Abstract
DNA methylation in transposons and other DNA repeats is conserved in plants as well as in animals. In Arabidopsis thaliana, an RNA-directed DNA methylation (RdDM) pathway directs de novo DNA methylation. We performed a forward genetic screen for suppressors of the DNA demethylase mutant ros1 and identified a novel Zinc-finger and OCRE domain-containing Protein 1 (ZOP1) that promotes Pol IV-dependent siRNA accumulation, DNA methylation, and transcriptional silencing. Whole-genome methods disclosed the genome-wide effects of zop1 on Pol IV-dependent siRNA accumulation and DNA methylation, suggesting that ZOP1 has both RdDM-dependent and -independent roles in transcriptional silencing. We demonstrated that ZOP1 is a pre-mRNA splicing factor that associates with several typical components of the splicing machinery as well as with Pol II. Immunofluorescence assay revealed that ZOP1 overlaps with Cajal body and is partially colocalized with NRPE1 and DRM2. Moreover, we found that the other development-defective splicing mutants tested including mac3a3b, mos4, mos12 and mos14 show defects in RdDM and transcriptional silencing. We propose that the splicing machinery rather than specific splicing factors is involved in promoting RdDM and transcriptional silencing.
Keywords: DNA methylation, RdDM, splicing, transcriptional silencing, ZOP1
Introduction
RNA-mediated repressive chromatin modifications and transcriptional silencing is a conserved mechanism that is required for maintenance of genome stability, repression of transposable elements (TEs), and regulation of genes in eukaryotes (Slotkin and Martienssen, 2007; Matzke et al, 2009; Law and Jacobsen, 2010). In Arabidopsis, an RNA-directed DNA methylation (RdDM) pathway has been characterized (Matzke et al, 2009; Law and Jacobsen, 2010). The transposons and other DNA repeats are transcribed by a plant-specific DNA-dependent RNA polymerase Pol IV and give rise to double-stranded RNAs by RNA-dependent RNA polymerase RDR2 (Xie et al, 2004; Herr et al, 2005; Kanno et al, 2005; Pontier et al, 2005; Ream et al, 2009). The double-stranded RNAs are cleaved into 24-nt small interfering RNAs (siRNAs) by a Dicer-like protein DCL3 (Xie et al, 2004). The Pol IV-interaction proteins, CLSY and SHH1/DTF1, are required for siRNA generation at a subset of RdDM target loci (Smith et al, 2007, Law et al, 2011; Liu et al, 2011). The siRNAs are loaded onto an ARGONAUTE protein AGO4, which interacts with NRPE1, the largest subunit of another DNA-dependent RNA polymerase, Pol V, and a transcription elongation factor-like protein, KTF1 (Pontes et al, 2006; Qi et al, 2006; Wierzbicki et al, 2008; He et al, 2009a). Pol V is required for the transcription of intergenic non-coding RNAs (Wierzbicki et al, 2008). DRD1, DMS3, and RDM1 form a tight complex, termed as DDR, that facilitates generation of Pol V-dependent RNA transcripts (Kanno et al, 2004, 2008; Gao et al, 2010; Law et al, 2010). The IWR1-like protein RDM4/DMS4 is a transcription factor that interacts with Pol II, Pol IV, and Pol V (He et al, 2009b; Kanno et al, 2010; Law et al, 2011). Pol V-dependent RNA transcripts are required for the association of AGO4 with chromatin (Wierzbicki et al, 2009). In the RdDM pathway, the DNA methyltransferase DRM2 is eventually recruited to RdDM target loci and catalyses DNA methylation (Cao and Jacobsen, 2002; Gao et al, 2010). Besides the canonical RdDM components, Pol II and its mediator (a multi-subunit regulator of Pol II) were also demonstrated to be required for RdDM and transcriptional gene silencing (Zheng et al, 2009; Kim et al, 2011). The non-coding scaffold RNAs produced by Pol II recruit AGO4 and Pol V to a subset of RdDM target loci in order to promote siRNA-mediated transcriptional gene silencing (Zheng et al, 2009).
The Arabidopsis RdDM system parallels the fission yeast RNAi-induced heterochromatin formation machinery in that they share several conserved RNAi components, including Dicer proteins, RNA-dependent RNA polymerases, Argonaute proteins, and WG/GW-containing proteins. The fission yeast RNAi-induced heterochromatin formation and transcriptional silencing is essential for the heterochromatin assembly on the dh and dg repeats of centromeric regions (Moazed, 2009; Grewal, 2010). Non-coding RNAs are transcribed from the repeat regions by Pol II and give rise to double-stranded RNAs that are processed into siRNAs by Dcr1 (Hall et al, 2002; Volpe et al, 2002). In fission yeast, Ago1, Tas3, and Chp1 are assembled into an RNAi-induced transcriptional silencing (RITS) effector complex containing siRNAs (Verdel et al, 2004). The RITS complex facilitates the recruitment of the RNA-dependent RNA polymerase complex (RDRC, containing Rdp1, cid12, and Hrr1). RDRC increases the production of double-stranded RNA precursors, reinforcing siRNA generation (Motamedi et al, 2004). The processing of RNA precursors into siRNAs ultimately recruits histone H3K9 dimethylase Clr4 to the centromeric repeat regions and promotes histone H3K9 dimethylation and heterochromatin formation (Buhler et al, 2006; Grewal, 2010).
The recognition and removal of introns from pre-mRNA is catalysed by spliceosome complexes in eukaryotes (Sharp, 1994). The major spliceosome complex is composed of five small nuclear ribonucleoproteins (snRNPs: U1, U2, U4, U6, and U5) and numerous non-snRNP splicing factors (Zhou et al, 2002). After the core proteins of snRNPs are assembled in the cytoplasm, they are transported to the nucleolus-adjacent Cajal body for assembly and modification (Cioce and Lamond, 2005; Morris, 2008). To assemble a mature spliceosome on pre-mRNA, U1 snRNP and U2AF (U2 snRNP auxiliary factor) bind to the 5′ splice site and the branchpoint of the 3′ splice site, respectively. Thus, the U4/U6-U5 tri-snRNP is added to the intermediate spliceosome, which finally leads to the displacement of U1 and U4 snRNPs and the formation of a catalytic mature spliceosome (Matlin and Moore, 2007). Assembly of snRNPs and spliceosome complexes is facilitated by many non-snRNP splicing factors.
Recent studies revealed that the spliceosome proteins and splicing factors affect not only pre-mRNA splicing but also Pol II transcription elongation, mRNA polyadenylation, and telomerase RNA biogenesis and processing, indicating that splicing-related proteins have multiple functions (Kaida et al, 2010; Shukla et al, 2011; Tang et al, 2012). Several specific splicing factors are also required for RNAi-induced heterochromatin formation and transcriptional silencing in fission yeast (Bayne et al, 2008; Chinen et al, 2010). The underlying mechanism remains to be elucidated. In Arabidopsis, the splicing factor SR45 was recently found to be required for RdDM, but an indirect role of SR45 via the splicing of RdDM regulator genes was not precluded in this report (Ausin et al, 2012).
The Cajal body is a dynamic nuclear structure involved in snRNP assembly, rRNA processing, and telomerase RNP formation (Pikaard, 2006; Morris, 2008). The immunolocalization of RdDM proteins revealed that AGO4, RDR2, and DCL3 localize in the Cajal body (Li et al, 2006, 2008). The Cajal body mutant coilin disrupts not only the formation of Cajal bodies but also the concentrated AGO4 foci in the Cajal body (Li et al, 2008). It is possible that proteins other than AGO4 in Cajal bodies are required for assembly and recruitment of the AGO4 effector complex in RdDM.
To increase our understanding of the RdDM mechanism in Arabidopsis, in the current study we searched for new RdDM mutants by screening for suppressors of ros1 from the previously described EMS-mutagenized library in the ros1 mutant background (Liu et al, 2011). The results showed that most of the identified mutants are new alleles of known RdDM components (unpublished data). In addition, the screen identified a previously uncharacterized gene encoding Zinc finger (ZnF) and OCRE domain-containing Protein 1 (ZOP1). We demonstrated that ZOP1 is a novel nucleic acid-binding protein that is required for both RdDM and pre-mRNA splicing. Moreover, we found that the four other development-defective splicing mutants that were tested (mac3a3b, mos4, mos12, and mos14) also show defects in siRNA accumulation and DNA methylation. This study reveals a novel function of the splicing machinery in siRNA accumulation and RdDM.
