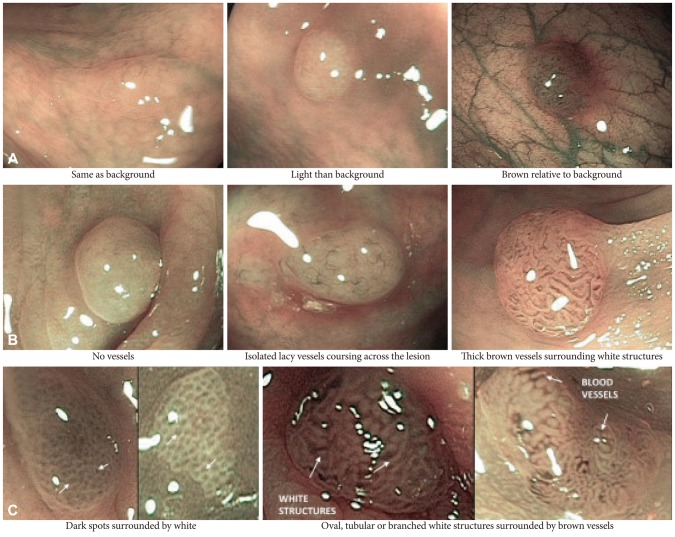
More recently, a group of international experts developed and validated a classification for the endoscopic diagnosis of colon polyps called the NBI International Colorectal Endoscopic (NICE) classification.21 They unified previous NBI classifications to create a simple and practical one that could be used without optical magnification. This classification has three criteria for polyp histology characterization; color of polyp, vessels, and surface pattern. Hyperplastic polyps have the following features: color, same or lighter than the background; vessels, none or isolated lacy vessels coursing across the lesion; surface pattern, dark or white spots of uniform size, or homogenous absence of pattern (Fig. 6). In contrast, adenomas have browner color relative to the background with brown vessels surrounding white structures and have a surface pattern of oval, tubular, or branched white structures surrounded by brown vessels. In a preliminary clinical evaluation of this classification, endoscopists showed an accuracy of 89% in diagnostic prediction of polyps <1 cm in size with a sensitivity and NPV of 98% and 95%, respectively.
Fig. 6.
Features of the narrow band imaging International Colorectal Endoscopic (NICE) criteria: (A) color, (B) vessels, and (C) surface pattern (courtesy Dr. D.K. Rex, MD).
With these numerous reports on performance of NBI for polyp histology characterization, several meta-analysis and systematic reviews have been published.22-24 In an earlier systematic review by van den Broek et al.,22 six studies were included with a total of 358 adenomas and 158 nonneoplastic lesions. These were differentiated by NBI with a sensitivity of 92% (95% confidence interval [CI], 89 to 94), specificity of 86% (95% CI, 89 to 94), and accuracy of 89% (95% CI, 87 to 91). In a more recent meta-analysis,23 10 studies were included in the pooled diagnostic assessment of NBI performance. Random effects pooled NBI test characteristics showed a sensitivity of 94% (95% CI, 91 to 97) and a specificity of 88% (95% CI, 83 to 89). In the latest meta-analysis published by Wu et al.,24 11 studies were included and the sensitivity and specificity of NBI in diagnosis of colorectal neoplastic polyps was 92% (95% CI, 90 to 93) and 83% (95% CI, 81 to 86). The area under the curve for NBI was 95%. Furthermore when the studies were separated based on the use of magnification, the pooled sensitivity and specificity was 92% and 81%, respectively, with magnification and 91% and 86% without the use of magnification.
