Abstract
The cellular inhibitor of apoptosis (c-IAP) proteins are E3 ubiquitin ligases that are critical regulators of tumour necrosis factor (TNF) receptor (TNFR)-mediated signalling. Through their E3 ligase activity c-IAP proteins promote ubiquitination of receptor-interaction protein 1 (RIP1), NF-κB-inducing kinase (NIK) and themselves, and regulate the assembly of TNFR signalling complexes. Consequently, in the absence of c-IAP proteins, TNFR-mediated activation of NF-κB and MAPK pathways and the induction of gene expression are severely reduced. Here, we describe the identification of OTUB1 as a c-IAP-associated deubiquitinating enzyme that regulates c-IAP1 stability. OTUB1 disassembles K48-linked polyubiquitin chains from c-IAP1 in vitro and in vivo within the TWEAK receptor-signalling complex. Downregulation of OTUB1 promotes TWEAK- and IAP antagonist-stimulated caspase activation and cell death, and enhances c-IAP1 degradation. Furthermore, knockdown of OTUB1 reduces TWEAK-induced activation of canonical NF-κB and MAPK signalling pathways and modulates TWEAK-induced gene expression. Finally, suppression of OTUB1 expression in zebrafish destabilizes c-IAP (Birc2) protein levels and disrupts fish vasculature. These results suggest that OTUB1 regulates NF-κB and MAPK signalling pathways and TNF-dependent cell death by modulating c-IAP1 stability.
Keywords: c-IAP1, DUB, OTUB1, ubiquitin, TWEAK
Introduction
The controlled regulation of protein stability by the ubiquitin-proteasome system is paramount for maintenance and modification of numerous cellular processes (Hershko and Ciechanover, 1998). Ubiquitination involves covalent modification of target proteins with ubiquitin, a 76-amino acid protein whose C-terminus can be linked to a lysine residue on a substrate (Hershko and Ciechanover, 1998). This process requires an E1 ubiquitin-activating enzyme, an E2 ubiquitin-conjugating enzyme, and an E3 ubiquitin ligase (Schulman and Harper, 2009). Covalent attachment of a single ubiquitin molecule to the substrate is referred to as monoubiquitination (Pickart and Fushman, 2004). However, the presence of seven lysines and a free N-terminus within ubiquitin allows for a variety of ubiquitin-ubiquitin linkages and the formation of polyubiquitin chains (Pickart and Fushman, 2004). The diverse topology of different polyubiquitin chains presents an opportunity to convey complex biological information that is instrumental for many cellular functions (Ikeda and Dikic, 2008). Hence, K63-linked chains, N-terminally linked linear chains, and in some cases K11-linked chains provide scaffolding for the recruitment and assembly of signalling complexes (Vucic et al, 2011). In contrast, K48-linked chains predominantly target substrates for proteasomal degradation (Hershko and Ciechanover, 1998). The remarkable combinatorial complexity of ubiquitination stems from tens of different E2 enzymes, which mostly dictate the selectivity of ubiquitin chain assembly, and hundreds of E3 ligases, which provide substrate specificity (Deshaies and Joazeiro, 2009). The diversity of ubiquitin modifications is complemented by numerous ubiquitin-binding domains that recognize ubiquitin chains with varying levels of specificity to allow transmission of intended biological information (Iwai and Tokunaga, 2009; Husnjak and Dikic, 2012). Ultimately, ubiquitination is a reversible process as deubiquitinases can cleave polyubiquitin chains to eliminate this post-translational modification and thereby alter its substrate protein’s stability and/or functional role (Reyes-Turcu et al, 2009).
Cellular inhibitor of apoptosis (c-IAP) proteins are really interesting new gene (RING) domain-containing E3 ligases that promote the assembly of polyubiquitin chains on themselves and on several signalling molecules including receptor-interaction protein 1 (RIP1) and NF-κB-inducing kinase (NIK) (Vucic et al, 2011). c-IAP1 and c-IAP2 are recruited to tumour necrosis factor receptor (TNFR) signalling complexes through their constitutive association with TNFR-associated factor 2 (TRAF2) (Rothe et al, 1995). Within the TNFR1 complex, c-IAPs undergo autoubiquitination and mediate ubiquitination of RIP1 predominantly with K63 and K11 ubiquitin linkages (Bertrand et al, 2008; Varfolomeev et al, 2008; Haas et al, 2009; Dynek et al, 2010). This allows the assembly of the inhibitor of kappa B kinase (IKK) and other signalling complexes such as the linear ubiquitin chain assembly complex (LUBAC) and subsequent activation of NF-κB and MAPK signalling pathways (Emmerich et al, 2011; Vucic et al, 2011). With the exception of TNFR1 and DR3, most of the other TNFR family members also activate the non-canonical NF-κB signalling pathway. The non-canonical NF-κB pathway is continuously inhibited by proteasomal degradation of NIK induced by a complex of E3 ligases c-IAP1 and 2 and adaptor proteins TRAF2 and TRAF3 (Varfolomeev et al, 2007; Vallabhapurapu et al, 2008; Zarnegar et al, 2008). Binding of TNF family ligands TWEAK or LIGHT to their respective receptors triggers recruitment and aggregation of TRAF2, TRAF3, and c-IAP proteins at the membrane-associated receptor complexes, which stimulates c-IAP E3 ligase activity and results in ubiquitination and degradation of c-IAP proteins as well as TRAF2 and TRAF3 (Varfolomeev et al, 2012).
TWEAK-stimulated degradation of c-IAP proteins activates non-canonical NF-κB signalling, but it also eventually limits TWEAK-induced activation of the canonical NF-κB and MAPK pathways and sensitizes to cell death (Vince et al, 2008; Varfolomeev et al, 2012). Activation of NF-κB and MAPK signalling pathways by TWEAK induces the expression of TNF, which can bind TNFR1 to activate its signalling (Silke and Brink, 2010). However, in the absence of c-IAP proteins, RIP1 and c-IAP ubiquitination cannot occur (Moulin et al, 2012; Varfolomeev et al, 2012). Instead, TNFR1 will cause caspase-8 activation leading to cell death (Bertrand et al, 2008). In a similar fashion, IAP antagonists, which mimic second mitochondrial activator of caspases (SMAC), bind c-IAP proteins and stimulate their E3 ligase activity, resulting in the activation of NF-κB signalling and TNF expression (Varfolomeev et al, 2007; Vince et al, 2007). Autoubiquitination of c-IAPs leads to their ultimate destruction by the proteasome, thus paving the way for TNF-mediated cell death. This principle of regulated c-IAP degradation is the foundation of IAP antagonist-induced death of cancer cells and tumour growth inhibition, and it is currently being tested as a novel anticancer therapeutic approach in clinical trials (Fulda and Vucic, 2012).
Gaining a more detailed understanding of processes and relevant cellular factors that modulate c-IAP protein stability should provide additional tools for deciphering complex signalling events. Here, we describe identification of the deubiquitinating enzyme otubain 1 (OTUB1) as a novel regulator of c-IAP1 stability. OTUB1 associates with c-IAP1 and removes K48-linked polyubiquitin from c-IAP1 in vitro and in vivo in the context of TWEAK signalling. Knockdown of OTUB1 enhances c-IAP1 degradation, and TWEAK- and IAP antagonist-stimulated caspase activation and cell death. In addition, downregulation of OTUB1 reduces TWEAK-induced activation of canonical NF-κB and MAPK signalling pathways and modulates TWEAK-induced gene expression. In zebrafish, inhibition of OTUB1 expression emulates suppression of c-IAP (BIRC2) expression and disrupts fish vasculature. Collectively, this study suggests that OTUB1 regulates survival and signalling pathways by modulating c-IAP1 stability.