Results
Identification and characterization of the zop1 mutant
Previous studies suggest that the RD29A promoter-driven luciferase (RD29A-LUC) transgene, endogenous RD29A, and the 35S promoter-driven NPTII (35S-NPTII) transgene are highly expressed in wild-type plants under stress conditions, whereas loss-of-function mutation in the DNA demethylase gene ROS1 silences the expression of all three genes (Gong et al, 2002). The previous forward genetic screens for suppressors of ros1 identified most of the RdDM components and other important proteins involved in transcriptional gene silencing (He et al, 2009c; Liu et al, 2011). The RdDM pathway is required for the silencing of the RD29A-LUC transgene as well as of the endogenous RD29A in ros1, but is dispensable for the silencing of the 35S-NPTII transgene from the same construct. In the current study, a new mutant, zop1, was identified as a suppressor of ros1 (Figure 1A). Like the previously identified RdDM mutants, zop1 suppresses the silencing of RD29A-LUC and endogenous RD29A but not of 35S-NPTII in the ros1 background, although suppression of silencing is less with zop1 than with nrpe1 (Figure 1A and B). The DNA methylation of both transgenic and endogenous RD29A promoters was tested by bisulphite sequencing. Similar to the results of previous reports, heavy DNA methylation in the ros1 mutant occurs in all three cytosine contexts (CG, CHG, and CHH) at both transgenic and endogenous RD29A promoters. The high DNA methylation is substantially reduced at CHG and CHH sites in both ros1zop1 and ros1nrpe1 (Figure 1C and D).
Figure 1.
RD29A-LUC transgene silencing and DNA methylation are affected by zop1. (A) The expression of RD29A-LUC was measured by luminescence imaging in the wild type, ros1, ros1zop1, and ros1nrpe1. (B) The RNA transcripts of LUC, RD29A, and NPTII were detected by real-time RT–PCR in the indicated genotypes. The relative expression levels of the genes are shown. Total RNA was extracted from 2-week old seedlings with or without cold treatment (48 h, 4°C). (C, D) The DNA methylation at both transgene (C) and endogenous (D) RD29A promoters was determined by bisulphite sequencing. The percentage of methylated cytosines at CG, CHG, and CHH sites is separately shown in the charts.
We mapped the zop1 mutation by using a F2 segregation population from the cross between the ros1zop1 mutant in the C24 background and a homozygous ros1 mutant (Salk_045303) in the Col-0 background. The plants that emitted high bioluminescence were selected from the F2 population for map-based cloning. The zop1 mutation was mapped to a ∼273-kb interval on Chromosome 1 (Supplementary Figure S1A). A single-nucleotide G to A substitution in AT1G49590 was identified by deep sequencing of the whole ros1zop1 genome (Supplementary Figure S1B). The substitution creates a premature stop codon and truncates the protein at Arg18 (Supplementary Figure S1B). The construct harbouring the full AT1G49590 genomic sequence in-frame to the 3 × Flag tag was transformed into ros1zop1 for a complementation test. The results show that the AT1G49590 transgene is able to restore the RD29A-LUC silencing (Supplementary Figure S1C). Moreover, the transgene complements the developmental defects of ros1zop1 (Supplementary Figure S1D). The results suggest that AT1G49590 is the ZOP1 gene that is not only required for RdDM and transcriptional gene silencing but also for proper development.
ZOP1 contains an N-terminal C2H2-type ZnF domain and a predicted octamer repeat (OCRE) domain (Supplementary Figure S1B and S2), and its name reflects its status as a zinc finger- and OCRE domain-containing protein. Previous studies reported that the OCRE-containing proteins are likely to be involved in nucleic acid binding and RNA metabolism (Callebaut and Mornon, 2005). However, the function of the ZnF and OCRE domains in ZOP1 remains to be elucidated. ZOP1 and its homologues are conserved from unicellular green algae to various angiosperms (Supplementary Figure S2), whereas there is no ZOP1 homologue in animals and fungi.
The zop1 mutation impairs RdDM at endogenous genome targets
We measured the effect of the zop1 mutation on accumulation of Pol IV-dependent siRNAs from RD29A-LUC transgene promoter and endogenous genome target loci. The 24-nt siRNA from the transgene promoter is blocked in ros1nrpd1 (He et al, 2009c), suggesting that it is a Pol IV-dependent siRNA. Our small RNA northern blot analysis indicates that the accumulation of the RD29A promoter siRNA is partially reduced in ros1zop1 as well as in ros1nrpe1 (Figure 2A). Moreover, we found that the zop1 mutation markedly reduces the accumulation of the Pol IV- and Pol V-dependent siRNAs, including AtSN1 siRNA and siRNA1003 (Figure 2A; Supplementary Table S1). Cluster4 siRNA and siRNA02 were previously recognized as Pol IV-dependent but Pol V-independent siRNAs (Mosher et al, 2008; Zheng et al, 2009), but our results indicate that both siRNAs are weakly reduced by nrpe1 in ros1nrpe1 (Figure 2A; Supplementary Table S1). Although Cluster4 siRNA and siRNA02 are partially dependent on Pol V, the dependence is much lower than that of previously characterized Pol IV- and Pol V-dependent siRNAs including AtSN1 siRNA and siRNA1003. The reduction of Cluster4 siRNA and siRNA02 in ros1zop1 is similar to that in ros1nrpe1 (Figure 2A; Supplementary Table S1). Interestingly, we found that the accumulation of AtSN1 siRNA and Cluster4 siRNA seems to be weakly reduced by ros1 (Figure 2A; Supplementary Table S1). This effect may be caused by the feedback effect of ros1 on RdDM.
Figure 2.
Effect of zop1 on small RNA accumulation and DNA methylation. (A) Effect of zop1 on small RNA accumulation was determined by small RNA northern blotting. The accumulation of 24-nt Pol IV-dependent siRNAs, 21-nt ta-siRNA255, and miRNA171 was detected. The ethidium bromide-stained small RNA gel is shown as a loading control. (B) Effect of zop1 on Pol IV-dependent siRNA accumulation as determined by small RNA deep sequencing. Numbers of uniquely matched Pol IV-dependent siRNA reads in ros1, ros1nrpd1, ros1zop1, and ros1nrpe1. siRNA abundance in each genotype is expressed as a percentage of siRNAs in ros1. (C, D) The DNA methylation of AtSN1 and MEA-ISR was determined by bisulphite sequencing in the wild type, ros1, ros1zop1, and ros1nrpe1. CG, CHG, and CHH methylation in each ecotype is shown in the charts. (E) The diagrams indicate the numbers of genes and TEs that show reduced CHG methylation in ros1nrpd1 and ros1zop1 relative to ros1. The number of overlapping loci and specific loci is shown. (F) The numbers of loci that show reduced CHH methylation.
Source data for this figure is available on the online supplementary information page.
The effect of zop1 on Pol IV-dependent siRNA accumulation was measured by small RNA deep sequencing. The results indicate that 201 658 Pol IV-dependent 24-nt siRNA reads are uniquely matched on the Arabidopsis nuclear genome (Figure 2B; Supplementary Table S2). These siRNAs are reduced to 18 654 reads (9.3%) and 115 604 reads (57.3%) in ros1nrpd1 and ros1nrpe1, respectively. The siRNAs in ros1zop1 are reduced to 146 555 reads (72.7%), which is comparable to that in ros1nrpe1 (Figure 2B; Supplementary Table S2). Plotting of the Pol IV-dependent 24-nt siRNA distribution in ros1, ros1zop1, and ros1nrpd1 (Supplementary Figure S3A–E) indicates that unlike nrpd1, zop1 only partially affects Pol IV-dependent siRNA accumulation. The effect of zop1 on siRNA accumulation is similar to that of nrpe1, indicating that the zop1 mutation may influence a downstream step at the RdDM pathway.
The effect of zop1 on the DNA methylation level of endogenous RdDM genomic targets was tested by bisulphite sequencing analysis. The results indicate that the DNA methylation of AtSN1 A and MEA-ISR at CHG and CHH sites is substantially reduced in ros1zop1 as well as in ros1nrpe1, whereas the DNA methylation at CG sites is not affected by either zop1 or nrpe1 (Figure 2C and D). The effect of zop1 on the DNA methylation of AtSN1 was further confirmed by chop-PCR. The results suggest that the AtSN1 DNA methylation is partially reduced in ros1zop1 compared to that in WT and ros1, but the reduction is less than that in ros1nrpd1 and ros1nrpe1 (Supplementary Figure S4). Moreover, the suppressive effect of zop1 on DNA methylation was demonstrated by chop-PCR at one more RdDM target IGN23 (Supplementary Figure S4).
To determine the genome-wide effect of zop1 on DNA methylation, we preformed whole-genome bisulphite sequencing in ros1, ros1nrpd1, and ros1zop1. The results show that zop1 as well as nrpd1 reduces DNA methylation especially at CHG and CHH sites at a whole-genome level (Supplementary Figure S5; Supplementary Table S3). The genome-wide effect of nrpd1 on DNA methylation determined by this study is consistent with the previous study (Wierzbicki et al, 2012). The reduction of CHH methylation in ros1zop1 is clearly less than that in ros1nrpd1, whereas the reduction of CHG methylation in ros1zop1 is comparable to that in ros1nrpd1 (Supplementary Table S3). The identified DNA methylation differences caused by zop1 and nrpd1 were measured by sequence-specific bisulphite sequencing analysis at three randomly selected loci (AT5G35540, At1G54750, and AT1G14247) and the results indicated that our whole-genome bisulphite sequencing results are reliable (Supplementary Figure S6A–C).
