Loss of wild-type p53 activity and acquisition of a multidrug resistance (MDR) phenotype, two apparently key factors in the resistance of human cancers to chemotherapy, may be interrelated. Wild-type p53 represses expression of both the MDR-1 gene (encoding the prototypical MDR protein P-glycoprotein [PGP]) and MRP-1 (encoding the MDR-associated protein) (1, 2). Using a temperature-sensitive p53 mutant, Sullivan et al. (3) recently claimed in the JCI that the introduction of a mutant p53 variant promoted MRP expression and activity in a prostate cancer cell line. Our experience with transfection of p53 into other tumor cell lines casts their data in a different light.
We transfected five previously characterized (4) human malignant glioma cell lines with the murine temperature-sensitive p53V135A mutant, which is dominant-negative over endogenous wild-type p53 at 38.5°C but assumes wild-type properties at 32.5°C, mediating the strong induction of p21 expression and causing G2/M arrest (5, 6). Control cell lines (hygro) were transfected with the same plasmid lacking a p53 cDNA insert. Changes in PGP or MRP protein levels were examined 48 hours after the cells were placed at the permissive temperature of 32.5°C (Table 1, upper part). At 32.5°C, expression of p53V135A caused a decline in PGP expression and activity in two cell lines (LN-18 and LN-308) but left these parameters unaffected in two cell lines (U87MG and LN-229). p53V135A caused an increase in PGP activity but no significant change in PGP expression in T98G cells. These changes mediated by p53V135A at wild-type conformation did not correlate with the endogenous p53 status of the cell lines. Under these same conditions, MRP expression and activity were increased by expression of p53V135A in three of the five cell lines (U87MG, T98G, and LN-229) and unaffected in two cell lines (LN-18 and LN-308) at 32.5°C. As with PGP, no correlation emerged between the p53V135A-mediated changes at 32.5°C and endogenous p53 status.
Table 1.
Modulation of PGP and MRP expression and activity by p53V135A at permissive (32.5°C) and restrictive (38.5°C) temperatures
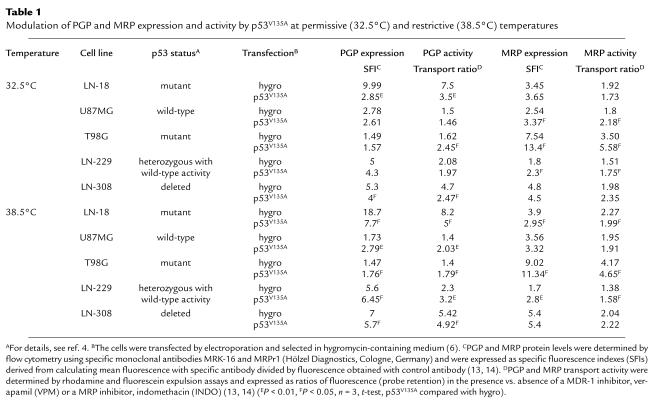
Since several studies have reported that mutant p53 may activate MDR gene expression (1, 7–9), probably by both dominant-negative and gain-of-function mechanisms, changes mediated by p53V135A in mutant conformation (38.5°C) were also carefully considered (Table 1, lower part). At 38.5°C, p53V135A enhanced PGP expression and activity in three of the five cell lines (U87MG, T98G, and LN-229), whereas the opposite pattern was observed in the other two cell lines (LN-18 and LN-308). Similarly, p53V135A had heterogeneous effects on MRP expression and activity. At 38.5°C, p53V135A reduced MRP expression and activity in LN-18 cells but increased MRP expression and activity in T98G and LN-229 cells.
We also examined the modulation of glioma cell sensitivity to vincristine (VCR), a classic substrate of both PGP and MRP, by p53V135A at 32.5°C or 38.5°C (Table 2). At 32.5°C, p53V135A induced dramatic resistance to VCR in four of the five cell lines, although
Table 2.