Results
Identification of potential regulators of c-IAP stability
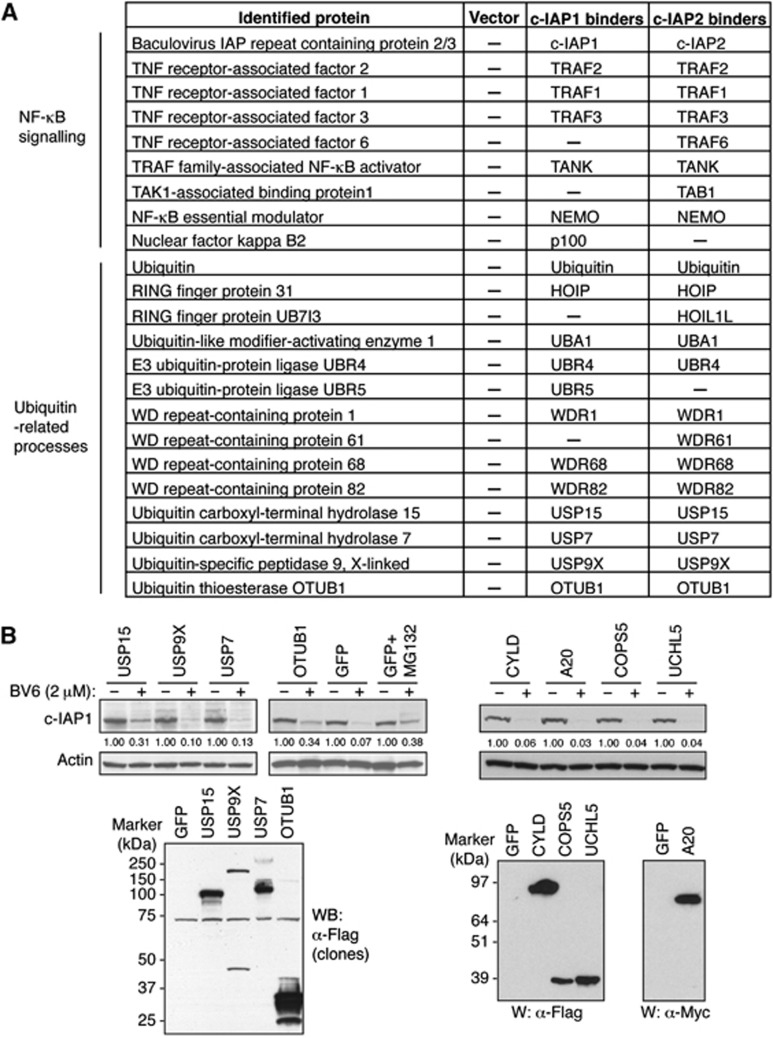
Several members of the TNF family including TWEAK and LIGHT, as well as IAP antagonists, stimulate autoubiquitination and subsequent proteasomal degradation of c-IAP proteins (Vucic et al, 2011; Varfolomeev et al, 2012). To search for proteins that might bind to c-IAP proteins and affect their stability, we have generated stably transfected KMS18 cell lines that express Flag-tagged c-IAP1, c-IAP2 or empty Flag vector plasmid. KMS18 cells do not express endogenous c-IAP proteins due to genetic deletion of the c-IAP locus (Annunziata et al, 2007; Keats et al, 2007). Pull-down with Flag-affinity resin followed by mass spectrometry analysis revealed the presence of several known c-IAP1 and c-IAP2 interacting partners and signalling proteins (Vucic et al, 2011; Varfolomeev et al, 2012) including TRAF2, TRAF3, TRAF1, and NEMO, as well as members of the LUBAC, HOIP, and HOIL-1L (Figure 1A). In addition to these regulators of NF-κB and MAPK signalling pathways, we have also identified a number of ubiquitin-related proteins including E3 ligases UBR4 and UBR5, and several deubiquitinating enzymes (DUBs): USP15, USP7, USP9X, and OTUB1 (Figure 1A; Supplementary Figure 1). Deubiquitinating enzymes were of special interest as they can efficiently regulate stability of the target proteins by removing ubiquitin moieties from their substrates (Reyes-Turcu et al, 2009).
Figure 1.
Identification of c-IAP-associated regulators of protein stability. (A) Proteins captured in pull-downs from KMS18 cells stably transfected with Flag-tagged c-IAP1 or c-IAP2 were identified by mass spectrometry and are grouped according to their reported roles in cellular processes. (B) DUB screen. A collection of DUB constructs including the DUBs identified in (A) was transfected into 293T cells. Forty-eight hours later cells were treated with BV6 (2 μM) for 10 min, and lysates were analysed by western blotting with c-IAP1, Actin, Flag (DUBs), and Myc (A20) antibodies. The intensities of c-IAP1 bands were quantified using densitometry and indicated under western blots.
To further explore the potential relevance of DUBs for c-IAP1 stability, we performed a DUB screen by ectopically expressing a library of DUB cDNAs and treating transfected cells with the IAP antagonist BV6 (Varfolomeev et al, 2007; Supplementary Figure S2; Supplementary Table S1). As a readout we evaluated the levels of endogenous c-IAP1 protein in treated cells (Figure 1B). Most of the examined DUBs did not affect c-IAP1 protein levels but expression of OTUB1 and USP15 stabilized c-IAP1 protein to a similar degree as the treatment with protease inhibitor MG132 (Figure 1B; Supplementary Figure S2). Several DUBs that have been implicated in NF-κB signalling pathways, such as CYLD or A20, and USP7 and USP9X that were identified in our pull-downs, did not stabilize c-IAP1 following IAP antagonist treatment and were not further examined (Figure 1B).
OTUB1 regulates c-IAP1 protein stability and TNF-dependent cell death
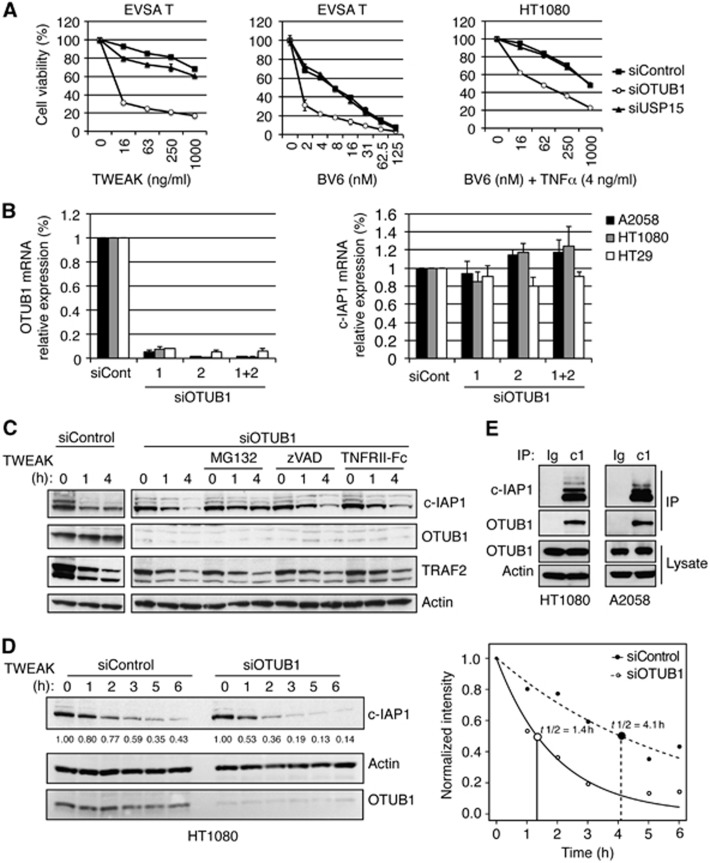
Given that c-IAP1 is an important regulator of cell death we investigated the roles of DUB candidates OTUB1 and USP15 in extrinsic and intrinsic apoptotic pathways. Knockdown of OTUB1 potentiated cell death induced by TWEAK or BV6, while downregulation of USP15 had no effect (Figure 2A). On the other hand, knockdown of OTUB1 did not affect etoposide-stimulated cell death (Supplementary Figure S3). These data suggest that OTUB1 might regulate TNF-dependent cell death pathways in which c-IAP1 plays a critical role, such as TWEAK- and IAP antagonist-induced apoptosis. Based on these results, we focused on OTUB1 as a potential deubiquitinase for c-IAP1.
Figure 2.