In the ros1nrpd1 mutant, there are 3522 genes and 3799 TEs that show reduced CHG methylation. Among them, 1211 genes (34.4%) and 1015 TEs (26.7%) also have reduced CHG methylation in ros1zop1 (Figure 2E; Supplementary Tables S4 and S5). ZOP1 may act on these loci through an RdDM-dependent pathway. Moreover, there are 2672 genes and 1683 TEs whose CHG methylation is reduced in ros1zop1 but not in ros1nrpd1, suggesting that the CHG methylation at these loci may be established and maintained through an RdDM-independent pathway (Figure 2E; Supplementary Tables S4 and S5). There are 1807 genes and 5709 TEs that have reduced CHH methylation in ros1nrpd1 relative to ros1, in which only 277 genes (15.3%) and 410 TEs (7.2%) have reduced CHH methylation in both ros1nrpd1 and ros1zop1 (Figure 2F; Supplementary Tables S4 and S5). There are 606 genes and 563 TEs that show reduced CHH methylation in ros1zop1 but not in ros1nrpd1. Totally, the zop1 mutation can reduce CHH methylation at 883 genes and 973 TEs, which are much less than 1807 genes and 5709 TEs that are affected by nrpd1 (Figure 2F; Supplementary Tables S4 and S5). The result is consistent with the weak reduction of the overall CHH methylation level in ros1zop1 (Supplementary Table S3). In the 5709 TEs that show NRPD1-dependent CHH methylation, only less than one tenth of these loci (410/5709) whose CHH methylation is markedly reduced by zop1 (Figure 2F; Supplementary Tables S4 and S5), suggesting that the effect of zop1 on CHH methylation is much less than nrpd1.
Like nrpd1, zop1 reduces DNA methylation substantially in euchromatic regions and only slightly in centromeric regions (Supplementary Figure S5). At the promoter regions of protein-coding genes, the DNA methylation level is generally reduced at CHG and CHH sites by both zop1 and nrpd1, although the reduction of CHH methylation caused by zop1 is less than that caused by nrpd1 (Supplementary Figure S7A). At TEs, the DNA methylation is reduced by nrpd1 especially at CHH sites, whereas the reduction of DNA methylation caused by zop1 is significant at CHG sites rather than at CHH sites (Supplementary Figure S7B). The results suggest that ZOP1 may act on DNA methylation through an uncharacterized mechanism that is different from the canonical RdDM pathway.
The transcriptional silencing at endogenous RdDM genome target loci is associated with DNA hypermethylation. Our quantitative RT–PCR results indicate that the silencing of AtSN1 A, AtGP1, and solo LTR is substantially released in either ros1nrpd1 or ros1nrpe1 and is released to a much lesser extent in ros1zop1 (Supplementary Figure S8A and B). Unlike the previously characterized RdDM mutants including nrpd1 and nrpe1, zop1 has no effect on ROS1 expression (Supplementary Figure S8A). The release of silencing at AtGP1 and solo LTR was also found in the zop1 single mutants (Supplementary Figure S8B), suggesting that the effect of zop1 on silencing is unrelated to the ros1 mutation in ros1zop1. The Pol V-dependent RNA transcripts AtSN1 B, IGN5 B, and IGN23 from RdDM target loci are blocked in ros1nrpe1 but are not affected in ros1zop1 (Supplementary Figure S8C), demonstrating that ZOP1 is not required for accumulation of Pol V-dependent RNA transcripts.
ZOP1 is a novel pre-mRNA splicing factor
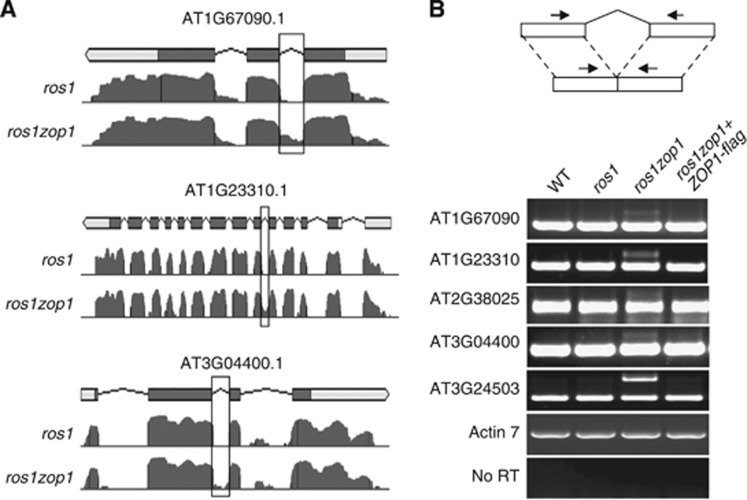
ZOP1 is a ZnF and OCRE domain-containing protein. The OCRE domain-containing proteins are usually involved in RNA processing (Callebaut and Mornon, 2005). We performed RNA deep sequencing to determine the possible effect of zop1 on RNA processing. From the ros1 and ros1zop1 RNA libraries, we obtained 25.5 million and 27.7 million RNA reads, respectively. Among them, 14.5 million and 13.5 million reads were mapped to the Arabidopsis genome. The intron-retention events were analysed in ros1 and ros1zop1, and 361 intron-retention events in 215 genes were identified in ros1zop1 but not in ros1 (P<0.01, intron read coverage >80%) (Supplementary Table S6). In contrast, only 31 intron-retention events in 27 genes were identified in ros1 but not in ros1zop1 (Supplementary Table S7). The intron-retention events in ros1zop1 were confirmed by RT–PCR using intron-flanking primers (Figure 3A and B), suggesting that zop1 may cause defects in pre-mRNA splicing. Moreover, we found that the defects can be complemented by the ZOP1 transgene in the ros1zop1 mutant background (Figure 3B). The results confirmed that zop1 causes defects in pre-mRNA splicing. Additionally, we found that the mature mRNAs are still present in ros1zop1 even some mRNAs are probably mildly reduced (Figure 3B). Together, the results indicate that ZOP1 is a protein related to pre-mRNA splicing.
Figure 3.
Pre-mRNA splicing is affected by zop1. (A) The splicing defects in ros1zop1 at three indicated loci are shown. In the gene diagrams, bars and lines represent exons and introns, respectively. Filled bars are encoding regions, whereas blank bars are 5′ UTR or 3′ UTR. Normalized reads for the three genes are plotted for ros1zop1 as well as for ros1. The intron-retention events in the three genes are labelled with frames. (B) The identified intron-retention events were confirmed by semiquantitative RT–PCR. The primers crossing introns were used to test the intron-retention events in ros1zop1 as well as in the wild type and ros1. The amplification of an intron-containing fragment of ACT7 as a control suggests the absence of DNA contamination. No RT represents amplification of ACT7 from total RNA without reverse transcription.
Source data for this figure is available on the online supplementary information page.
According to RNA deep sequencing in ros1 and ros1zop1, the RNA transcripts of hundreds of genes were affected by zop1 (Supplementary Table S8). A possible explanation for the involvement of ZOP1 in Pol IV-dependent siRNA accumulation and DNA methylation is that the splicing defects caused by zop1 impair the accumulation of the mature mRNAs encoding typical RdDM components. However, our RNA deep sequencing result shows that no RdDM component-encoding gene is affected in ros1zop1 (Supplementary Table S8). Furthermore, our quantitative RT–PCR results confirmed that the expression of major RdDM components is not reduced in ros1zop1 (Supplementary Figure S9). Thus, the effect of zop1 on RdDM is unlikely to be the result of indirect regulation of RdDM component-encoding genes. The splicing factor ZOP1 is likely to directly contribute to DNA methylation and transcriptional gene silencing.
To better understand the function of ZOP1, we carried out affinity purification of the ZOP1-Flag fusion protein from the ZOP1-Flag transgenic plants. The copurified proteins were analysed by mass spectrometry. Many peptides corresponding to components of spliceosome complexes and splicing factors were identified from the ZOP1-Flag copurified proteins (Supplementary Figure S10; Supplementary Table S9), suggesting the association between ZOP1 and these splicing proteins. In the list of ZOP1 copurified proteins, STA1 is the homologue of a yeast splicing factor PRP6 that interacts with both the U4/U6 and U5 snRNPs and facilitate the formation of U4/U6-U5 tri-snRNPs (Makarov et al, 2000). Our coimmunoprecipitation assay confirmed that ZOP1 can specifically associate with STA1 (Figure 4A), indicating that ZOP1 is likely to be a novel splicing factor. Subcellular localization of ZOP1 was determined by immunolocalization assay with the Flag antibody in the ZOP1-Flag transgenic plants. The result shows that ZOP1 is a nuclear protein (Supplementary Figure S11), which is consistent with its roles in pre-mRNA splicing as well as in DNA methylation and transcriptional silencing.
