Abstract
OBJECTIVES
Only patients with a complete resection of non-small-cell lung cancer (NSCLC) may expect long-term survival. Despite the recent progress in imaging and induction therapy, a thoracotomy may remain exploratory or with incomplete resection (R2). Our purpose was to revisit these situations.
METHODS
A total of 5305 patients who underwent surgery for NSCLC between 1980 and 2009 were reviewed. We compared the epidemiology, pathology, causes and prognosis characteristics of exploratory thoracotomy (ET) and R2 resections.
RESULTS
ET and R2 resections were observed in 223 (4%) and 197 (4%) patients, respectively. The frequency of ET decreased with time, while the frequency of R2 resection remained almost stable. The indications for ET and R2 resections were not significantly different. In comparison with ET, R2 resections were characterized by a significantly higher frequency of induction therapy (22 vs 17%, P < 10−3), adenocarcinomas (49 vs 15%, P < 10−6), T1–T2 (53 vs 29%, P < 10−6) and N0–N1 extension (67 vs 42%, P = 10−6). R2 resections were also characterized by a higher rate of postoperative complications (19.1 vs 9.9%, P = 0.014), with no significant difference in postoperative mortality (6.9 vs 4.9%, P = non significant). R2 resections resulted in a higher 5-year survival compared with ET (11.1 vs 1.2%, P = 10−3). There was no long-term survivor after ET, except during the last decade.
CONCLUSIONS
ET and R2 remain unavoidable. In comparison with ET, R2 resection is associated with a higher rate of postoperative complications, but a higher long-term survival.
Keywords: Lung cancer, Surgery, Exploratory thoracotomy, R2 resection, Induction therapy
INTRODUCTION
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related mortality in the western world [1]. Its management has undergone a rapid evolution over the last decade, but its prognosis showed only a slight improvement, with 5-year survival rates increasing from 15 to 17% in population-based studies [2]. Due to the lack of screening protocols and the constant improvement of imaging techniques, 40–50% of newly diagnosed NSCLC are now classified as metastatic stages. At the same time, only patients who benefit from a local treatment, using surgery, chemoradiotherapy or a combination of both, may expect 5-year survival rates ranging from 20 to 80% [1]. Therefore, the possibility of obtaining local treatment through complete surgical resection remains of tremendous importance and may be considered even in advanced disease due to tumoural, lymphatic or metastatic extension, either as a first-line therapy or after induction treatments.
However, despite the improvement in imaging techniques, tumoural extension may be preoperatively underestimated. A surgical procedure scheduled with curative intent may ultimately be limited to an exploratory thoracotomy (ET) because of intraoperatively established non-resectability or an incomplete resection (R2) due to the impossibility of removing the whole tumour, resulting in a macroscopic part of the tumour remaining [3]. Aiming to help the surgeons in their choice while facing uncertain resections, we sought to analyse the indications for ET and R2, their evolution over time and the prognosis associated with these unexpected conditions.
PATIENTS AND METHODS
All clinical records of patients who underwent surgery for NSCLC between January 1980 and December 2009 in the Thoracic Surgery Department of Georges Pompidou European Hospital (Paris) and Cedar Surgery Centre (Bois Guillaume) were reviewed. The preoperative diagnostic work-up included chest X-ray, bronchoscopy, computed tomography scan of the chest, spirometry, lung perfusion scan and a thorough search for distant metastases (including positron emission tomography in recent years). Supplemental examinations were performed according to tumour location (i.e. magnetic resonance imaging for vascular and bone infiltration, and ultrasound oesophagoscopy for oesophageal infiltration, etc.). Mediastinoscopy was routinely performed to exclude N3 disease and to confirm N2 involvement in some patients included in various induction therapy protocols depending on the referring centre. Initial evidence of N3 disease and multiple distant metastases precluded surgery. The staging system was the International Staging System for NSCLC recently modified concerning the T and M [1]. For mediastinal lymph node involvement, the classification of Mountain and Dresler was used [4]. All patients deemed suitable for surgery underwent a thoracotomy.