Modulation of VCR cytotoxicity in the absence or presence of verapamil or indomethacin by p53V135A at permissive (32.5°C) and restrictive (38.5°C) temperatures
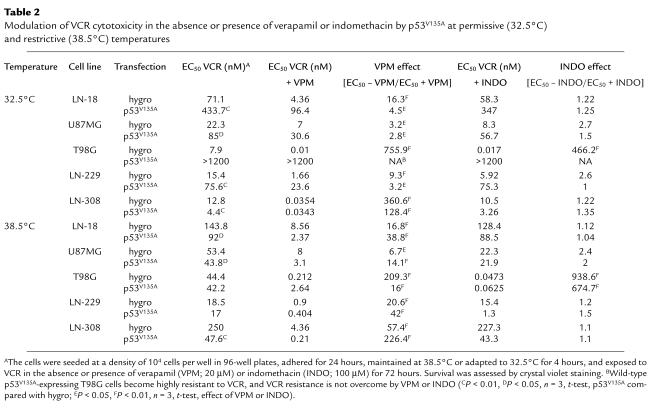
LN-308 cells were sensitized to this drug. Further, we asked how p53V135A affected the ability of a MDR-1 inhibitor, verapamil (VPM), or a MRP inhibitor, indomethacin (INDO), to enhance VCR sensitivity. At 32.5°C, p53V135A uniformly decreased the sensitizing properties of VPM compared with isogenic control transfectants (hygro) under identical experimental conditions. In contrast, p53V135A had little effect on the sensitizing effect of INDO. Note that INDO has biologically relevant effects only in T98G cells, as predicted from the strong expression of MRP in these cells but not in the other cell lines (Table 1). At 38.5°C, p53V135A uniformly increased VCR sensitivity in the glioma cell lines, albeit moderately in all but LN-308 cells. Moreover, p53V135A enhanced the ability of VPM to increase VCR sensitivity in four of the five cell lines (the exception being T98G), whereas the effects of INDO on VCR sensitivity were largely unaffected at 38.5°C. All studies reported here were performed with pooled transfectants, precluding cloning artifacts as an explanation for unexpected data.
Our data highlight several important issues that need to be considered when using temperature-sensitive p53 mutants to study cancer cell sensitivity to chemotherapy or irradiation. First, it is imperative to always examine isogenic, empty vector control cell lines under identical experimental conditions. Shifting the control cells from 38.5°C to 32.5°C alone significantly (a) reduced PGP expression and activity in LN-18 and LN-308 cells but increased PGP expression in U87MG cells, (b) reduced MRP expression in four of the five (LN-18, U87MG, T98G, and LN-308) and MRP activity in two of the five (LN-18, T98G) cell lines, and (c) increased VCR sensitivity in all cell lines. There is no information in the work of Sullivan et al. (3) indicating whether such controls were performed and whether temperature had any effect on MRP expression or activity in the cells examined in their study. Importantly, heat may transcriptionally activate MDR expression (10), but hyperthermia has also been suggested as one strategy to overcome MDR-type drug resistance (11), further supporting the need for careful controls when examining the modulation of drug resistance using temperature-sensitive p53 mutants.
Second, in contrast to the proposed negative regulation of MDR transcription by wild-type p53 (7), we found that p53V135A at wild-type conformation (32.5°C) fails to reduce PGP expression and activity in three of the five cell lines. Moreover, in the two cell lines in which p53V135A reduced PGP expression and activity at 32.5°C, LN-18 and LN-308, the same effects were observed at 38.5°C, where p53V135A assumes mutant conformation and would be expected to enhance rather than reduce MDR expression and activity. Thus, these observations dissociate p53V135A-dependent modulation of MDR from the conformation of the sequence-specific DNA-binding domain of p53V135A in cell lines lacking p53 function. On the other hand, the effects of p53V135A in p53 wild-type cell lines, U87MG and LN-229, conform to current concepts of MDR modulation by p53 (1). This is because p53V135A had no effect on MDR expression at wild-type conformation (32.5°C) but enhanced MDR expression and activity at 38.5°C, acting as a dominant-negative mutant (5). Finally, although activation of rat MDR1b transcription by wild-type p53 has been described (12), the only cell line responding to p53V135A with moderately enhanced PGP activity at 32.5°C, T98G, behaved accordingly at 38.5°C, where p53V135A assumes mutant conformation.
Third, in contrast to the proposed negative regulation of MRP gene transcription by wild-type p53 (2), p53V135A at wild-type conformation (32.5°C) not only failed to reduce MRP expression and activity in any of the cell lines examined but enhanced MRP expression and activity in three of the five cell lines. Again, p53V135A also promoted MRP expression and activity in two of these three cell lines at 38.5°C. Since no such effect was seen in U87MG cells at either temperature, and since p53V135A promoted MRP expression and activity in
LN-229 cells at both temperatures (Table 1), the modulation of MRP by p53 is different from the modulation of MDR by p53 in glioma cells. Moreover, these data contrast sharply with the effects of temperature-sensitive p53 on MRP in a prostate carcinoma cell line (3). Thus, there is striking heterogeneity of the MDR/MRP responses to p53 alterations among cell lines of the same histogenetic origin, and studies of p53 and MDR/MRP interactions must not be extrapolated from one cell type to another.