Figure 4.
Detection of the interaction between ZOP1 and related proteins. (A) Detection of the interaction between ZOP1 and STA1 by coimmunoprecipitation assay. Total protein extracts from STA1-Flag transgenic plants were immunoprecipitated with anti-Flag antibody. Immunoprecipitated proteins were tested by western blotting. (B) Coimmunoprecipitation testing the interaction between ZOP1 and NRPB1 or AGO4. Total protein extracts from wild-type and ZOP1-Flag transgenic plants were immunoprecipitated with anti-Flag antibody, and the precipitated proteins were subjected to western blotting with the antibodies aFlag, aNRPB1, and aAGO4. (C) Immunolocalization of ZOP1-Flag and U2B in nuclei of ZOP1-Flag transgenic plants using anti-Flag and the anti-U2B antibodies. Nuclei were counterstained with DAPI. Colocalization between ZOP1-Flag and U2B generates yellow signals. The percentage of the nuclei with indicated patterns is shown. (D) Immunolocalization of ZOP1-Myc and NRPE1-Flag in nuclei of plants expressing both ZOP1-Myc and NRPE1-Flag transgenes. (E) Immunolocalization of ZOP1-Myc and DRM2-Flag in nuclei of plants expressing both ZOP1-Myc and DRM2-Flag transgenes. (F) Immunolocalization of ZOP1-Myc and NRPD1-Flag in nuclei.
Source data for this figure is available on the online supplementary information page.
Because ZOP1 belongs to a member of OCRE domain-containing proteins that are usually involved in nucleic acid binding, we carried out electrophoretic mobility shift assays (EMSAs) to determine whether ZOP1 is capable of binding to nucleic acids. The results show that the recombinant His-tagged ZOP1 protein can bind double-stranded DNA as well as double-stranded RNA but not single-stranded DNA or RNA (Supplementary Figure S12A). ZOP1 can bind both blunt-end double-stranded RNA (bdsRNA) and 5′ overhanging double-stranded RNA (odsRNA) (Supplementary Figure S12A). ZOP1 binds to double-stranded DNA or RNA in a dose-dependent manner, and the binding is reduced by competition with unlabelled double-stranded DNA or RNA (Supplementary Figure S12B and C), suggesting that the nucleic acid-binding ability of ZOP1 is specific. To identify the domain that is required for the nucleic acid binding of ZOP1, we generated a series of truncated ZOP1 proteins and used these proteins in EMSA (Supplementary Figure S12D). The results show that the previously uncharacterized C-terminal domain but not the ZnF and OCRE domains is responsible for ZOP1 binding to both double-stranded DNA and double-stranded RNA (Supplementary Figures S12D and E). Positively charged amino acids are rich in the ZOP1 C-terminal domain (Supplementary Figure S2), which is consistent with its nucleic acid-binding ability.
The relationship between ZOP1 and other RdDM-related proteins
AGO4 protein levels are reduced in the RdDM mutants that are directly responsible for primary siRNA biogenesis, including nrpd1, rdr2, and dcl3 (Li et al, 2006; Supplementary Figure S13). Our western blotting result indicates that neither zop1 nor nrpe1 affects AGO4 protein levels (Supplementary Figure S13), suggesting that like NRPE1, ZOP1 is not directly involved in primary siRNA biogenesis, but how ZOP1 is involved in DNA methylation remains to be elucidated. We used coimmunoprecipitation to determine whether ZOP1 interacts with the proteins that promote DNA methylation. The results suggest that ZOP1 cannot interact with the tested canonical RdDM components, including AGO4, NRPD1, NRPE1, DRM2, and RDM4 (Figure 4B; Supplementary Figure S14A–D). Coimmunoprecipitation indicates, however, that ZOP1 can interact in vivo with Pol II (Figure 4B). Furthermore, the interaction between ZOP1 and Pol II was tested in the presence of RNase and DNase. The result demonstrated that the interaction is insensitive to RNase and DNase (Supplementary Figure S15). Pol II was previously demonstrated to be involved in transcriptional silencing (Zheng et al, 2009), suggesting that the function of ZOP1 in transcriptional silencing is related to Pol II.
The localization pattern of ZOP1 in nuclei was investigated by immunofluorescence assay. Anti-Flag and anti-Myc antibodies did not produce any visible signals in the nuclei of wild-type plants (Supplementary Figure S16), but the anti-Flag antibody produced specific ZOP1 signals in the nuclei of ZOP1-Flag transgenic plants (Figure 4C). The results show that the ZOP1 is present in the form of either condensed nucleolus-adjacent foci or dispersed nucleoplasmic speckles (Figure 4C–F). The condensed nucleolus-adjacent foci of ZOP1 include the signals that overlap with U2B in the Cajal bodies (Figure 4C). The Cajal body, an snRNP assembly centre, was previously reported to be required for the assembly of the AGO4 effector complex of RdDM (Li et al, 2008). U2B, a component of U2 snRNP, was used as a marker for the Cajal body, and AGO4 colocalizes with U2B in the Cajal body (Li et al, 2006). Our results suggest that ZOP1 can colocalize with AGO4 at the Cajal body. We tested the relationship between ZOP1 and other canonical RdDM components by immunofluorescence assay. The ZOP1-Myc transgenic plants were crossed to NRPE1-Flag, DRM2-Flag, and NRPD1-Flag transgenic plants, respectively. Anti-Flag and anti-Myc antibodies were used to detect the signals in the nuclei of their F1 plants. The results indicated that ZOP1 partially colocalizes with NRPE1 as well as with DRM2 but not with NRPD1 in the nucleolus-adjacent foci (Figure 4D–F). NRPE1 and DRM2 directly associate with chromatin at RdDM target loci. Partial colocalization between ZOP1 and the two RdDM components is consistent with the finding that ZOP1 shares a large number of chromatin targets with the RdDM pathway (Figure 2E; Supplementary Tables S4 and S5).
To investigate the genetic relationship between ZOP1 and known RdDM proteins, we crossed ros1zop1 to ros1nrpd1, ros1nrpe1, and ros1ago4, and generated ros1zop1nrpd1, ros1zop1nrpe1, and ros1zop1ago4. The luminescence analysis shows that the combination of zop1 with nrpd1, nrpe1, or ago4 in the ros1 background can enhance the expression of the RD29A-LUC transgene (Figure 5A). Moreover, we found that zop1 can also enhance the expression of endogenous RD29A in ros1zop1nrpd1, ros1zop1nrpe1, and ros1zop1ago4 (Figure 5B). These results suggest that zop1 has an additive effect with the RdDM mutants nrpd1, nrpe1, and ago4. Involvement of ZOP1 in transcriptional silencing is at least partially independent of the previously characterized RdDM pathway.
Figure 5.
Effect of the zop1 mutation on transcriptional silencing in the ros1nrpd1, ros1nrpe1, and ros1ago4 backgrounds. (A) The expression of the RD29A-LUC transgene was evaluated by luminescence imaging in the indicated genotypes at the top panel. The averages of three independent experiments with standard deviations are shown at the bottom panel. (B) Relative expression levels of endogenous RD29A, SDC, and AtGP1 were determined by quantitative RT–PCR.
To evaluate the function of ZOP1 on the silencing of endogenous RdDM targets, we measured the transcript levels of endogenous RdDM target loci in ros1zop1, ros1nrpd1, ros1nrpe1, and ros1ago4 as well as in ros1nrpd1zop1, ros1nrpe1zop1, and ros1ago4zop1. The results show that the zop1 mutation induces the transcript level of a typical RdDM target SDC in the ros1nrpd1 and ros1ago4 backgrounds, whereas the zop1 mutation has no effect on SDC in the ros1nrpe1 background (Figure 5B). Moreover, the zop1 mutation reduces the transcript levels of AtGP1 in the ros1nrpd1, ros1nrpe1, and ros1ago4 backgrounds (Figure 5B). Combination of the zop1 mutation with the RdDM mutants is likely to complicate the transcriptional regulation of RdDM target loci. But we have now known that ZOP1 is likely to act in transcriptional silencing at least partially through an RdDM-independent pathway.