OTUB1 modulates c-IAP1 stability and cellular viability. (A) EVSA T and HT1080 cells were transfected with control, OTUB1-specific, or USP15-specific siRNA oligos and 48 h later treated with TWEAK or BV6 (EVSA T), or BV6 and TNFα (HT1080). Cell death was assessed using CellTiter-Glo luminescent cell viability assay 24 h following the start of treatment. (B) Indicated cell lines were transfected with control or OTUB1-specific siRNA oligos and 48 h later mRNA levels of OTUB1 and c-IAP1 were determined by quantitative RT–PCR. All values were normalized to an RPL19 RNA internal control. Columns represent mean from triplicate experiments and bars represent standard deviation. (C) HT1080 cells were transfected with control or OTUB1-specific siRNA oligos and 48 h later with TWEAK (100 ng/ml) for 1 or 4 h in the presence of MG132 (20 μM), zVAD (20 μM), or TNFRII-Fc (20 μg/ml). Protein levels were determined by western blotting with c-IAP1, OTUB1, TRAF2, or Actin-specific antibodies. (D) HT1080 cells were transfected with control or OTUB1-specific siRNA oligos and 32 h later pre-incubated with transcription inhibitor Actinomycin D (1 μg/ml) for 16 h and then treated with TWEAK (100 ng/ml) for 1–6 h. The effect of OTUB1 knockdown on c-IAP1 half-life was examined by western blotting (left panel) and subsequent densitometry of band intensities was indicated under c-IAP1 western blot and plotted as a function of time using RGraph (t1/2 differences are significant with Z=5.815 and P<0.0001) (right panel). (E) Endogenous association of c-IAP1 and OTUB1. A2058 and HT1080 cells were treated with MG132 (20 μM) for 1 h, lysed and immunoprecipitated with c-IAP1-specific (c1) or isotype control (Ig) antibodies. Cellular lysates and immunoprecipitates were examined by western blotting using antibodies specific for c-IAP1, OTUB1, or Actin.
Previous reports suggested that OTUB1 might regulate the stability of several signalling molecules including TRAF3 and TRAF6 (Li et al, 2010). The importance of OTUB1 for the stability of TRAFs and other signalling proteins was evaluated by downregulation of OTUB1 expression with two different siRNA oligos separately and together. Knockdown of OTUB1 did not affect protein levels of IκBα, p100, TRAF2, TRAF3, TRAF6, or TAK1 (Supplementary Figure 4A). Steady-state levels of c-IAP1 and XIAP proteins were also unaffected by OTUB1 knockdown (Supplementary Figure 4A). Importantly, downregulation of OTUB1 did not affect the mRNA levels of c-IAP1 (Figure 2B). Nevertheless, knockdown of OTUB1 potentiated BV6-stimulated degradation of c-IAP1, in agreement with the results from the DUB screen (Supplementary Figure S4B). Next, we evaluated the stability of c-IAP1 following TWEAK treatment when OTUB1 was downregulated and observed that knockdown of OTUB1 enhanced c-IAP1 degradation (Figure 2C; Supplementary Figure S4C). Treatment with MG132 stabilized c-IAP1 protein levels, while the pan-caspase inhibitor zVAD or TNF blockade had no effect, suggesting that knockdown of OTUB1 increased proteasomal degradation of c-IAP1 (Figure 2C). Evaluation of c-IAP1 half-life following TWEAK treatment revealed that knockdown of OTUB1 significantly potentiated c-IAP1 degradation (Figure 2D). Similarly, c-IAP1 degradation induced by treatment with the related TNF-family ligand LIGHT was potentiated in the absence of OTUB1 (Supplementary Figure S4D). In accordance with its ability to modulate c-IAP1 stability, endogenous binding of OTUB1 to c-IAP1 was detected in examined cell lines (Figure 2E). Knockdown of OTUB1 also destabilized related c-IAP2 protein following TWEAK treatment, although not as pronounced as c-IAP1 (Supplementary Figure 4E).
Knockdown of OTUB1 enhanced cell death induced by TWEAK or BV6 in several cell lines (Figure 3A; Supplementary Figure S5A and B). Conversely, overexpression of OTUB1 diminished TWEAK-mediated c-IAP1 degradation and cell death induction (Supplementary Figure S5C). Thus, the apoptosis-regulatory activity of OTUB1 is limited to stimuli that destabilize c-IAP1. On the other hand, downregulation of OTUB1 did not affect cell death induction by TNFα, FasL, or agonistic anti-DR5 antibody (Supplementary Figure 5D and E). To investigate the mechanistic aspects of this increase in cell death, we co-treated cells with the TNF-blocking reagent TNFRII-Fc or with the pan-caspase inhibitor zVAD. Both reagents provided significant protection from OTUB1-potentiated cell death (Figure 3B). Examination of cellular lysates after TWEAK treatment revealed enhanced caspase-8 and caspase-3 processing following OTUB1 knockdown, further supporting the role of caspases in OTUB1-stimulated cell death (Figure 3C). Thus, OTUB1 knockdown destabilizes c-IAP1 and promotes caspase- and TNF-dependent cell death.
Figure 3.
Knockdown of OTUB1 promotes TNF-dependent apoptosis. (A) Indicated cell lines were transfected with control or OTUB1-specific siRNA oligos and 48 h later treated with TWEAK. Cell death was assessed using CellTiter-Glo luminescent cell viability assay 24 h following the start of treatment. (B) A2058 cells were transfected with control or OTUB1-specific siRNA oligos and 48 h later treated with TWEAK (100 ng/ml) in combination with TNFRII-Fc, zVAD, or DMSO. Cell death was assessed as in (A). (C) A2058 cells were transfected with control or OTUB1-specific siRNA oligos and 48 h later treated with TWEAK (100 ng/ml) for indicated time periods. Cellular lysates were examined by western blotting with antibodies against OTUB1, caspase-8, caspase-3, and Actin.
OTUB1 removes K48-linked polyubiquitin chains from c-IAP1
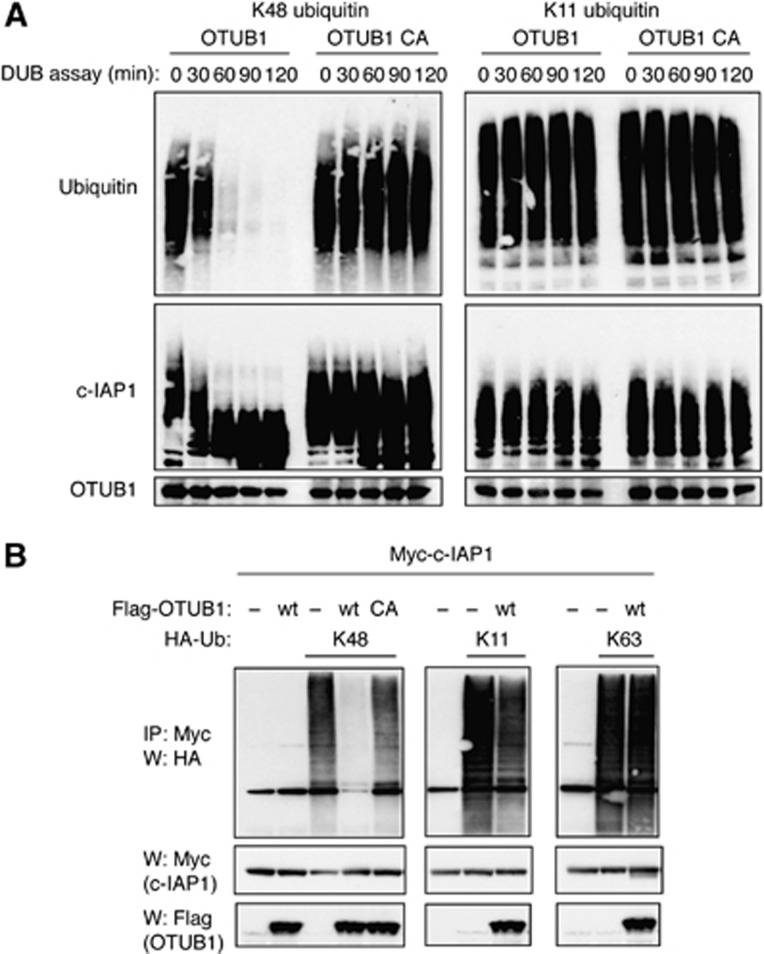
Structural and biochemical studies have shown that OTUB1 has a strong preference for cleaving K48-linked over K63-linked polyubiquitin chains (Edelmann et al, 2009). We extended those observations by testing the ability of OTUB1 to cleave K11-linked and linear chains in addition to K48- and K63-linked polyubiquitin chains in reconstituted deubiquitination reactions. Our results unambiguously show that wild-type but not catalytically dead Cys91Ala (C91A) mutant OTUB1 exclusively cleaves K48-linked polyubiquitin chains (Supplementary Figure S6). Importantly, OTUB1 shows the same selectivity in removing K48-linked polyubiquitin chains from in vitro autoubiquitinated c-IAP1 (Figure 4A). At the same time, OTUB1 did not disassemble K11-linked polyubiquitin chains from c-IAP1 and the C91A mutant of OTUB1 did not cleave any of the examined polyubiquitin chains (Figure 4A). Next, we investigated OTUB1-mediated DUB activity on autoubiquitinated c-IAP1 in cells. Ectopic expression of c-IAP1 with tagged ubiquitin constructs that can assemble only K11-, K48-, or K63-linked polyubiquitin chains yielded autoubiquitination of c-IAP1 with selective linkages (Figure 4B). Co-expression of OTUB1 resulted in a stark reduction in K48-linked c-IAP1 autoubiquitination while sparing other polyubiquitin chain linkages (Figure 4B). Thus, OTUB1 can deubiquitinate c-IAP1 in vitro and in vivo, with a clear selectivity for K48-linked chains.