PERFORMANCE OF NONEXPERTS AND GASTROENTEROLOGISTS IN COMMUNITY PRACTICE WITH NBI
Majority of the data on NBI for polyp histology characterization has been published by experts in academics with experience in NBI and research interest in novel imaging technologies. However, for any new technology to gain widespread acceptance in practice, it is imperative that nonexperts become proficient in it and be able to implement it in practice. Preliminary assessment of whether physicians with less or no experience in NBI can learn to distinguish adenomatous and hyperplastic polyps was encouraging. In a study by Raghavendra et al.,25 37 physicians (12 medical residents, 12 gastroenterology fellows, and 13 gastroenterology faculty) participated in a 20 minute teaching session by an expert, in which criteria for differentiating adenomas and hyperplastic polyps with NBI were demonstrated in an interactive format. All participants completed a pretest before and a posttest after this training in which images of polyps under NBI without magnification were shown and they were asked to differentiate the histology. The mean overall correct responses improved significantly from 48% in the pretest to 91% in the posttest (p=0.0001). The multiple-rater kappa coefficients for interobserver agreement in the pretest and posttest was 0.05 and 0.69, respectively (p<0.0001). Thus after a short didactic teaching session even those without experience in NBI could achieve high accuracy in determining the histology of colon polyps. As didactic interactive session are time consuming, expensive and not practical for wider implementation, Ignjatovic et al.26 developed a 15 minute computer based training module on the use of NBI in the differentiation of adenomas from hyperplastic polyps. Twenty-one participants with varying colonoscopy experience (novices, trainees, and experienced gastroenterologists) evaluated NBI images of 30 polyps <10 mm before and after reviewing the training module. The accuracy improved significantly for all three groups after training: novices, 62% vs. 84%; trainees, 75% vs. 90%; experienced gastroenterologists, 68% vs. 84%. Similarly there was a significant improvement in the interobserver agreement post training in all three groups. These studies though encouraging were not a true representation of real life performance as they did not assess the performance of nonexperts during live colonoscopy procedures.
More recently investigators in Europe and the United States have conducted studies to assess the live performance of gastroenterologists in community practice. A study was conducted in two nonacademic centers in Amsterdam27 with three endoscopists who received a short training on polyp characterization with NBI following which their accuracy in optical diagnosis of polyps <1 cm was assessed during colonoscopy. Of the 281 lesions <1 cm in size, 231 were characterized with high confidence with NBI and the sensitivity, specificity and accuracy were 75%, 76%, and 76%, respectively. These levels were subpar compared to the previously presented data with experts. A similar study was conducted in a large community practice in the United States.28 in which 12 gastroenterologists first reviewed a computerized training session comprised of a pretest, learning module and posttest. Following this, they enrolled patients undergoing colonoscopy with the aim of assessing the accuracy in real-time optical diagnosis of diminutive (≤5 mm) polyps. While 12 of 13 gastroenterologists identified adenomas with >90% accuracy at the end of the computer study (ex vivo phase), only three of 12 were able to do so in the in vivo phase of the study. The in vivo test performance characteristics for diminutive (≤5 mm) polyps were: sensitivity, 87%; spe-cificity, 65%; and accuracy, 78% and for small (6 to 9 mm) polyps were: sensitivity, 96%; specificity, 28%; and accuracy, 79%. These results were disappointing compared to the published performance data for experts in NBI in the academic setting. Another fact that emerges from these studies is that the high accuracy in differentiating adenomas from nonadenoma on ex vivo review of images does not really translate to similar high accuracies in real-time optical diagnosis of polyps during colonoscopy. The selection bias of choosing high quality images of polyps for the ex vivo review may account for this discordance in the performance in the two settings. Another factor may be the training provided prior to assessing the performance. It is likely that merely reviewing a short training module and some NBI images of polyps may not be enough for those without prior experience to achieve accuracies >90%. Perhaps a more detailed and longer training period with feedback may be necessary.
CONFIDENCE LEVEL IN OPTICAL DIAGNOSIS
This concept of the level of confidence in optical diagnosis was introduced by Rex.16 When assessing the polyp histology, the endoscopist may have a "high" or "low" level of confidence in the optical diagnosis. High confidence essentially means that the polyp has one or more features associated with one histology and no features associated with the other histology. While low confidence means that there is uncertainty regarding the features or if there are features of both histology seen on the polyp. These definitions have been further expanded and refined by a group of experts29 according to which high confidence means that clinical judgment can be used in deciding whether the histology of a given polyp can be assessed accurately using an endoscopic technology. If a polyp lacks features associated with confident endoscopic assignment of histology, then the level of confidence in the diagnosis will be low. These polyps diagnosed with low confidence would require histopathology evaluation and would not qualify for the "resect and discard" or the "do not resect" strategy. In one study, the accuracy in optical diagnosis was significantly higher when the diagnosis was made with high confidence compared to low confidence.16 Another study28 showed that the adjusted odds ratio for high confidence as a predictor of accuracy was 1.8 (95% CI, 1.3 to 2.5).