Other development-defective splicing mutants affect RdDM
To investigate the possible role of other splicing-related proteins in RdDM and transcriptional silencing, we surveyed four other splicing mutants (mac3a3b, mos4, mos12, and mos14) that show defects in both pre-mRNA splicing and development (Monaghan et al, 2009; Xu et al, 2011, 2012). MAC3A and MAC3B are the homologues of the conserved yeast PRP19 splicing factor, and MOS4 is a component of the PRP19 complex, an evolutionarily conserved spliceosome-associated complex (Monaghan et al, 2009). MOS12 associates with the MOS4 and is required for proper pre-mRNA splicing (Xu et al, 2012). MOS14 is a nuclear-import receptor for serine/arginine-rich splicing factors (Xu et al, 2011). We first tested whether the splicing sites affected by zop1 are also differentially spliced in these four splicing mutants. The results show that in the five indicated zop1-affected splicing sites, two of them in AT2G38025 and AT3G04400 are affected in mac3a3b, mos4, mos12, but not in mos14, whereas the other three splicing sites in AT1G67090, AT1G23310, and AT3G24503 are correctly spliced in all the four tested splicing mutants (Supplementary Figure S17). The splicing defects of SNC1 and RPS4 caused by mos14 are not present in ros1zop1 (Supplementary Figure S17). Affected splicing sites in the four splicing mutants (mac3a3b, mos4, mos12, and mos14) are much different from that in zop1.
By small RNA northern blotting, we found that in the four the tested splicing mutants (mac3a3b, mos4, mos12, and mos14), the Pol IV-dependent 24-nt siRNAs, including solo LTR siRNA and siRNA1003, are reduced (Figure 6A). The results demonstrate that like ZOP1, these splicing-related proteins are required for siRNA accumulation. The reduction of siRNAs in mac3a3b is comparable to that in nrpe1 and is greater than that in mos4, mos12, or mos14 (Figure 6A). The mos4 mutant has the least effect on siRNA accumulation, whereas the effects of mos12 and mos14 are intermediate (Figure 6A). Together, the four splicing proteins contribute to accumulation of Pol IV-dependent siRNAs. Interestingly, we found that both miRNA171 and ta-siRNA255 are reduced in mac3a3b, mos4, mos12, and mos14, whereas they are not affected in nrpd1 and nrpe1 as expected (Figure 6A). These splicing proteins are likely to be involved in biogenesis of miRNAs and ta-siRNAs. It will be interesting to determine how miRNAs and ta-siRNAs are affected in these splicing mutants in future.
Figure 6.
The splicing mutants mac3a3b, mos4, mos12, and mos14 affect siRNA accumulation, DNA methylation, and transcriptional gene silencing. (A) Effects of the indicated splicing mutants on accumulation of 24-nt siRNAs, 21-nt microRNA171, ta-siRNA255, and Met-tRNA were determined by small RNA northern blotting. Met-tRNA signals are shown as a loading control. The quantitative results of small RNAs are indicated. (B) Effects of the splicing mutants on DNA methylation of AtSN1 and the newly identified RdDM target AT5G35540 were detected by bisulphite sequencing. (C) Effects of the splicing mutants on transcriptional silencing of solo LTR and AtGP1 were determined by real-time RT-PCR.
Source data for this figure is available on the online supplementary information page.
Bisulphite sequencing indicated that in the four splicing mutants (mac3a3b, mos4, mos12, and mos14), the DNA methylation levels at the CHH sites of AtSN1 are weakly reduced relative to those in wild type, and the reduction is less than that in nrpd1 (Figure 6B). Moreover, the reduction of DNA methylation in these mutants was also confirmed at the newly identified RdDM target AT5G35540 (Figure 6B). The results suggest that not only ZOP1 but also other splicing proteins including MAC3A, MAC3B, MOS4, MOS12, and MOS14 are involved in regulation of DNA methylation.
Furthermore, quantitative RT–PCR showed that mac3a3b, mos4, and mos14 partially induce the transcript levels of both solo LTR and AtGP1, whereas mos12 induces the transcript level of solo LTR but has no effect on AtGP1 (Figure 6C). Generally, all four of the tested splicing mutants partially release the silencing of RdDM targets, although the effect is less than that in the RdDM mutants nrpd1 and nrpe1 (Figure 6C). The weak effect of these splicing mutants on the silencing of RdDM targets is comparable to that of zop1. Together, these results suggest that the fully functional splicing machinery is probably involved in Pol IV-dependent siRNA accumulation, DNA methylation, and transcriptional silencing in Arabidopsis.
We carried out quantitative RT–PCR to test whether the transcript levels of major RdDM component-encoding genes are reduced in the splicing mutants mac3a3b, mos4, mos12, and mos14. The results indicated that DCL3 is weakly reduced in all the four splicing mutants, whereas NRPE1 and RDM1 are weakly reduced in mos12 and mos14 (Supplementary Figure S18). The other RdDM component-encoding genes are generally not reduced in the four splicing mutants (Supplementary Figure S18). Further studies are required to determine whether the effect of these splicing mutants on RdDM is completely due to the indirect effect of the mutants on the transcript levels of RdDM component-encoding genes.
Discussion
We have identified a novel plant-specific ZnF and OCRE domain-containing protein, ZOP1. The protein contains an OCRE domain that is usually related to RNA processing (Callebaut and Mornon, 2005). By affinity purification of ZOP1, we copurified many splicing-related proteins (Supplementary Figure S10; Supplementary Table S9), suggesting a possible role of ZOP1 in pre-mRNA splicing. RNA deep sequencing results indicated that mutation of ZOP1 affects the pre-mRNA splicing of hundreds of protein-coding genes (Supplementary Table S6). Coimmunoprecipitation assay demonstrated that ZOP1 can associate with the PRP6-like protein STA1 (Figure 4A). PRP6 is an snRNP-associated protein that facilitates the assembly of U4/U6-U5 tri-snRNPs (Makarov et al, 2000). The association between ZOP1 and STA1 suggests that ZOP1 may act during the assembly of U4/U6-U5 tri-snRNPs. The core snRNPs are preliminarily assembled in the cytoplasm and then transported to the nuclear Cajal body, in which many splicing-related proteins are assembled on the core snRNPs (Cioce and Lamond, 2005; Morris, 2008). Our immunolocalization assay revealed that the ZOP1 signals overlap with the Cajal body (Figure 4C), which is consistent with the view that ZOP1 functions in snRNP assembly. In addition, ZOP1 signals also appear in the nucleoplasm, suggesting that ZOP1 may continue to associate with snRNPs after snRNPs move out of the Cajal body and may form mature spliceosome complexes on pre-mRNA targets. The detailed function of ZOP1 in pre-mRNA splicing remains to be studied.
Ausin et al (2012) recently reported that the previously characterized splicing factor SR45 affects the RdDM pathway in Arabidopsis, but they did not determine whether SR45 affects RdDM directly or indirectly through the splicing of RdDM factor genes. In our study, we demonstrated that the novel pre-mRNA splicing factor ZOP1 is involved in both pre-mRNA splicing and RdDM in Arabidopsis. To test whether ZOP1 is involved in regulation of RdDM factor genes, we compared gene expression in ros1 and ros1zop1 by RNA deep sequencing. The result indicated that no RdDM factor-encoding gene is markedly reduced by zop1 (Supplementary Table S8). Our quantitative RT–PCR results confirmed that the transcript levels of all major RdDM factor-encoding genes in ros1zop1 are not reduced compared to those in ros1 (Supplementary Figure S9). Furthermore, the zop1 mutation can even repress the transcript levels of a subset of RdDM target loci in the presence of the RdDM mutations, nrpd1, nrpe1, and ago4 (Figure 5B). These results suggest that ZOP1 is directly involved in RdDM regulation and is different from canonical RdDM regulators. In addition to ZOP1, five other tested splicing proteins MAC3A, MAC3B, MOS4, MOS12, and MOS14 are also involved in RdDM and transcriptional silencing (Figure 6). It is possible that the splicing machinery rather than specific splicing factors is involved in promoting RdDM and transcriptional silencing.
The two RNA-binding proteins FPA and FCA are involved in RNA 3′ processing and FLC chromatin silencing (Hornyik et al, 2010; Liu et al, 2010). FPA and FCA can also promote de novo DNA methylation and chromatin silencing at some specific RdDM target loci (Baurle et al, 2007). Depression of the RdDM target AtSN1 in the fpa mutant is caused by defective RNA 3′ end formation at an upstream RNA polymerase II-dependent gene (Hornyik et al, 2010), suggesting that FPA-mediated RNA 3′ processing contributes to the silencing of AtSN1. Our study indicates that RNA splicing factors promote DNA methylation and chromatin silencing at some RdDM target loci. It is possible that many RNA processing proteins are shared by both pre-mRNAs and non-coding RNAs. Although the detailed role of non-coding RNA processing in chromatin silencing remains to be elucidated, our results have demonstrated that siRNAs and DNA methylation are all affected in the splicing mutants, suggesting that the proper status of non-coding RNAs may play an important role during the silencing of RdDM target loci.