Figure 4.
OTUB1 disassembles K48-linked polyubiquitin chains from c-IAP1. (A) OTUB1 removes K48-linked chains from c-IAP1 in vitro. Recombinant c-IAP1 was incubated in reconstituted ubiquitination reaction for 20 min at 30°C with K48-only or K11-only ubiquitin followed by the addition of apyrase, for 1 h to stop the ubiquitination reaction. Recombinant OTUB1 wild-type or C91A catalytic mutant was then added and deubiquitination reactions were carried out for indicated time periods and subjected to western blotting with ubiquitin, c-IAP1, or OTUB1-specific antibodies. (B) 293T cells were transiently transfected with Myc-c-IAP1, Flag-OTUB1 (wild-type or C91A mutant), K48-, K11-, or K63-only ubiquitin constructs and vector plasmid for 40 h. Cells were lysed and lysates were immunoprecipitated with anti-Myc affinity resin. Inputs from cellular lysates and immunoprecipitates were examined by western blotting with Myc, Flag, and HA antibodies.
In addition to acting as a canonical deubiquitinating enzyme that cleaves polyubiquitin chains, OTUB1 can also inhibit ubiquitination in a non-canonical fashion by binding E2 enzymes and preventing ubiquitin chain synthesis (Nakada et al, 2010; Juang et al, 2012; Sato et al, 2012; Wiener et al, 2012). To examine possible non-canonical activity of OTUB1 in c-IAP1 deubiquitination, we pre-incubated OTUB1 with E2 enzyme UbcH5c in the presence of E1 enzyme and ubiquitin reaction components and subsequently added c-IAP1 to the reconstituted ubiquitination reaction. The presence of OTUB1 did not affect c-IAP1 autoubiqutination (Supplementary Figure S7A). Similarly, OTUB1 did not affect c-IAP1-mediated assembly of K48-linked or K11-linked polyubiquitin chains (Supplementary Figure S7B). Lastly, we tested if OTUB1 could disrupt c-IAP1 E3 ligase activity in vivo. Knockdown of OTUB1 did not diminish TNF-stimulated RIP1 ubiquitination, and expression of OTUB1 did not prevent c-IAP1-mediated NIK degradation, suggesting that OTUB1 does not affect E3 ligase activity of c-IAP1 in a non-canonical fashion (Supplementary Figure S7C and D). In addition, OTUB1 was not recruited to TNFR1 complex and its absence did not affect the engagement of RIP1 and c-IAP1 into this complex either (Supplementary Figure S7C).
OTUB1 modulates TWEAK-stimulated signalling and K48-specific c-IAP1 ubiquitination
To investigate the nature of endogenous c-IAP1 ubiquitination, we took advantage of recently developed ubiquitin chain-specific antibodies (Newton et al, 2008; Matsumoto et al, 2010). We first treated HT1080 cells with a well-established stimulator of c-IAP1 E3 ligase activity, IAP antagonist BV6, and observed K11-, K63-, and K48-specific autoubiquitination of c-IAP1 (Figure 5A). Such ubiquitination profile confirmed earlier studies with recombinant c-IAP1 and validated these reagents for the investigation of endogenous c-IAP1 ubiquitination (Blankenship et al, 2009; Phu et al, 2011). Next, we treated HT1080 cells with TNFα or TWEAK and, after denaturing lysis, immunoprecipitated cellular lysates with ubiquitin chain-specific antibodies. TWEAK treatment stimulated marked K11- and K63-, as well as K48-linked polyubiquitin chain assembly, while TNFα triggered moderate but visible K11- and K63-specific and no K48-specific c-IAP1 ubiquitination (Figure 5B). We also examined endogenous ubiquitination of RIP1, a well-established ubiquitination substrate in TNF signalling. As previously reported (Dynek et al, 2010), TNFα treatment prompted the assembly of all three examined ubiquitin linkages on RIP1, while TWEAK did not stimulate RIP1 ubiquitination—consistent with the absence of RIP1 from TWEAK-stimulated receptor complex (Figure 5B; Varfolomeev et al, 2012). We observed similar pattern of c-IAP1 ubiquitination when we treated Ramos cells with αCD40, IAP antagonist MV1 or TNFα: CD40 and MV1 stimulated K63- and K48-specific c-IAP1 ubiquitination, while TNFα triggered only K63-linked ubiquitination (Supplementary Figure 8A). To evaluate the importance of OTUB1 for endogenous c-IAP1 ubiquitination, OTUB1 was knocked down and cells were treated with TWEAK. As a result of OTUB1 absence, endogenous K48-linked polyubiquitination of c-IAP1 was much more apparent within the TWEAK signalling complex (Figure 5C). Accordingly, OTUB1 is recruited to TWEAK receptor FN14 with the same kinetics as c-IAP1 and its constitutive partner TRAF2 (Figure 5D). These results suggest strongly that TWEAK and IAP antagonists promote endogenous K48-specific c-IAP1 ubiquitination that can be regulated by OTUB1.
Figure 5.
OTUB1 regulates endogenous K48-linked polyubiquitination of c-IAP1. (A) c-IAP1 undergoes polyubiquitination with multiple linkages in response to IAP antagonist BV6. HT1080 cells were pre-treated with MG132 for 30 min and then treated with BV6 (2 μM) for 5 min. Cells were lysed in 6 M urea buffer, lysates were diluted twice and immunoprecipitated using linkage-specific anti-ubiquitin antibodies or control antibody (anti-Herceptin). Immunoprecipitated material was detected using anti-c-IAP1 antibody. (B) TWEAK stimulates K48-linked c-IAP1 polyubiquitination. HT1080 cells were pre-treated with MG132 for 30 min followed by the TNFα (50 ng/ml) or TWEAK (100 ng/ml) treatment for 7 min. Cells were lysed and immunoprecipitated as in (A) and immunoprecipitated proteins were detected using anti-RIP1 and c-IAP1 antibodies. (C) A2058 cells were transfected with control or OTUB1-specific siRNA oligos and 48 h later treated with MG132 (20 μM) for 1 h and subsequently with α-Flag antibody cross-linked TWEAK for indicated time periods. Cellular lysates were immunoprecipitated with TWEAK as described in Materials and methods, precipitates disrupted in 6 M urea and re-immunoprecipitated with K48 ubiquitin chain-specific antibodies. Lysates and precipitates were examined by western blotting with indicated antibodies. (D) A2058 cells were treated with MG132 (20 μM) for 1 h and with TWEAK for indicated time periods. Cellular lysates were immunoprecipitated with TWEAK as in (B) and examined by western blotting with indicated antibodies.