OPTICAL DIAGNOSIS AND SURVEILLANCE INTERVALS
The major potential impact of optical diagnosis of polyps would be the cost savings entailed if the "resect and discard" strategy or the "do not resect" strategy were implemented in clinical practice. Majority of the polyps detected during colonoscopy are diminutive, i.e., ≤5 mm in diameter and advanced histology like high grade dysplasia, villous features, or cancers is rare in these polyps.4,5 Therefore, the main purpose of sending these polyps for histopathological assessment is to differentiate whether they are adenomatous or not as this information impacts the recommendations for the future surveillance colonoscopy intervals. If this information is ascertained by the endoscopist during the procedure, then histopathologic al evaluation of these polyps would become superfluous. The upfront cost savings of forgoing the pathologic assessment of diminutive polyps have been estimated to be in excess of a billion dollars annually in the United States.30 For this major change in paradigm to be accepted by gastroenterologist in practice, there are several intermediate steps that need to be accomplished. The principle ones include achieving competence in real-time optical diagnosis of polyps and inclusion of this strategy in the standards of practice guidelines endorsed by the major gastroenterology societies.
As a step towards this goal, the American Society for Gastrointestinal Endoscopy (ASGE) established the Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) initiative to identify important clinical questions related to endoscopy and to establish a priori diagnosis and/or therapeutic thresholds for endoscopic technologies designed to resolve these clinical questions. An ad hoc committee of experts under the auspices of the existing ASGE Technology and Standards of Practice Committees Chairs published the benchmarks to be achieved before real-time endoscopic assessment of histology of diminutive polyps can be incorporated into clinical practice.29 For diminutive polyps to be "resected and discarded" without pathological assessment, their high confidence endoscopic diagnosis when combined with the histopathologic assessment of polyps >5 mm in size should provide a ≥90% agreement in assignment of postpolypectomy surveillance intervals when compared to decisions based on pathology assessment of all identified polyps. Furthermore, for the "do not resect" strategy for suspected rectosigmoid hyperplastic polyps ≤5 mm in size, the high confidence real-time diagnosis should provide ≥90% NPV for adenomatous histology.
Several studies have assessed whether these benchmarks can be achieved with NBI. Gupta et al.31 performed a retrospective analysis of data from three prospective clinical trials that were conducted between two tertiary referral centers on in vivo optical diagnosis of polyp histology using NBI. Of the total of 410 patients meeting the inclusion criteria, using the in vivo optical diagnosis of diminutive polyps combined with the histopathology results of rest of the polyps, predicted the correct surveillance interval in 86% or 94% patients depending upon whether those with one to two adenomas <1 cm and without advanced histology were brought back in 5 or 10 years, respectively.31 Moreover, when optical diagnosis was limited to diminutive polyps in the rectosigmoid only, the NPV for diagnosing adenomatous histology with NBI was 95%. Therefore the threshold NPV for diagnosing adenomatous histology in diminutive polyps was achieved while the threshold for accurate surveillance intervals was achieved only if patients with one to two small (<1 cm) adenomas without advanced features were to have a repeat colonoscopy in 10 years. One caveat in this study was that confidence level in optical diagnosis was not accounted for. The cost saving was estimated to be $228 per patient if all the diminutive polyps were not sent for histopathological evaluation. Another study by experts in NBI evaluated the accuracy of predicting histology with NBI in real-time for distal colorectal polyps.32 A total of 220 diminutive polyps were detected in the rectosigmoid and a high confidence diagnosis was made with NBI without magnification in 201 out of 220 polyps (91%). The NPV for adenomatous histology in these high confidence predictions was 99.4%, thus achieving the >90% threshold set forth in the PIVI document. The sensitivity, specificity and accuracy were 96%, 99.4%, and 99%, respectively.