Fourth, previous studies have shown that two critical hydrophobic amino acids in the NH2-terminal domain of p53, as well as the oligomerization and non–sequence-specific DNA-binding domains, are required for the transcriptional activation of the MDR gene by tumor-derived p53 mutants (8, 9). These two amino acids and the mentioned domain of p53 are preserved on p53V135A. The uniform effects of p53V135A on T98G cells at both temperatures, for instance, could be attributed to such effects. On the other hand, most of the effects of p53V135A observed here could not have been predicted by the current understanding of MDR/MRP regulation by p53, suggesting that other genetic alterations, which differ among the glioma cell lines, determine how the MDR/MRP systems are modulated by p53V135A at either conformation.
Fifth, the use of temperature-sensitive p53 mutants for the study of chemoresistance requires careful consideration of controls (6). Thus, we show here that although p53V135A in its mutant conformation at 38.5°C uniformly increased VCR sensitivity in the glioma cell lines, it had heterogenous, indeed opposing, effects on MDR/MRP expression and activity. Clearly, p53-dependent changes in cellular sensitivity to the classic MDR and MRP substrates are not due exclusively to the modulation of MDR or MRP expression.
References
- 1.Chin KV, Ueda K, Pastan I, Gottesman M. Modulation of activity of the promoter of the human MDR1 gene by ras and p53. Science. 1992;255:459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Beck WT. Transcriptional suppression of multidrug resistance-associated protein (MRP) gene expression by wild-type p53. Cancer Res. 1998;58:5762–5769. [PubMed] [Google Scholar]
- 3.Sullivan GF, et al. Regulation of expression of the multidrug resistance protein MRP1 by p53 in human prostate cancer cells. J Clin Invest. 2000;105:1261–1267. doi: 10.1172/JCI9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller M, et al. Predicting chemoresistance in human malignant glioma cells: the role of molecular genetic analyses. Int J Cancer. 1998;79:640–644. doi: 10.1002/(sici)1097-0215(19981218)79:6<640::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Naumann U, Durka S, Weller M. Dexamethasone-mediated protection from drug toxicity linked to p21WAF/CIP1 protein accumulation. Oncogene. 1998;17:1567–1575. doi: 10.1038/sj.onc.1202071. [DOI] [PubMed] [Google Scholar]
- 6.Trepel M, et al. Chemosensitivity of human malignant glioma: modulation by p53 gene transfer. J Neurooncol. 1998;39:19–32. doi: 10.1023/a:1005910323338. [DOI] [PubMed] [Google Scholar]
- 7.Zastawny RL, Salvino R, Chen J, Benchimol S, Ling V. The core promoter region of the P-glycoprotein gene is sufficient to confer differential responsiveness to wild-type and mutant p53. Oncogene. 1993;8:1529–1535. [PubMed] [Google Scholar]
- 8.Lin J, Teresky AK, Levine AJ. Two critical hydrophobic amino acids in the N-terminal domain of the p53 protein are required for the gain of function phenotypes of human p53 mutants. Oncogene. 1995;10:2387–2390. [PubMed] [Google Scholar]
- 9.Lányi Á, et al. ”Gain of function” phenotype of tumor-derived mutant p53 requires the oligomerization/nonsequence-specific nucleic acid-binding domain. Oncogene. 1998;16:3169–3176. doi: 10.1038/sj.onc.1201857. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, et al. Involvement of heat shock factor in regulating transcriptional activation of MDR1 gene in multidrug-resistant cells. Cancer Lett. 1997;115:9–14. doi: 10.1016/s0304-3835(97)04725-3. [DOI] [PubMed] [Google Scholar]
- 11.Larrivee B, Averill DA. Melphalan resistance and photoaffinity labelling of P-glycoprotein in multidrug-resistant Chinese hamster ovary cells: reversal of resistance by cyclosporin A and hyperthermia. Biochem Pharmacol. 1999;58:291–302. doi: 10.1016/s0006-2952(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Kuo MT. Wild-type p53-mediated induction of rat mdr1b expression by the anticancer drug daunorubicin. J Biol Chem. 1998;273:15387–15394. doi: 10.1074/jbc.273.25.15387. [DOI] [PubMed] [Google Scholar]
- 13.Rieger L, et al. Evidence for a constitutive, verapamil-sensitive, non-Pgp multidrug resistance phenotype that is unaltered by radiochemotherapy in vivo. Acta Neuropathol. 2000;99:555–562. doi: 10.1007/s004010051160. [DOI] [PubMed] [Google Scholar]
- 14.Roller A, et al. Selective potentiation of drug cytotoxicity by NSAID in human glioma cells: the role of COX-1 and MRP. Biochem Biophys Res Commun. 1999;259:600–605. doi: 10.1006/bbrc.1999.0825. [DOI] [PubMed] [Google Scholar]