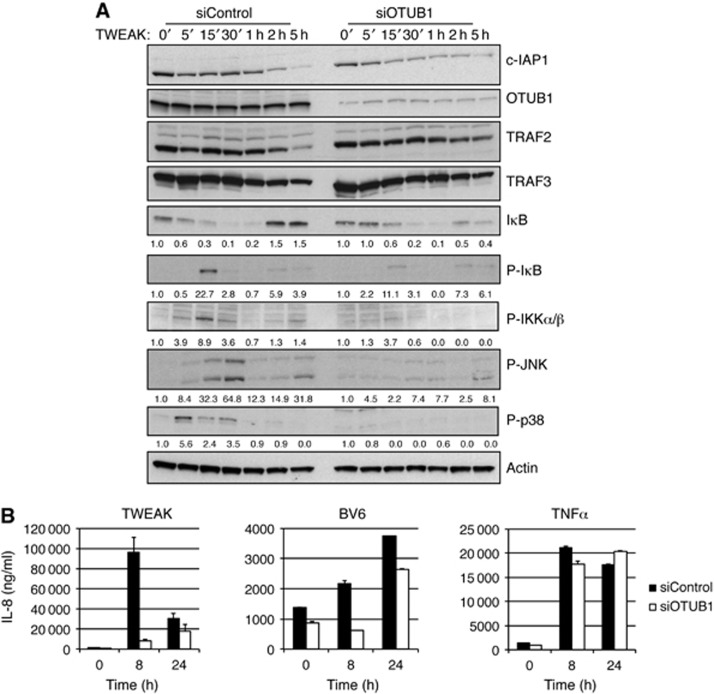
Given that OTUB1 can regulate c-IAP1 stability and TWEAK-induced cell death (Figure 2), we asked whether OTUB1 can affect TWEAK-stimulated signalling. Transient or stable knockdown of OTUB1 did not affect non-canonical NF-κB signalling stimulated by TWEAK or CD40 treatment (Supplementary Figure S8B–D). However, downregulation of OTUB1 significantly diminished TWEAK-induced activation of canonical NF-κB and MAPKs JNK and p38 (Figure 6A). We suspected that this blunted signalling might be associated with enhanced c-IAP1 degradation following TWEAK stimulation. Indeed, downregulation of OTUB1 prompted enhanced c-IAP1 degradation (Figure 6A). Since NF-κB and JNK activation induce expression of multiple genes, we assessed the role of the OTUB1 in gene induction by real-time PCR analysis and cytokine ELISA. TWEAK and BV6 induced robust increases in IL-8 production that were attenuated at early time points, but not at later time points, by OTUB1 knockdown (Figure 6B). Similarly, OTUB1 downregulation lead to a more pronounced decrease in TWEAK-stimulated mRNA production at earlier time points (Supplementary Figure S9). These results are not surprising given that TWEAK-induced activation of non-canonical NF-κB signalling was not affected by OTUB1 downregulation (Supplementary Figure S8C and D). At the same time TNF stimulation, which does not result in K48-linked c-IAP1 autoubiquitination, induced gene expression that was not affected by OTUB1 knockdown (Figure 6B). Thus, absence of OTUB1 dampens initial but not persistent TWEAK- and BV6-stimulated gene expression, likely because of differential effects of OTUB1 on the activation of the canonical NF-κB and MAPK pathways versus non-canonical NF-κB signalling.
Figure 6.
Downregulation of OTUB1 diminishes TWEAK signalling. (A) A2058 cells were transfected with control or OTUB1-specific siRNA oligos and 48 h later treated with TWEAK (100 ng/ml) for indicated time periods. Cellular lysates were examined by western blotting with indicated antibodies. (B) HT1080 cells were transfected with control or OTUB1-specific siRNA oligos and 48 h later treated with TWEAK (100 ng/ml), BV6 (2.5 μM), or TNFα (20 ng/ml) for indicated time periods. Supernatant from cells was examined for production of IL-8 cytokine by Luminex Technology.
OTUB1 regulates the stability of zebrafish vascular structures
An elegant study by the Stainier group has shown an essential role for Birc2 (zebrafish orthologue of human c-IAP1 and c-IAP2) in the maintenance of vascular integrity in the zebrafish, thus making the zebrafish a suitable model to study Birc2 biological function and stability (Santoro et al, 2007). To investigate the role of Otub1 in Birc2 stability, we used morpholino antisense olionucleotides (MO) that target Otub1 and monitored vascular development. During zebrafish vascular development, the axial and intersegmental vessels (ISV) develop in a stereotypical pattern and establish a functional vascular network by 72 h post fertilization (hpf). Vascular development was examined in Tg(fli1:EGFP) zebrafish that express GFP in the vasculature, while vascular function and blood flow were analysed by Dextran-rhodamine injection into the common cardinal vein.
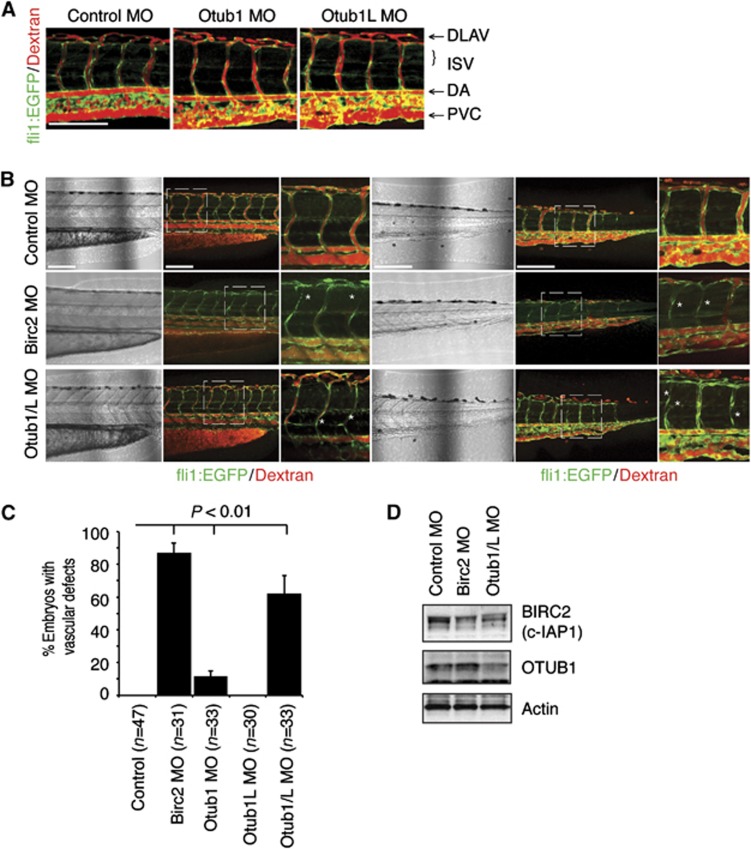
Zebrafish contains two Otub1 isoforms, Otub1a (Otub1L) and Otub1b (Otub1). Morpholino knockdown of Otub1 or Otub1L individually reduced their protein levels but had minimal impact on vascular development or stability (Figure 7A; Supplementary Figure S10A), as reported previously (Tse et al, 2009). To test the functional redundancy of Otub1 and Otub1L, we targeted them both simultaneously. Analysis of vascular integrity of embryos with combined Otub1/L knockdown at 72 hpf demonstrated a significant regression and loss of blood flow through ISV along the axis (Supplementary Figure S10). Confocal imaging of the zebrafish ISV revealed that Otub1/L combination knockdown results in ISV that are non-functional for blood flow, display luminal narrowing and ISV detachment from the axial vessels (Figure 7B). Capillary luminal narrowing and detaching from a larger vessel is a hallmark of vascular regression (reviewed in Wacker and Gerhardt, 2011). Importantly, the Otub1/L knockdown resulted in a phenotype that is very similar to that observed in the embryos injected with Birc2 MO, as well as in lowered levels of Birc2 protein (Figure 7B–D), although not as severe. Birc2 knockdown caused complete regression of ISV in the anterior trunk regions (Figure 7B, left three panels) and produced severe defects in blood flow through the ISV. We did not observe a significant decrease in embryo viability or gross defects in anatomical development from any of the morpholinos used. In ∼10% of Otub1-injected embryos, we observed a defect in blood flow, although ISV and axial structure appeared normal (Figure 7C; Supplementary Figure S10; Tse et al, 2009). These blood flow defects are possibly the first signs of regression and reflect the potential effect of Otub1 loss (Lobov et al, 2011). Taken together, our data suggest that Otub1 is an important regulator of vascular integrity and Birc2 (c-IAP) stability in zebrafish.