In case of unexpected tumoural or nodal extension diagnosed during thoracotomy, the surgeon remains the sole decision-maker in choosing between performing a doubtful resection (risking R1 or R2 resections) and closing the chest (performing an ET). Resection margins were routinely controlled by perioperative frozen section studies when technically feasible. Resection was classified as R0 if complete, R1 if microscopic tumour was found on the edge of the operative specimen and R2 if gross residual disease was left behind after surgical excision of the tumour. R2 resections usually concern a small amount of tumour remaining on a non-resectable structure, or if a larger resection was deemed functionally unacceptable. R2 resections should be distinguished from debulking, which was not routinely performed.
Altogether, 5707 patients underwent thoracotomy with curative intent during the mentioned period. Patients with R1 resection (n = 264) and oligometastastic disease (M1b, n = 138) were excluded from the study. The overall population included 5305 patients. R0 resections, ET and R2 resections were performed in 4885 (92%), 223 (4%) and 197 (4%) patients, respectively.
Postoperatively, ET and R2 patients were treated by chemotherapy, radiation therapy or with a combination of both, depending on the type of neoadjuvant therapy and the cause of unsuccessful surgery. Three decades were considered: 1980–89, 1990–99 and 2000–09. We particularly analysed and compared the epidemiology, pathology and prognotic characteristics of ET and R2 patients treated during these periods.
The study was approved by the French Thoracic Surgery Society Ethic Committee (CERC-SFCTCV) that waived need for informed consent. Follow-up information was obtained from the hospital case records, from a questionnaire completed by either the chest physician or the general practitioner or from death certificates. The main outcome was the overall survival, defined as the time interval between the date of operation and the date of death or the last follow-up visit for censored patients. Follow-up was completed for 5116 (96.4%) patients. The mean duration of follow-up was 94 months for patients still alive. Actuarial survival curves were estimated by the Kaplan–Meier method. Statistical comparisons between survival distributions were made using the log-rank test. All data analyses were conducted with the two-sided test: a P-value <0.05 was considered as statistically significant. The statistical software used for the analysis was SEM (Statistiques Épidémiologie Médecine), (Anticancer Centre Jean Perrin, Clermont-Ferrand, France) [5].
RESULTS
The main demographic and surgical characteristics of the patients are summarized in Table 1. According to the period of time, the proportion of ET decreased, while the proportion of R0 resections increased and the proportion of R2 resections remained stable (P < 10−6). Induction therapy was more frequently performed in ET (n = 39, 18%) and R2 (n = 43, 22%) than in R0 patients (n = 624, 13%, P < 10−3). In case of surgical resection, its extension differed slightly, with R2 patients undergoing more wedge resections and segmentectomy (n = 42, 21%) than R0 patients (n = 378, 8%, P < 10−6). Postoperative complications were observed in 22.2% patients and less often after ET (n = 22, 9.9%) than after surgical resection (R2 n = 54, 19.1%; R0 n = 180, 22.2%; P < 10−3). R2 resection showed a higher postoperative mortality rate (6.9%) compared with ET (4.9%) and R0 resections (3.7%, P < 10−3).
Table 1:
Demographic and surgical characteristics
| ET (n = 223) | R2 (n = 197) | R0 (n = 4885) | Total (n = 5305) | P-value | |
|---|---|---|---|---|---|
| Male | 191 (86%) | 164 (83%) | 3951 (81%) | 4306 (81%) | 0.150 |
| Age | 62.6 ± 10 | 59 ± 11 | 61.9 ± 10 | <10−6 (R2 vs R0) | |
| Perioda | |||||
| 1980–1989 | 97 (8%) | 35 (3%) | 1096 (89%) | 1228 (100%) | <10−6 |
| 1990–1999 | 89 (4%) | 97 (5%) | 1934 (91%) | 2120 (100%) | |
| 2000–2009 | 37 (2%) | 65 (3%) | 1855 (95%) | 1957 (100%) | |
| Smoker | 194 (87%) | 172 (87%) | 4383 (90%) | 4749 (90%) | 0.013 |
| Previous cancer history | 23 (10%) | 33 (17%) | 1000 (21%) | 1056 (20%) | <10−3 |
| Induction treatment | 39 (18%) | 43 (22%) | 624 (13%) | 706 (13%) | <10−3 |
| Resectionb | <10−6 | ||||
| Sublobar | 42 (21%) | 378 (8%) | 420 (8%) | ||
| Lobectomy | 104 (53%) | 2933 (60%) | 3037 (57%) | ||
| Pneumonectomy | 51 (26%) | 1574 (32%) | 1625 (31%) | ||
| Postoperative mortality | 11 (4.9%) | 15 (6.9%) | 180 (3.7%) | 206 (3.9%) | 0.014 |
| Postoperative complications | 22 (9.9%) | 54 (19.1%) | 1121 (22.9%) | 1197 (22.2%) | <10−3 |
ET: exploratory thoracotomy; R2: incomplete resection thoracotomy.