Splicing factors were previously reported to be involved in RNA-induced chromatin silencing in fission yeast (Bayne et al, 2008; Bernard et al, 2010; Chinen et al, 2010). The role of the splicing machinery in the Arabidopsis RdDM pathway resembles that of the splicing factors in RITS of fission yeast (Bayne et al, 2008), but the mechanism remains elusive. Recent work revealed that RNA processing activities that utilize both non-coding and coding RNAs are involved in histone H3K9 methylation and chromatin silencing in fission yeast (Reyes-Turcu et al, 2011; Zofall et al, 2012). Given the contribution of the splicing machinery to RdDM and chromatin silencing in Arabidopsis, the involvement of RNA processing in chromatin silencing is likely to be an evolutionarily conserved mechanism from fungi to plants. We propose that the splicing machinery, in addition to contributing to pre-mRNA splicing on protein-coding genes, may function in non-coding RNA processing on RdDM target loci, by which it facilitates recruitment of RdDM effecter to chromatin and contributes to de novo DNA methylation and chromatin silencing at non-coding regions of the genome.
ZOP1 is a pre-mRNA splicing factor that can associate with several known splicing proteins (Supplementary Table S9; Figure 4A). Although no association between ZOP1 and canonical RdDM components was found (Figure 4B; Supplementary Figure S14), the association between ZOP1 and Pol II was demonstrated by coimmunoprecipitation (Figure 4B). Previous reports suggested that Pol II is required for recruitment of AGO4 to RdDM target loci on chromatin (Zheng et al, 2009). The coupling of Pol II and the splicing machinery by ZOP1 may facilitate recruitment of AGO4 to RdDM target loci on chromatin. The Cajal body is required for assembling AGO4 effector and snRNPs (Li et al, 2006; Pikaard, 2006), and ZOP1 may be involved in the assembly of snRNPs and AGO4 effector in the Cajal body. Our EMSA results indicated that ZOP1 is capable of binding to double-stranded RNAs (Supplementary Figure S12). Double-stranded RNAs are present in the second structures of snRNAs and the base pairing between snRNPs and pre-mRNAs (Matlin and Moore, 2007). The siRNAs bound by AGO4 can form double-stranded RNAs with Pol V-dependent scaffold non-coding RNAs (Wierzbicki et al, 2008). Thus, the double-stranded RNA-binding ability of ZOP1 may be helpful in both pre-mRNA splicing and RdDM. The double-stranded DNA binding ability of ZOP1 described in this study suggests that ZOP1 may directly associate with chromatin in vivo. ZOP1 partially colocalizes with NRPE1 and DRM2 at nucleoplasmic speckles, indicating that ZOP1 is likely to act on the chromatin regions that are occupied by NRPE1 and DRM2. However, further studies are required to clarify the details of ZOP1 function in vivo.
Co-transcriptional splicing and Pol II transcription elongation are coordinated by the cooperation of the splicing machinery and Pol II (Shukla et al, 2011). A recent finding indicated that inclusion of introns in transgenes can increase the transcript levels of the transgenes in Arabidopsis (Christie et al, 2011), which is consistent with the possible role of the splicing machinery in the regulation of RNA transcript levels. The transcript level of the RdDM target AtGP1 was partially derepressed by the zop1 mutation in ros1zop1 (Supplementary Figure S8A and B). When the zop1 mutation was combined with RdDM mutations in ros1zop1nrpd1, ros1zop1nrpe1, and ros1zop1ago4; however, the transcript level of AtGP1 was repressed by the zop1 mutation (Figure 5B). The results suggest that ZOP1 may have two inverse functions at RdDM targets. One is to contribute to the silencing of RdDM targets, and the other is to promote RNA transcript levels at RdDM targets in association with Pol II (and probably Pol III). The two inverse functions of ZOP1 may partially explain the weak effect of zop1 on silencing of RdDM targets in ros1zop1 compared to that in ros1nrpd1, ros1nrpe1, and ros1ago4 (Supplementary Figure S8A and B).
In conclusion, this study has found a novel function of the splicing machinery in de novo DNA methylation. Further studies are needed to identify additional splicing-related proteins that are required for DNA methylation and to elucidate how these proteins are involved in DNA methylation and transcriptional silencing.
Materials and methods
Plant materials, mutant screening, and cloning
Both wild-type C24 and ros1 mutant plants carry a homozygous RD29A promoter-driven luciferase transgene and a 35S promoter-driven NPTII transgene. The ros1 mutant plants with the two transgenes were mutagenized with ethyl methanesulphonate and screened for suppressors of ros1. Plants that emitted a high luminescence were crossed to the ros1 mutant in the Col-0 background (Salk_045303). The selfed F2 plants were used for map-based cloning of the mutant. Deep sequencing (Illumina) was carried out to detect the mutation in the localized genome interval of the mutant. The ZOP1 genomic sequence was cloned into the modified plant expression vector pCAMBIA1305 and transformed into ros1zop1 for a complementation test.
Analysis of RNA transcripts and small RNA accumulation
Total RNA was extracted from the indicated plant materials using Trizol reagent (Invitrogen). After contaminating DNA was removed by DNase, total RNA was used for semiquantitative RT–PCR and real-time RT–PCR. The oligo-dT or sequence-specific reverse primers were used for reverse transcription. The single-stranded cDNA was amplified by Ex-Taq DNA polymerase (Takara) for semiquantitative RT–PCR and quantitative RT-PCR. For quantitative RT-PCR, the results shown were based on at least three replications. Small RNA was extracted as previously described and separated on a 15% polyacrylamide gel at 200 V for 4 h. The separated small RNA was transferred onto hybond-N+ membranes (Amersham) for small RNA hybridization. The probes of DNA oligonucleotides and PCR products were radiation labelled by [γ-32P]ATP and [α-32P]dCTP, respectively. Small RNA hybridization was carried out in PerfectHyb buffer (Sigma) overnight at 38°C. The DNA oligonucleotides that were used are listed in Supplementary Table S10.
DNA methylation assay
Genomic DNA of indicated samples was extracted by CTAB and purified by phenol:chloroform (1:1). The DNA in the supernatant was precipitated by ethanol. DNA methylation was tested by bisulphite sequencing and chop-PCR. For bisulphite sequencing, 2 μg of genomic DNA was sodium bisulphite-converted and purified using the EpiTect Bisulfite Kit (Qiagen). The purified DNA was amplified and cloned for bisulphite sequencing. For each ecotype, at least 15 samples were sequenced. For chop-PCR, genomic DNA was digested by DNA methylation-sensitive restriction enzymes (HaeIII), followed by amplification of the digested DNA at indicated DNA loci. The DNA oligonucleotides used for DNA methylation assay are listed in Supplementary Table S10.
Electrophoretic mobility shift assay
The full length of ZOP1 and its truncated forms were cloned in-frame to the N-terminal His tag in the pET28a vector, and the constructs were transformed into E. coli strain BL21 (Invitrogen) for expression. The bacterially expressed His fusion proteins were purified by Ni-NTA His Bind Resin (Novagen) and used for EMSA. EMSA was carried out as described previously with minor modifications (He et al, 2009a). The complemented single-stranded oligonucleotides were annealed to generate double-stranded DNA and RNA oligonucleotides. All probes were end labelled by [γ-32P]ATP with T4 polynucleotide kinase. The labelled probes were purified with G-25 columns (GE Healthcare). The binding assay was carried out in buffer containing 25 mM HEPES (pH 7.6), 50 mM KCl, 0.1 mM EDTA (pH 8.0), 12.5 mM MgCl2, 1 mM DTT, 0.5% (w/v) BSA, and 5% (w/v) glycerol. The binding reaction was incubated at 25°C for 30 min. The reaction mixtures were separated on 4% non-denaturing polyacrylamide gels at 200 V for 2 h, and the gels were exposed to X-ray film for analysis.
Immunolocalization
Protoplasts were isolated from young Arabidopsis leaves as described (Yoo et al, 2007), and nuclei were fixed in 4% formaldehyde and applied to slides. Immunostaining was performed as previously described (Onodera et al, 2005). After nuclei were blocked with 3% BSA in PBS, primary antibodies were incubated with the nuclei overnight at 4°C. Primary antibodies were diluted as follows: rabbit or mouse anti-Flag (1:200), rabbit or mouse anti-cMyc (1:200), and mouse anti-U2B (1:50, lifespan). Secondary anti-mouse TRITC (Invitrogen) and anti-rabbit FITC (Invitrogen) were used at 1:200 dilutions. Chromatin was counterstained with DAPI in mounting medium. Images were acquired by SPINNING DISK confocal microscopy, analysed with Volocity software, and processed with Adobe Photoshop (Adobe Systems).