Figure 7.
OTUB1 regulates zebrafish vascular stability. (A) Tg(fli1:EGFP) zebrafish were injected with Control (2 ng), OTUB1 (0.8 ng), or OTUB1L (2 ng) morpholinos. At 72 hpf angiography was performed and vascular development and blood flow were analysed by confocal microscopy. ISV, intersegmental vessel; DLAV, dorsal longitudinal vessel; DA, dorsal aorta; PVC, posterior cardinal vein. Scale bar: 150 μm. (B) Tg(fli1:EGFP) zebrafish were injected with Control (4 ng), Otub1/L (2.8 ng), or Birc2 (4 ng) morpholinos. At 72 hpf angiography was performed and vascular development and blood flow were analysed by confocal microscopy in two zebrafish trunk regions. *Indicates sites of lumen narrowing and retraction of ISV vessels. Scale bar: 150 μm. (C) Quantification of ISV blood flow. Percentage of embryos with two or more ISV vessels that have no observable blood flow (Dextran-rhodamine). Error bars represent the standard deviation. Statistical significance of experimental groups versus control, P<0.01, t-test. (D) Western blot analysis of Birc2 (with α-c-IAP1/2 pan antibody from R&D Systems) and Otub1 (with α-OTUB1 antibody from Bethyl) protein expression at 48 hpf in Tg(fli1:EGFP) zebrafish were injected with Control (4 ng), Otub1/L (2.8 ng), or Birc2 (4 ng) morpholinos.
Discussion
Turnover of cellular proteins regulates the availability of key components of signalling pathways, which allows proper functioning of cellular machinery (Hershko and Ciechanover, 1998). This is often accomplished through carefully orchestrated assembly and disassembly of polyubiquitin chains (Ikeda and Dikic, 2008). Cleavage of polyubiquitin chains is carried out by deubiquitinases, a group of enzymes that remove ubiquitin modifications from substrate proteins (Reyes-Turcu et al, 2009). Our search for regulators of c-IAP1 stability identified the deubiquitinase OTUB1 as a c-IAP interacting protein that can cleave K48-linked polyubiquitin chains from c-IAP1. The validation of OTUB1 as a DUB for c-IAP1 stems from its identification as a c-IAP binding partner in mass spectrometry analysed pull-downs, its ability to modulate IAP antagonist-stimulated proteasomal degradation of c-IAP1, and its role in TNF-dependent cell death pathways. Although several other deubiquitinating enzymes were identified in pull-downs or in our DUB screen, OTUB1 was the only candidate that consistently demonstrated the capacity to bind to and regulate K48-linked polyubiquitination of c-IAP1. OTUB1 has garnered a lot of attention lately because of its ability to block ubiquitination in a canonical fashion—by removing K48-linked chains, and in a non-canonical fashion—by binding a select group of ubiquitin conjugating enzymes and suppressing their enzymatic function (Edelmann et al, 2009; Nakada et al, 2010; Juang et al, 2012; Sato et al, 2012; Wiener et al, 2012). Although OTUB1 appears to rely on its non-canonical E2-blocking activity to regulate ubiquitination in response to DNA damage (Nakada et al, 2010; Sun et al, 2012), our data suggest that OTUB1 regulates c-IAP1 stability by disassembling K48-linked chains. This was demonstrated in reconstituted in vitro ubiquitination and deubiquitination reactions, as well as in cellular studies where the presence or absence of OTUB1 did not affect c-IAP1-mediated ubiquitination of RIP1 or NIK kinases. However, it is possible that c-IAP1 mediates some other, yet unidentified, biological processes where the non-canonical function of OTUB1 may play an important regulatory role.
Ligands and receptors of the TNF superfamily are instrumental for the immunity, development, and homeostasis of metazoan organisms (Locksley et al, 2001). TNFR signalling activates NF-κB and MAPK pathways in a ubiquitin-dependent manner and c-IAP proteins are critical E3 ligases responsible for proper functioning of these signalling pathways. In the absence of the c-IAP proteins, TWEAK-stimulated signalling and gene expression are drastically reduced (Varfolomeev et al, 2012). Downregulation of OTUB1 and the consequent enhancement of c-IAP1 K48-linked polyubiquitination led to faster c-IAP1 degradation and dampened TWEAK-induced signalling. Absence of OTUB1 affected canonical NF-κB and MAPK signalling but not non-canonical NF-κB pathway activation; this is likely because c-IAP1 levels drop following TWEAK signalling, even in the presence of OTUB1 (although not as rapidly). The loss of c-IAP1 liberates NIK from degradative ubiquitination and allows activation of non-canonical NF-κB signalling (Varfolomeev et al, 2007). This differential effect of OTUB1 on signalling pathways is reflected in gene expression and cytokine production—the reduction in canonical NF-κB and MAPK activation results in lowered early cytokine production but not at later time periods when non-canonical NF-κB activation allows comparable induction of gene expression. One of the cytokines upregulated by these signalling pathways is TNFα. When c-IAP proteins are present and stable TNFα does not stimulate cell death (Moulin et al, 2012). However, the absence of OTUB1 enhances TWEAK- or IAP antagonist-stimulated c-IAP1 degradation and lowers the threshold of apoptotic sensitivity, leading to caspase activation and cell death. TWEAK and IAP antagonists promote K48-linked ubiquitination of c-IAP1 leading to its degradation (Figure 5; Varfolomeev et al, 2007). TNFα, however, does not stimulate c-IAP1 K48-specific ubiquitination nor does it promote c-IAP1 degradation (Figure 5; Supplementary Figure S8; Varfolomeev et al, 2012). A K48-linked ubiquitin-specific DUB that associates with c-IAP1, OTUB1, ensures proper level of K48-linked c-IAP1 ubiquitination and allows the fine-tuning of signalling and cell death pathways that rely on c-IAP proteins. Collectively, these results suggest that stimuli that promote c-IAP1 K48-specific ubiquitination and destabilization can be regulated by OTUB1 resulting in TNF-dependent cell death. In zebrafish, downregulation of OTUB1 orthologues destabilized the c-IAP orthologue Birc2 resulting in severe defects in vascular integrity and blood flow in ISV. Therefore, OTUB1 regulates c-IAP stability in mammalian cells and in lower vertebrates with considerable consequences for TNFR-mediated signalling pathways.
IAP antagonists are a class of IAP protein-targeted compounds that show great promise for the treatment of human malignancies and are currently under investigation in clinical trials (Flygare et al, 2012; Fulda and Vucic, 2012). Our data indicate that OTUB1 is an important modulator of c-IAP1 stability with potential to regulate IAP antagonist-stimulated cell death. Indeed, in a number of cancer cells, knockdown of OTUB1 potentiated IAP antagonist-induced apoptosis suggesting that targeting OTUB1 might have beneficial effects in IAP targeting treatment scenarios. Thus, future studies involving combined targeting of OTUB1 and IAP proteins might pave a way for novel therapeutic strategies.
Materials and methods
Cell lines, reagents, and transfections
HEK 293T human embryonic kidney cells, SKOV3 ovarian carcinoma cells, HT29 human colon carcinoma cells, HT1080 human fibrosarcoma cells, Panc1 human pancreatic carcinoma cells, Hs294T and A2058 human melanoma cells, and Ramos B cell lymphomas were obtained from American Type Culture Collection. EVSA T human breast carcinoma cells were obtained from DSMZ (German Collection of Microorganisms and Cell Cultures). All cells were grown and maintained in 50:50 RPMI-1640/DMEM medium supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin.
KMS18 multiple myeloma cells were from Japanese Collection of Research Bioresources (JCRB). The pBabe-puro empty vector or vector containing human c-IAP1 or c-IAP2 cDNA tagged at N-terminus with 3 × Flag tag constructs were introduced into KMS18 cells after retroviral infection and KMS18 stable cell lines expressing 3 × Flag c-IAP1, c-IAP2, or vector were selected and maintained in the media with puromycin.