aThe percentages represent the proportion of patients in each group during a given time period.
bThe sum is >100% due to the 223 patients undergoing exploratory thoracotomy (ET).
Pathology and TNM characteristics are shown in Table 2. Definite histology included 2444 squamous cell carcinomas (46%), 2180 adenocarcinomas (41%), 419 undifferentiated large cell carcinomas (8%), 128 adenosquamous (2%) and 134 other cancers (3%). squamous cell carcinomas were more frequently associated with ET (n = 97, 44% of ET and 4% of squamous cell carcinoma patients) than with R2 resections (n = 63, 32% of R2 resections and 2% of squamous cell carcinoma patients). Adenocarcinomas were more frequently associated with R2 resection (n = 96, 49% of R2 resections and 4% of adenocarcinoma patients) than with ET (n = 33, 15% of ET and 2% of adenocarcinoma patients). T1–T2 tumours were more frequent in R2 resections (n = 79, 53%) than in ET (n = 81, 40%, P < 10−6). Mediastinal lymph nodes invasion (N2) was more frequent in ET (n = 134, 67%) than in R2 resection (n = 97, 42%, P = 10−6).
Table 2:
Pathological classification and TNM extension
| ET (n = 223) | R2 (n = 197) | R0 (n = 4885) | Total (n = 5305) | P-value | |
|---|---|---|---|---|---|
| Histology | <10−6 | ||||
| Squamous cell | 97 (44%) | 63 (32%) | 2284 (47%) | 2444 (46%) | |
| Adenocarcinoma | 33 (15%) | 96 (49%) | 2051 (42%) | 2180 (41%) | |
| Large cell | 27 (12%) | 22 (11%) | 370 (8%) | 419 (8%) | |
| Adenosquamous | 0 (0%) | 8 (4%) | 120 (2%) | 128 (2%) | |
| Miscellanousa | 66 (29%) | 8 (4%) | 60 (1%) | 134 (3%) | |
| Tumour | <10−6 | ||||
| pT1 | 10 (4%)b | 23 (13%) | 1456 (31%) | 1489 (30%) | |
| pT2 | 71 (35%) | 56 (40%) | 2337 (55%) | 2464 (54%) | |
| pT3 | 23 (11%) | 68 (31%) | 912 (13%) | 1003 (14%) | |
| pT4 | 101 (49%) | 50 (17%) | 180 (1%) | 331 (3%) | |
| Nodes | 10−6 | ||||
| pN0 | 51 (23%)c | 74 (43%) | 2864 (59%) | 2989 (56%) | |
| pN1 | 14 (7%) | 26 (15%) | 937 (19%) | 977 (18%) | |
| pN2 | 134 (67%) | 97 (42%) | 1085 (22%) | 1316 (25%) |
ET: exploratory thoracotomy; R2: incomplete resection thoracotomy.
aInclude pathological reports with a diagnosis of primary non-small-cell lung cancer but with no mention of the subtype.
bEighteen patients were not available.
cTwenty-four patients were not available.
The causes of ET and R2 resections are depicted in Table 3 and were not significantly different, except that ET was more frequently related to both T and N major involvements than R2 resection (15 vs 5%, P < 10−3). T4 involvements leading to R2 resections concerned the oesophagus (n = 2), the pulmonary artery at its origin (n = 2), the aorta (n = 2), the left atrium (n = 7), the vertebra and foramen (n = 12), and the superior vena cava (n = 5, in association with N2 involvement).