Analysis of RNA deep sequencing results
Total RNA was extracted from 1-month-old seedlings of ros1 and ros1zop1. The mRNA was purified for RNA-seq library construction and whole transcriptome analysis. The Arabidopsis genome sequences and annotated gene models were downloaded from TAIR10 ( www.arabidopsis.org). Tophat v1.3.1 was used to align the raw reads to genome sequences. Cufflinks (v1.1.0 http://cufflinks.cbcb.umd.edu/) was performed to assemble transcripts and calculate transcript abundances. Differences in RNA transcript levels between ros1 and ros1zop1 were identified by using the Cuffdiff. The presence of more than three FPKM in total exons of a gene was the criterion for gene detection. The cutoff of differential expression depended on the P-value and ln (FC) (ln of Fold Change). For intron-retention analysis, CoverageBed was performed to find reads located in intron regions, and Exact Testes in R was used to identify reliable expressed introns. The introns with >80% read coverage and P-values <0.01 were regarded as intron-retention events. This method was also used for the analysis of intron deletion events in ros1zop1.
Data processing and analysis of whole-genome bisulphite sequencing results
Raw Arabidopsis sequence data provided by Illumina were mapped to the modified Tair10 reference genome using Bismark (Krueger and Andrews, 2011). According to our previously sequenced Arabidopsis C24 genome, ∼461 666 SNP sites were identified compared to Tair10 genome. Because ros1, ros1zop1, and ros1nrpd1 are in the C24 background, Tair10 genome was modified according to the identified SNPs. Sequences that mapped to more than one position were removed to retain only reads that mapped uniquely. The methylation level of each cytosine site was represented by the percentage of the number of reads reporting a C relative to the total number of reads reporting a C or T. Only sites with at least five-fold coverage were included in the results. Gene annotations were downloaded from The Arabidopsis Information Resource (TAIR). The DNA methylation of annotated protein-coding genes and TEs was calculated. DNA methylation at CG, CHG, and CHH sites was separately evaluated.
Analysis of small RNA deep sequencing
Small RNA raw data were processed and mapped to the modified Arabidopsis TAIR10 reference genome. Only perfectly matched 24-nt siRNAs were extracted for analysis in ros1, ros1nrpd1, ros1zop1, and ros1nrpe1. The small RNAs in 100-nt-long windows were counted along the chromosomes, and the counts were adjusted according to the total library size for the comparison and graphic presentation. When the number of combined 24-nt small RNA reads in 100-bp windows in WT is significantly higher than that in nrpd1, the siRNAs in the windows are defined as Pol IV-dependent siRNAs.
Affinity purification, mass spectrometry, and coimmunoprecipitation
A 3-g quantity of flowers from ZOP1-Flag transgenic plants and wild-type control plants were used to prepare protein extracts as described previously (Pontes et al, 2006). In brief, the flowers were ground in liquid nitrogen and homogenized in 15 ml of protein extraction buffer (50 mM Tris (pH 7.6), 150 mM NaCl, 5 mM MgCl2, 10% glycerol, 0.1% NP-40, 0.5 mM DTT, 1 mM PMSF, and one protease inhibitor cocktail tablet/50 ml (Roche)). Cell debris was removed by three centrifugations. The supernatant of each sample was incubated with Anti-Flag M1 agarose (Sigma, A4596) at 4°C for 2.5 h and washed five times with the protein extraction buffer. The affinity-purified proteins were eluted from the agarose with 3 × Flag peptides (Sigma). The eluted proteins were run on a 12% SDS–PAGE gel and subjected to silver staining with the ProteoSilver Silver Stain Kit (Sigma, PROT-SIL1).
For mass-spectrometric analysis, proteins on SDS–PAGE gels were de-stained and digested in-gel with sequencing grade trypsin (10 ng/μl trypsin, 50 mM ammonium bicarbonate, pH 8.0) at 37°C overnight. The digested peptides were eluted on a capillary column and sprayed into an LTQ mass spectrometer equipped with a nano-ESI ion source (Thermo Fisher Scientific, USA). Identified peptides were searched in the IPI (International Protein Index) Arabidopsis protein database on the Mascot server (Matrix Science Ltd, UK).
For coimmunoprecipitation, the protein extracts were incubated with protein A agarose beads conjugated with the indicated antibody, washed five times, and boiled in SDS–PAGE sample buffer. The boiled extracts were run on a 12% SDS–PAGE gel for western blotting. For coimmunoprecipitation analysis between ZOP1 and NRPD1, NRPE1, or AGO4, the ZOP1-Flag or ZOP1-Myc transgenic plants were crossed to NRPD1-Flag, NPRE1-Flag, and Myc-AGO4 transgenic plants, respectively. The offspring plants expressing both fusion proteins were subjected to coimmunoprecipitation.
Supplementary Material
Acknowledgments
We thank Yuelin Zhang and Xin Li (Department of Botany, University of British Columbia) for the splicing mutants mac3a3b, mos4, mos12, and mos14, and She Chen (National Institute of Biological Sciences, Beijing, China) for the technique support of mass spectrometry. This work was supported by the National Basic Research Program of China (973 Program) (2012CB910900) and the 973 Program (2011CB812600) from the Chinese Ministry of Science and Technology.
Author contributions: Xin-Jian He designed experiments. Cui-Jun Zhang performed the experiments shown in Figures 1, 2A, 2C, 2D, 3, 4A, 4B, 6A, and 6C, and Supplementary Figures S4, S8–S10, S12–S15, S17, and S19. Jin-Xing Zhou performed the experiments shown in Figures 5 and 6B and Supplementary Figures S1 and S6. Jun Liu identified the ZOP1 gene by map-based cloning. Ze-Yang Ma performed immunofluorescence experiments shown in Figures 4C–F and Supplementary Figures S11 and S16. Su-Wei Zhang and Kun Dou helped with luminescence assay and in vivo coimmunoprecipitation assay. Huan-Wei Huang, Tao Cai, and Renyi Liu performed statistic analysis on small RNA deep sequencing data and genome-wide DNA methylation data. Cui-Jun Zhang, Jian-Kang Zhu, and Xin-Jian He analysed results. Cui-Jun Zhang and Xin-Jian He wrote the manuscript. Xin-Jian He supervised the work.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ausin I, Greenberg MV, Li CF, Jacobsen SE (2012) The splicing factor SR45 affects the RNA-directed DNA methylation pathway in Arabidopsis. Epigenetics 7: 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurle I, Smith L, Baulcombe DC, Dean C (2007) Widespread role for the flowering-time regulators FCA and FPA in RNA-mediated chromatin silencing. Science 318: 109–112 [DOI] [PubMed] [Google Scholar]
- Bayne EH, Portoso M, Kagansky A, Kos-Braun IC, Urano T, Ekwall K, Alves F, Rappsilber J, Allshire RC (2008) Splicing factors facilitate RNAi-directed silencing in fission yeast. Science 322: 602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Drogat J, Dheur S, Genier S, Javerzat JP (2010) Splicing factor Spf30 assists exosome-mediated gene silencing in fission yeast. Mol Cell Biol 30: 1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Verdel A, Moazed D (2006) Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell 125: 873–886 [DOI] [PubMed] [Google Scholar]
- Callebaut I, Mornon JP (2005) OCRE: a novel domain made of imperfect, aromatic-rich octamer repeats. Bioinformatics 21: 699–702 [DOI] [PubMed] [Google Scholar]
- Cao X, Jacobsen SE (2002) Role of the arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12: 1138–1144 [DOI] [PubMed] [Google Scholar]
- Chinen M, Morita M, Fukumura K, Tani T (2010) Involvement of the spliceosomal U4 small nuclear RNA in heterochromatic gene silencing at fission yeast centromeres. J Biol Chem 285: 5630–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M, Croft LJ, Carroll BJ (2011) Intron splicing suppresses RNA silencing in Arabidopsis. Plant J 68: 159–167 [DOI] [PubMed] [Google Scholar]
- Cioce M, Lamond AI (2005) Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol 21: 105–131 [DOI] [PubMed] [Google Scholar]
- Gao Z, Liu HL, Daxinger L, Pontes O, He X, Qian W, Lin H, Xie M, Lorkovic ZJ, Zhang S, Miki D, Zhan X, Pontier D, Lagrange T, Jin H, Matzke AJ, Matzke M, Pikaard CS, Zhu JK (2010) An RNA polymerase II- and AGO4-associated protein acts in RNA-directed DNA methylation. Nature 465: 106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Morales-Ruiz T, Ariza RR, Roldán-Arjona T, David L, Zhu JK (2002) ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111: 803–814 [DOI] [PubMed] [Google Scholar]
- Grewal SI (2010) RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev 20: 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IM, Shankaranarayana GD, Noma K, Ayoub N, Cohen A, Grewal SI (2002) Establishment and maintenance of a heterochromatin domain. Science 297: 2232–2237 [DOI] [PubMed] [Google Scholar]