These results by experts in NBI were encouraging, but the enthusiasm has been dampened by more recent studies employing nonexperts in academic and community setting. In the study by Ladabaum et al.28 with 12 community gastroenterologists, the agreement between surveillance recommendations informed by the high confidence diagnosis of diminutive polyps and the histopathologic analysis of rest of the polyps with that directed by histology of all polyps was 80%. This was well short of the >90% threshold. However the threshold of >90% NPV for adenomatous histology in diminutive rectosigmoid polyps was achieved. In the Amsterdam study27 in which three community endoscopists participated, the accuracy of on-site surveillance intervals was 81% in patients in whom all lesions were characterized with high confidence. In this study, however, the investigators did not restrict optical diagnosis to diminutive polyps and performed endoscopic diagnosis of polyps of all sizes. A large study from Italy33 prospectively enrolled patients with <10 mm polyps, all diagnosed with high confidence using NBI by six gastroenterologists in a community hospital. A total of 511 polyps were evaluated with NBI in 286 patients. The endoscopy directed surveillance strategy was concordant with the histology-directed strategy in 83% patients. The common theme that emerges from the results of these studies is that although the PIVI benchmarks can be achieved by experts in NBI and those with interest in novel imaging technologies, the same cannot be said for nonexperts in the community setting. Perhaps the degree and duration of training plays a role in this shortfall. All the studies with nonexperts have had a short training session that perhaps is not enough to augment their diagnostic capabilities for optical diagnosis of polyps to acceptable levels. Therefore further research is needed in developing training programs that are more exhaustive and perhaps with feedback that can be widely implemented for community gastroenterologists. These programs need to be validated to see if they are successful in assisting gastroenterologists achieve the PIVI thresholds.
AUTOMATED POLYP HISTOLOGY CHARACTERIZATION
The subjective nature of the assessment of surface patterns on polyps seen with NBI has resulted in variable rates of accuracies. Development of a computer based automated tool for polyp histology can potentially obviate this variance by making real-time endoscopic histology more objective and thereby reducing the interobserver and intraobserver variability. Several initial reports on this have been very encouraging although no such tool is as yet commercially available to be implemented in practice. In one study,34 images of 434 polyps <10 mm in size with magnification NBI were evaluated by two experts, two nonexperts and a computer-based algorithm based on vessel segmentation by phase congruency filter and hysteresis thresholding. Experts in NBI and the computer-based algorithm achieved similar diagnostic performance: sensitivity, 93% vs. 95%; specificity, 92% vs. 90%; accuracy, 93% vs. 93%. Both were significantly superior to the nonexpert group. A subgroup analysis of 255 polyps that were ≤5 mm in size showed results comparable to the analysis of all polyps thereby confirming that the computer-based classification can achieve good diagnostic performance. A Japanese study35 evaluated a computer-aided system called HuPAS version 3.1 (Hiroshima University, Hiroshima, Japan) on 371 polyp images with NBI and magnification. The diagnostic concordance between the computer-aided classification system and the two experienced endoscopists was 98.7%. Further refinement will be needed to test these algorithms and software programs on polyp images with NBI without magnification and also for making these applicable for real-time assessment during colonoscopy. These programs will need further validation in different practice settings. Computer-aided diagnosis has the potential for making this endeavor of optical diagnosis of polyps more objective by taking away the element of human error. This can allow novices and nonexperts to achieve parity with experts by automatic prediction of histology without having to exercise their discriminatory ability.
OPTICAL DIAGNOSIS IN CLINICAL PRACTICE
With considerable work already accomplished in optical diagnosis of colon polyps over the last few years, the obvious next question is that what will it take for this to be implemented in clinical practice? There are several prerequisites for this that merit discussion. First and foremost there should be robust data showing that the technology to be used for optical diagnosis is accurate for in vivo polyp histology characterization. It should also enable the endoscopist to make an accurate assessment of the surveillance interval compared to the recommendation guided by the histopathology of polyps. The technology should be hassle free, easy to learn, practical and not requiring excessive capital investment. Studies and data from both academic and community practice settings would be required to assess the generalizability. Formal training program and curriculum needs to be developed so that nonexperts can be taught the ability to characterize histology of polyps during colonoscopy. Furthermore, it is imperative to create mechanisms for reinforcement of skills, auto validation and assessment of individual performance as well as certification of maintaining competence. For optical diagnosis of polyps to be accepted by the gastroenterology community, it will also require establishing appropriate current procedural terminology (CPT) codes, lobbying government and payers for reimbursement36 and endorsement from the professional gastrointestinal societies by way of incorporating this in their standards of practice guidelines. As discussed in the preceding paragraphs, some of these steps have been either completely or at least partially accomplished with NBI. The most favorable aspect of NBI compared to other technologies in optical diagnosis like chromoendoscopy, confocal endomicroscopy, and spectroscopy is that it is easy to use, hassle free and does not require excessive capital investment as it is already incorporated into the current generation of colonoscopes.