Human recombinant soluble TNFα, TNFRII-Fc fusion protein, TWEAK, FasL, αDR5 (Drozitumab), and BV6 were produced at Genentech, Inc., LIGHT and αCD40 antibody were purchased from R&D Systems. For all treatments, Flag-tagged TWEAK ligand was cross-linked with anti-Flag antibody, His-tagged LIGHT was cross-linked with anti-His antibody, and agonistic αCD40 antibody was cross-linked with secondary anti-IgG2b antibody. MG132 was purchased from American Peptide Company, z-VAD-Fmk from Calbiochem, etoposide, iodoacitomide, Actinomycin D, N-ethylmaleimide (NEM), and Apyrase from Sigma. Antibodies against actin (Sigma), Myc (Upstate), Flag (Sigma), HA (Roche), His (Qiagen), ubiquitin (P4D1, Cell Signaling), RIP1, P-JNK, Mcl1, TRAF2 (BD Biosciences), c-IAP1 (Enzo and R&D Systems), c-IAP1/2 pan, TAK1 (R&D Systems), c-IAP2 (AbCam), XIAP, IκBα, P-IκBα, P-p38, P-IKKα/β-Ser 176/180, caspase-3, caspase-8, FN14 (Cell Signaling), OTUB1 (Everest Biotech and Bethyl), USP15 and NIK (Novus), p53 (NeoMarkers), TRAF3 (Invitrogen), TRAF6, p100 (Millipore) were purchased, and K11-, K48- and K63-linkage-specific anti-ubiquitin antibodies were described previously (Newton et al, 2008; Matsumoto et al, 2010). Plasmids expressing Flag-c-IAP1, Flag-c-IAP2, Myc-c-IAP1, Myc-NIK, and HA-ubiquitin variants (K11-, K48-, K63-only) have been described previously (Varfolomeev et al, 2007; Dynek et al, 2010). Expression constructs for Flag-tagged OTUB1 were from DUB library collection in pRK2001 vector (Pereg et al, 2010). Mutation for active site of OTUB1 C91A was introduced using QuikChange site-directed mutagenesis kit (Stratagene). HEK293T and A2058 cells were transiently transfected with plasmids using Geneporter 2 reagent (Genlantis) or Lipofectamine 2000 (Invitrogen), respectively. All siRNA transfections were done using Lipofectamine RNA iMax (Invitrogen); siRNA sequences are included in Supplementary data.
DUB screen
A collection of DUB constructs was provided by Vishva Dixit (Pereg et al, 2010). Flag-tagged deubiquitinating enzyme constructs were transfected into 293T cells using FuGene 6 (Roche). Forty-eight hours later cells were treated with BV6 (2 μM) for 10 min, lysed, and lysates were analysed by western blotting with c-IAP1, Actin, and Flag (DUBs) antibodies. The Myc-A20 expression construct was provided by Ingrid Wertz.
Western blot analysis and immunoprecipitation
Western blot analyses and immunoprecipitations were performed as described previously with the following lysis buffer: 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitor cocktail (Roche) (Varfolomeev et al, 2012). For immunoprecipitation of endogenous TWEAK receptor-associated complex indicated cells (5 × 107 cells per time point) were left untreated or treated with α-Flag antibody cross-linked Flag-TWEAK (1 μg/ml) for indicated period of time. Following PBS washes, cells were lysed in modified lysis buffer with 0.1% SDS (40 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1% Triton X-100, 20 μM MG132, 4 mM NEM, 20 mM iodoacetomide and EDTA-free protease inhibitor cocktail; Roche). Cellular lysates were sonicated briefly to break DNA, pre-cleared with agarose beads, and immunoprecipitated with protein-A/G beads. Immunoprecipitated protein complexes were washed several times in lysis buffer, resolved on SDS–PAGE and immunoblotted with the indicated antibodies. Detection of endogenous K11-, K48-, and K63-linked c-IAP1 ubiquitination was performed as described previously (Dynek et al, 2010). For detection of K48-specific ubiquitination of c-IAP1 within TWEAK complex, the immunoprecipitated endogenous TWEAK receptor complexes were washed in lysis buffer and then disrupted in 6 M Urea containing buffer (20 mM Tris–HCl (pH 7.5), 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, 20 μM MG132, 4 mM NEM, 20 mM iodoacetomide and protease inhibitor cocktail; Roche), diluted two times, and re-immunoprecipitated with K48-linkage-specific anti-ubiquitin antibody as described previously (Newton et al, 2008). Immunoprecipitated proteins and lysates were resolved on SDS–PAGE and immunoblotted with c-IAP1 antibody. Nuclear fractionation for p52 detection was performed using NE-PER nuclear and cytoplasmic extraction reagents according to manufacturer instructions (Pierce Biotechnology).
Ubiquitination and deubiquitination assays
Reconstituted c-IAP1 auto-ubiquitination assays were performed as described previously (Varfolomeev et al, 2007; Blankenship et al, 2009) using 0.5 μg of ubiquitin (wild type, K48-only, or K11-only), 1 μg E2 (UbcH5c) and 1 μg of c-IAP1 with the following modifications: 2 μg of recombinant OTUB1 wild-type protein or C91A mutant was added to the reconstituted ubiquitination reaction lacking E3 enzyme, pre-incubated for 15 min at 30°C, followed by the addition of c-IAP1. Autoubiquitination reactions were stopped at indicated times by adding 4 × LDS loading buffer, boiled at 90°C for 10 min and resolved on SDS–PAGE. To assess in vitro OTUB1 DUB activity on ubiquitinated c-IAP1 as a substrate, 1 μg of c-IAP1 was autoubiquitinated in assay using 1 μg of K48-only, or K11-only ubiquitin at 30°C for 20 min. An aliquot was taken and prepared for western blot analysis. The ubiquitination reaction was stopped by addition of 1 U/μl of apyrase (ATP diphosphohydrolase). After 1 h of incubation at 37°C, reactions with autoubiquitinated c-IAP1 were diluted six times in DUB buffer containing recombinant 2 μg of OTUB1 wild-type or C91A catalytic mutant. The deubiquitination assay was carried out for indicated time periods at 37°C and samples were analysed by western blotting.
Mass spectrometry analysis
KMS18 cells stably transfected with Flag-tagged c-IAP1 or c-IAP2, or vector were lysed in 1% Triton buffer and lysates were incubated with a Flag resin for 2 h followed by elution with Flag peptides overnight. Eluates were concentrated and protein samples were separated by SDS–PAGE and Coomassie stained. In gel digestions were performed and samples injected on an LTQ-Orbitrap mass spectrometer as previously described (Phu et al, 2011). The mass spectrometer was operated in data-dependent top8 mode with a full MS scan collected at 60 000 resolution in the Orbitrap and fragment ion MS/MS collected in the ion trap. Acquired MS/MS scans were searched against a concatenated target-decoy database compiled from Uniprot protein sequences and common contaminants across a 50-ppm mass window. Peptide spectral matches were filtered to a peptide level false discovery rate (FDR) of <0.5% using a two-stage linear discriminant analysis, initially filtering to 5% at the peptide level on a run by run basis and subsequently to 2% FDR at the protein level for aggregated data from each sample.
Zebrafish studies
Adult Tg(fli1:EGFP) fish were obtained from P Crosier, University of Auckland. Fish were maintained according to standard laboratory practices and all experiments were approved by the Genentech Inc. Institutional Animal Care and Use Committee. Morpholinos were diluted in ultrapure water +0.2% phenol red and 5 nl was injected into 1–4 cell embryos using a Eppendorf FemtoJet. Embryos were maintained at 28°C in 1X Danieu’s solution with 0.003% PTU. At 24 hpf embryos that failed to develop or have noticeable anatomical defects were removed. For microangiographic assessment of blood flow, morpholino-injected embryos were anaesthetized 72 hpf in 0.04% tricaine pH 7 (Sigma E10521), and injected with 5 nl of 2 MDa rhodamine-dextran (Invitrogen D7139) into the common cardinal vein. Embryos were mounted in 1% low-melt agarose in Danieau buffer containing tricaine on a coverslip. All images were acquired using a laser scanning confocal microscope (TCS SP5, Leica) with a 500-mW Argon-Ion laser (LASOS) and a 20 × objective (Apochromat 0.7 NA, Leica).