- He XJ, Hsu YF, Zhu S, Wierzbicki AT, Pontes O, Pikaard CS, Liu HL, Wang CS, Jin H, Zhu JK (2009a) An effector of RNA-directed DNA methylation in arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell 137: 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Hsu YF, Zhu S, Liu HL, Pontes O, Zhu J, Cui X, Wang CS, Zhu JK (2009b) A conserved transcriptional regulator is required for RNA-directed DNA methylation and plant development. Genes Dev 23: 2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Hsu YF, Pontes O, Zhu J, Lu J, Bressan RA, Pikaard C, Wang CS, Zhu JK (2009c) NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation. Genes Dev 23: 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr AJ, Jensen MB, Dalmay T, Baulcombe DC (2005) RNA polymerase IV directs silencing of endogenous DNA. Science 308: 118–120 [DOI] [PubMed] [Google Scholar]
- Hornyik C, Terzi LC, Simpson GG (2010) The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell 18: 203–213 [DOI] [PubMed] [Google Scholar]
- Kaida D, Berg MG, Younis I, Kasim M, Singh LN, Wan L, Dreyfuss G (2010) U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 468: 664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Bucher E, Daxinger L, Huettel B, Böhmdorfer G, Gregor W, Kreil DP, Matzke M, Matzke AJ (2008) A structural-maintenance-of-chromosomes hinge domain-containing protein is required for RNA-directed DNA methylation. Nat Genet 40: 670–675 [DOI] [PubMed] [Google Scholar]
- Kanno T, Bucher E, Daxinger L, Huettel B, Kreil DP, Breinig F, Lind M, Schmitt MJ, Simon SA, Gurazada SG, Meyers BC, Lorkovic ZJ, Matzke AJ, Matzke M (2010) RNA-directed DNA methylation and plant development require an IWR1-type transcription factor. EMBO Rep 11: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Huettel B, Mette MF, Aufsatz W, Jaligot E, Daxinger L, Kreil DP, Matzke M, Matzke AJ (2005) Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat Genet 37: 761–765 [DOI] [PubMed] [Google Scholar]
- Kanno T, Mette MF, Kreil DP, Aufsatz W, Matzke M, Matzke AJ (2004) Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr Biol 14: 801–805 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Zheng B, Yu Y, Won SY, Mo B, Chen X (2011) The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J 30: 814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Andrews SR (2011) Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27: 1571–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JA, Ausin I, Johnson LM, Vashisht AA, Zhu JK, Wohlschlegel JA, Jacobsen SE (2010) A protein complex required for polymerase V transcripts and RNA-directed DNA methylation in Arabidopsis. Curr Biol 20: 951–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11: 204–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JA, Vashisht AA, Wohlschlegel JA, Jacobsen SE (2011) SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV. PLoS Genet 7: e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CF, Henderson IR, Song L, Fedoroff N, Lagrange T, Jacobsen SE (2008) Dynamic regulation of ARGONAUTE4 within multiple nuclear bodies in Arabidopsis thaliana. PLoS Genet 4: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CF, Pontes O, El-Shami M, Henderson IR, Bernatavichute YV, Chan SW, Lagrange T, Pikaard CS, Jacobsen SE (2006) An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 126: 93–106 [DOI] [PubMed] [Google Scholar]
- Liu F, Marquardt S, Lister C, Swiezewski S, Dean C (2010) Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327: 94–97 [DOI] [PubMed] [Google Scholar]
- Liu J, Bai G, Zhang C, Chen W, Zhou J, Zhang S, Chen Q, Deng X, He XJ, Zhu JK (2011) An atypical component of RNA-directed DNA methylation machinery has both DNA methylation-dependent and -independent roles in locus-specific transcriptional gene silencing. Cell Res 21: 1691–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov EM, Makarova OV, Achsel T, Luhrmann R (2000) The human homologue of the yeast splicing factor prp6p contains multiple TPR elements and is stably associated with the U5 snRNP via protein-protein interactions. J Mol Biol 298: 567–575 [DOI] [PubMed] [Google Scholar]
- Matlin AJ, Moore MJ (2007) Spliceosome assembly and composition. Adv Exp Med Biol 623: 14–35 [DOI] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ (2009) RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21: 367–376 [DOI] [PubMed] [Google Scholar]
- Moazed D (2009) Small RNAs in transcriptional gene silencing and genome defence. Nature 457: 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J, Xu F, Gao M, Zhao Q, Palma K, Long C, Chen S, Zhang Y, Li X (2009) Two Prp19-like U-box proteins in the MOS4-associated complex play redundant roles in plant innate immunity. PLoS Pathog 5: e1000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GE (2008) The Cajal body. Biochim Biophys Acta 1783: 2108–2115 [DOI] [PubMed] [Google Scholar]
- Mosher RA, Schwach F, Studholme D, Baulcombe DC (2008) PolIVb influences RNA-directed DNA methylation independently of its role in siRNA biogenesis. Proc Natl Acad Sci USA 105: 3145–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi MR, Verdel A, Colmenares SU, Gerber SA, Gygi SP, Moazed D (2004) Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119: 789–802 [DOI] [PubMed] [Google Scholar]
- Onodera Y, Haag JR, Ream T, Costa Nunes P, Pontes O, Pikaard CS (2005) Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120: 613–622 [DOI] [PubMed] [Google Scholar]
- Pikaard CS (2006) Cell biology of the Arabidopsis nuclear siRNA pathway for RNA-directed chromatin modification. Cold Spring Harb Symp Quant Biol 71: 473–480 [DOI] [PubMed] [Google Scholar]
- Pontes O, Li CF, Costa Nunes P, Haag J, Ream T, Vitins A, Jacobsen SE, Pikaard CS (2006) The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell 126: 79–92 [DOI] [PubMed] [Google Scholar]
- Pontier D, Yahubyan G, Vega D, Bulski A, Saez-Vasquez J, Hakimi MA, Lerbs-Mache S, Colot V, Lagrange T (2005) Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev 19: 2030–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, He X, Wang XJ, Kohany O, Jurka J, Hannon GJ (2006) Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature 443: 1008–1012 [DOI] [PubMed] [Google Scholar]
- Ream TS, Haag JR, Wierzbicki AT, Nicora CD, Norbeck AD, Zhu JK, Hagen G, Guilfoyle TJ, Pasa-Tolić L, Pikaard CS (2009) Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol Cell 33: 192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Turcu FE, Zhang K, Zofall M, Chen E, Grewal SI (2011) Defects in RNA quality control factors reveal RNAi-independent nucleation of heterochromatin. Nat Struct Mol Biol 18: 1132–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PA (1994) Split genes and RNA splicing. Cell 77: 805–815 [DOI] [PubMed] [Google Scholar]
- Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S (2011) CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479: 74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin RK, Martienssen R (2007) Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 8: 272–285 [DOI] [PubMed] [Google Scholar]
- Smith LM, Pontes O, Searle I, Yelina N, Yousafzai FK, Herr AJ, Pikaard CS, Baulcombe DC (2007) An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell 19: 1507–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Kannan R, Blanchette M, Baumann P (2012) Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes. Nature 484: 260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D (2004) RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303: 672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA (2002) Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297: 1833–1837 [DOI] [PubMed] [Google Scholar]
- Wierzbicki AT, Haag JR, Pikaard CS (2008) Non-coding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135: 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AT, Ream TS, Haag JR, Pikaard CS (2009) RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nat Genet 41: 630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AT, Cocklin R, Mayampurath A, Lister R, Rowley MJ, Gregory BD, Ecker JR, Tang H, Pikaard CS (2012) Spatial and functional relationships among Pol V-associated loci, Pol IV-dependent siRNAs, and cytosine methylation in the Arabidopsis epigenome. Genes Dev 26: 1825–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2: e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Xu S, Wiermer M, Zhang Y, Li X (2012) The cyclin L homolog MOS12 and the MOS4-associated complex are required for the proper splicing of plant resistance genes. Plant J 70: 916–928 [DOI] [PubMed] [Google Scholar]
- Xu S, Zhang Z, Jing B, Gannon P, Ding J, Xu F, Li X, Zhang Y (2011) Transportin-SR is required for proper splicing of resistance genes and plant immunity. PLoS Genet 7: e1002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zheng B, Wang Z, Li S, Yu B, Liu JY, Chen X (2009) Intergenic transcription by RNA polymerase II coordinates Pol IV and Pol V in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes Dev 23: 2850–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Licklider LJ, Gygi SP, Reed R (2002) Comprehensive proteomic analysis of the human spliceosome. Nature 419: 182–185 [DOI] [PubMed] [Google Scholar]
- Zofall M, Yamanaka S, Reyes-Turcu FE, Zhang K, Rubin C, Grewal SI (2012) RNA elimination machinery targeting meiotic mRNAs promotes facultative heterochromatin formation. Science 335: 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.