OBSTACLES FOR OPTICAL DIAGNOSIS OF POLYPS
As this concept of optical diagnosis of polyps is relatively novel and represents a major paradigm shift from conventional practice, there will be hurdles and roadblocks in its adoption into clinical practice.37 The major obstacle will be the perceived medicolegal liability by the gastroenterologists as they bear the responsibility of characterizing the polyp histology instead of the pathologists. Saving a high-definition photo of the polyp in the colonoscopy report will serve as the equivalent of histology slides if the polyp is not sent to pathology. Inclusion into clinical practice guidelines by the major gastroenterology societies will provide backing and establish legal standards that may alleviate the anxiety of gastroenterologists. Any major change from conventional practice is bound to meet with resistance due to skepticism, trepidation and inertia to change especially if it is without financial compensation. There may also be a financial disincentive to adopt the "resect and discard" or "do not resect" strategy if the gastroenterology practice owns a financial interest in the pathology services by employing a pathologist. Development of a CPT code with reimbursement for the gastroenterologist can be a helpful strategy. Finally lack of histopathological evaluation of polyps raises the potential for the gastroenterologists to err too much on the side of caution by characterizing more polyps as adenomas thereby leading to unnecessary shorter surveillance intervals that will negate the cost savings of optical dia-gnosis of polyps.
CONCLUSIONS
In conclusion, optical diagnosis of colon polyps with NBI has been studied extensively over the last decade. Variety of surface pattern classifications have been described that show high accuracy in the optical diagnosis of polyp histology. Data on accuracy of predicted histology and surveillance intervals from academic centers with experts in NBI have been very encouraging. The same, however, cannot be said about the results from community practices. Real-time optical diagnosis of polyps has the potential for significant cost savings and thus can make colonoscopy more cost-effective in the prevention of colorectal cancer. However, before it can be ready for prime time we will need to educate nonexperts in the community and academics and conduct more studies outside the auspices of academic centers focusing on accuracy of surveillance interval recommendations as dictated by the ASGE PIVI document. Further refinement and validation of computer aided classification could make optical diagnosis more objective and therefore more reproducible. The future appears bright and optimistic and the gastroenterology community should forge forwards and continue to build upon the work that has been already accomplished to make optical diagnosis of colon polyps a reality.
Footnotes
Received research grant support from Olympus America Inc.
References
- 1.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi A, Keighley J, Singh V, et al. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol. 2009;104:2422–2430. doi: 10.1038/ajg.2009.403. [DOI] [PubMed] [Google Scholar]
- 3.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Overhiser AJ, Chen SC, Cummings OW, Ulbright TM. Estimation of impact of American College of Radiology recommendations on CT colonography reporting for resection of high-risk adenoma findings. Am J Gastroenterol. 2009;104:149–153. doi: 10.1038/ajg.2008.35. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N, Bansal A, Rao D, et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. 2012;75:1022–1030. doi: 10.1016/j.gie.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 7.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 8.Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094–1098. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 9.Hirata M, Tanaka S, Oka S, et al. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988–995. doi: 10.1016/j.gie.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 10.East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310–316. doi: 10.1016/j.gie.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 11.East JE, Suzuki N, Bassett P, et al. Narrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensity. Endoscopy. 2008;40:811–817. doi: 10.1055/s-2008-1077586. [DOI] [PubMed] [Google Scholar]