Supplementary Material
Acknowledgments
We thank Wayne Fairbrother, Vishva Dixit, Nobuhiko Kayagaki, Kim Newton, Ingrid Wertz, Kerry Zobel, Erin Dueber, Elizabeth Helgason, Jinfeng Liu, Nicholas Lewin-Koh, Somasekar Seshagiri, Andrea Cochran, Zora Modrusan, Yaron Pereg, Jasmin Dynek, Leon Parker, Scott Marsters, Karen O’Rourke, members of the Early Discovery Biochemistry department, and the Oligo Synthesis, FACS and Sequencing facilities at Genentech that provided help with insightful discussions, suggestions and reagents.
Author contributions: TG designed and performed most of the biochemical and cellular signalling studies, viability assays, and expression analysis; AIT and DK designed and performed Mass Spectrometry studies; KN designed and performed zebrafish studies; EV and MCdeA performed signalling studies; AVF and KD purified recombinant proteins; DV conceived and supervised the study and wrote most of the manuscript with input from TG, KN, DK, EV, and MCdeA.
Footnotes
All of the authors are employees of Genentech, Inc.
References
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JD Jr. et al. (2007) Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12: 115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30: 689–700 [DOI] [PubMed] [Google Scholar]
- Blankenship JW, Varfolomeev E, Goncharov T, Fedorova AV, Kirkpatrick DS, Izrael-Tomasevic A, Phu L, Arnott D, Aghajan M, Zobel K, Bazan JF, Fairbrother WJ, Deshayes K, Vucic D (2009) Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2. Biochem J 417: 149–160 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78: 399–434 [DOI] [PubMed] [Google Scholar]
- Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, Helgason E, Fairbrother WJ, Deshayes K, Kirkpatrick DS, Vucic D (2010) c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J 29: 4198–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann MJ, Iphofer A, Akutsu M, Altun M, di Gleria K, Kramer HB, Fiebiger E, Dhe-Paganon S, Kessler BM (2009) Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem J 418: 379–390 [DOI] [PubMed] [Google Scholar]
- Emmerich CH, Schmukle AC, Walczak H (2011) The emerging role of linear ubiquitination in cell signaling. Sci Signal 4: re5. [DOI] [PubMed] [Google Scholar]
- Flygare JA, Beresini M, Budha N, Chan H, Chan IT, Cheeti S, Cohen F, Deshayes K, Doerner K, Eckhardt SG, Elliott LO, Feng B, Franklin MC, Reisner SF, Gazzard L, Halladay J, Hymowitz SG, La H, LoRusso P, Maurer B et al. (2012) Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152). J Med Chem 55: 4101–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Vucic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 11: 109–124 [DOI] [PubMed] [Google Scholar]
- Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, Koschny R, Komander D, Silke J, Walczak H (2009) Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell 36: 831–844 [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479 [DOI] [PubMed] [Google Scholar]
- Husnjak K, Dikic I (2012) Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem 81: 291–322 [DOI] [PubMed] [Google Scholar]
- Ikeda F, Dikic I (2008) Atypical ubiquitin chains: new molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series. EMBO Rep 9: 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai K, Tokunaga F (2009) Linear polyubiquitination: a new regulator of NF-kappaB activation. EMBO Rep 10: 706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YC, Landry MC, Sanches M, Vittal V, Leung CC, Ceccarelli DF, Mateo AR, Pruneda JN, Mao DY, Szilard RK, Orlicky S, Munro M, Brzovic PS, Klevit RE, Sicheri F, Durocher D (2012) OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol Cell 45: 384–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P et al. (2007) Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12: 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zheng H, Mao AP, Zhong B, Li Y, Liu Y, Gao Y, Ran Y, Tien P, Shu HB (2010) Regulation of virus-triggered signaling by OTUB1- and OTUB2-mediated deubiquitination of TRAF3 and TRAF6. J Biol Chem 285: 4291–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Cheung E, Wudali R, Cao J, Halasz G, Wei Y, Economides A, Lin HC, Papadopoulos N, Yancopoulos GD, Wiegand SJ (2011) The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood 117: 6728–6737 [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487–501 [DOI] [PubMed] [Google Scholar]
- Matsumoto ML, Wickliffe KE, Dong KC, Yu C, Bosanac I, Bustos D, Phu L, Kirkpatrick DS, Hymowitz SG, Rape M, Kelley RF, Dixit VM (2010) K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol Cell 39: 477–484 [DOI] [PubMed] [Google Scholar]
- Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, Vaux DL (2012) IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J 31: 1679–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada S, Tai I, Panier S, Al-Hakim A, Iemura S, Juang YC, O'Donnell L, Kumakubo A, Munro M, Sicheri F, Gingras AC, Natsume T, Suda T, Durocher D (2010) Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 466: 941–946 [DOI] [PubMed] [Google Scholar]
- Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, Komuves L, French DM, Ferrando RE, Lam C, Compaan D, Yu C, Bosanac I, Hymowitz SG, Kelley RF, Dixit VM (2008) Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134: 668–678 [DOI] [PubMed] [Google Scholar]
- Pereg Y, Liu BY, O'Rourke KM, Sagolla M, Dey A, Komuves L, French DM, Dixit VM (2010) Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat Cell Biol 12: 400–406 [DOI] [PubMed] [Google Scholar]
- Phu L, Izrael-Tomasevic A, Matsumoto ML, Bustos D, Dynek JN, Fedorova AV, Bakalarski CE, Arnott D, Deshayes K, Dixit VM, Kelley RF, Vucic D, Kirkpatrick DS (2011) Improved quantitative mass spectrometry methods for characterizing complex ubiquitin signals. Mol Cell Proteomics 10: M110.003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM, Fushman D (2004) Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8: 610–616 [DOI] [PubMed] [Google Scholar]
- Reyes-Turcu FE, Ventii KH, Wilkinson KD (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78: 363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV (1995) The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83: 1243–1252 [DOI] [PubMed] [Google Scholar]
- Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY (2007) Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet 39: 1397–1402 [DOI] [PubMed] [Google Scholar]
- Sato Y, Yamagata A, Goto-Ito S, Kubota K, Miyamoto R, Nakada S, Fukai S (2012) Molecular basis of K63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem 287: 25860–25868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman BA, Harper JW (2009) Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol 10: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Brink R (2010) Regulation of TNFRSF and innate immune signalling complexes by TRAFs and cIAPs. Cell Death Differ 17: 35–45 [DOI] [PubMed] [Google Scholar]
- Sun XX, Challagundla KB, Dai MS (2012) Positive regulation of p53 stability and activity by the deubiquitinating enzyme Otubain 1. EMBO J 31: 576–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse WK, Eisenhaber B, Ho SH, Ng Q, Eisenhaber F, Jiang YJ (2009) Genome-wide loss-of-function analysis of deubiquitylating enzymes for zebrafish development. BMC Genomics 10: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M (2008) Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol 9: 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131: 669–681 [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D (2008) c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFα)-induced NF-κB activation. J Biol Chem 283: 24295–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Goncharov T, Maecker H, Zobel K, Komuves LG, Deshayes K, Vucic D (2012) Cellular inhibitors of apoptosis are global regulators of NF-kappaB and MAPK activation by members of the TNF family of receptors. Sci Signal 5: ra22. [DOI] [PubMed] [Google Scholar]
- Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, McKinlay M, Benetatos CA, Condon SM, Chunduru SK, Yeoh G, Brink R, Vaux DL, Silke J (2008) TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol 182: 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J (2007) IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131: 682–693 [DOI] [PubMed] [Google Scholar]
- Vucic D, Dixit VM, Wertz IE (2011) Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol 12: 439–452 [DOI] [PubMed] [Google Scholar]
- Wacker A, Gerhardt H (2011) Endothelial development taking shape. Curr Opin Cell Biology 23: 676–685 [DOI] [PubMed] [Google Scholar]
- Wiener R, Zhang X, Wang T, Wolberger C (2012) The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 483: 618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G (2008) Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol 9: 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.