- 12.Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711–2716. doi: 10.1111/j.1572-0241.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 13.Chiu HM, Chang CY, Chen CC, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373–379. doi: 10.1136/gut.2006.099614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rastogi A, Bansal A, Wani S, et al. Narrow-band imaging colonoscopy: a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc. 2008;67:280–286. doi: 10.1016/j.gie.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 15.Rastogi A, Pondugula K, Bansal A, et al. Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: interobserver and intraobserver agreement and prediction of polyp histology. Gastrointest Endosc. 2009;69(3 Pt 2):716–722. doi: 10.1016/j.gie.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174–1181. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Sano Y, Ikematsu H, Fu KI, et al. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278–283. doi: 10.1016/j.gie.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 18.Katagiri A, Fu KI, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269–1274. doi: 10.1111/j.1365-2036.2008.03650.x. [DOI] [PubMed] [Google Scholar]
- 19.Henry ZH, Yeaton P, Shami VM, et al. Meshed capillary vessels found on narrow-band imaging without optical magnification effectively identifies colorectal neoplasia: a North American validation of the Japanese experience. Gastrointest Endosc. 2010;72:118–126. doi: 10.1016/j.gie.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Tischendorf JJ, Schirin-Sokhan R, Streetz K, et al. Value of magnifying endoscopy in classifying colorectal polyps based on vascular pattern. Endoscopy. 2010;42:22–27. doi: 10.1055/s-0029-1215268. [DOI] [PubMed] [Google Scholar]
- 21.Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607. doi: 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 22.van den Broek FJ, Reitsma JB, Curvers WL, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos) Gastrointest Endosc. 2009;69:124–135. doi: 10.1016/j.gie.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Hayashino Y, Jackson JL, Takagaki N, Hinotsu S, Kawakami K. Diagnostic performance of chromoendoscopy and narrow band imaging for colonic neoplasms: a meta-analysis. Colorectal Dis. 2012;14:18–28. doi: 10.1111/j.1463-1318.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Li Y, Li Z, Cao Y, Gao F. Diagnostic accuracy of narrow-band imaging for the differentiation of neoplastic from non-neoplastic colorectal polyps: a meta-analysis. Colorectal Dis. 2013;15:3–11. doi: 10.1111/j.1463-1318.2012.02947.x. [DOI] [PubMed] [Google Scholar]
- 25.Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572–576. doi: 10.1016/j.gie.2010.03.1124. [DOI] [PubMed] [Google Scholar]
- 26.Ignjatovic A, Thomas-Gibson S, East JE, et al. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011;73:128–133. doi: 10.1016/j.gie.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Kuiper T, Marsman WA, Jansen JM, et al. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol. 2012;10:1016–1020. doi: 10.1016/j.cgh.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Ladabaum U, Fioritto A, Mitani A, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81–91. doi: 10.1053/j.gastro.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex DK, Kahi C, O'Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Kessler WR, Imperiale TF, Klein RW, Wielage RC, Rex DK. A quantitative assessment of the risks and cost savings of forgoing histologic examination of diminutive polyps. Endoscopy. 2011;43:683–691. doi: 10.1055/s-0030-1256381. [DOI] [PubMed] [Google Scholar]
- 31.Gupta N, Bansal A, Rao D, et al. Accuracy of in vivo optical diagnosis of colon polyp histology by narrow-band imaging in predicting colonoscopy surveillance intervals. Gastrointest Endosc. 2012;75:494–502. doi: 10.1016/j.gie.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Hewett DG, Huffman ME, Rex DK. Leaving distal colorectal hyperplastic polyps in place can be achieved with high accuracy by using narrow-band imaging: an observational study. Gastrointest Endosc. 2012;76:374–380. doi: 10.1016/j.gie.2012.04.446. [DOI] [PubMed] [Google Scholar]
- 33.Paggi S, Rondonotti E, Amato A, et al. Resect and discard strategy in clinical practice: a prospective cohort study. Endoscopy. 2012;44:899–904. doi: 10.1055/s-0032-1309891. [DOI] [PubMed] [Google Scholar]
- 34.Gross S, Trautwein C, Behrens A, et al. Computer-based classification of small colorectal polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2011;74:1354–1359. doi: 10.1016/j.gie.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Takemura Y, Yoshida S, Tanaka S, et al. Computer-aided system for predicting the histology of colorectal tumors by using narrow-band imaging magnifying colonoscopy (with video) Gastrointest Endosc. 2012;75:179–185. doi: 10.1016/j.gie.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 36.Rex DK, Fennerty MB, Sharma P, Kaltenbach T, Soetikno R. Bringing new endoscopic imaging technology into everyday practice: what is the role of professional GI societies? Polyp imaging as a template for moving endoscopic innovation forward to answer key clinical questions. Gastrointest Endosc. 2010;71:142–146. doi: 10.1016/j.gie.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Hogan RB, 3rd, Brill JV, Littenberg G, Demarco DC. Predict, resect, and discard ... really? Gastrointest Endosc. 2012;75:503–505. doi: 10.1016/j.gie.2011.09.047. [DOI] [PubMed] [Google Scholar]