Table 3:
Causes of exploratory thoracotomy (ET) and incomplete (R2) resections
| ET (n = 223) | R2 (n = 197) | ET + R2 (n = 420) | P-value | |
|---|---|---|---|---|
| T-related | 67 (30%) | 76 (39%) | 143 (34%) | 0.076 |
| N-related | 55 (25%) | 55 (28%) | 110 (26%) | 0.48 |
| T + N-related | 34 (15%) | 10 (5%) | 44 (11%) | 0.00061 |
| Pleura involvement | 51 (23%) | 37 (19%) | 88 (21%) | 0.28 |
| Pulmonary function | 16 (7%) | 19 (10%) | 35 (8%) | 0.21 |
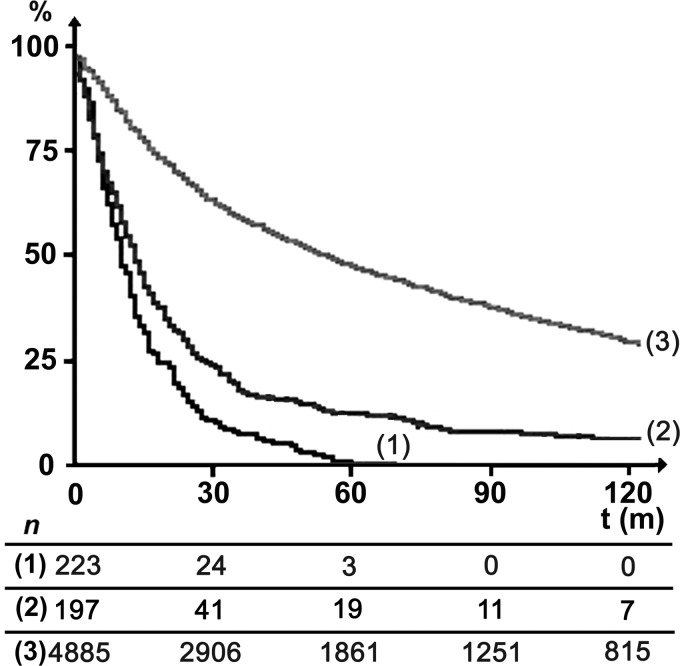
Long-term survival curves are shown in Fig. 1. Five-year survival rates were 1.2, 11.1 and 47.1% for ET, R2 and R0 resections, respectively. The 5-year survival rate was significantly lower with ET compared with R2 resection (P = 10−3). The five-year survival rates concerning ET and R2 according to the cause of those futile operations are shown in Tables 4 and 5, respectively. Five-year survival of ET patients was anecdotic (2.5%), except when the cause was T-involvement and it was during the last decade. Five-year survival rates of R2 patients ranged between 6.8 and 21.1%, except in the case of both T- and N-involvements resulting in no 5-year survivors. When comparing the 5-year survival rates of R2 and ET patients, a significant difference was found in the case of N-involvement (11.1 vs 1.9%, respectively, P = 0.033) and when the patients did not physiologically support the demanding resection (19.7 vs 0%, respectively, P = 0.024).
Figure 1:
Long-term survival of exploratory thoracotomy (curve 1), R2 (curve 2) and R0 (curve 3) resections (P = 10–3).
Table 4:
Survival of exploratory thoracotomy (ET) according to selected characteristics
| Number | Median survival (months) | 5-year survival (%) | 10-year survival (%) | |
|---|---|---|---|---|
| Cause of ET | ||||
| T | 67 | 10 | 2.5 | 2.5 |
| N | 55 | 10 | 1.9 | 0 |
| T + N | 34 | 8 | 0 | 0 |
| Pleura | 51 | 12 | 0 | 0 |
| Miscellaneous | 14 | 10 | 0 | 0 |
| Induction therapy | ||||
| Yes | 39 | 10 | 0 | 0 |
| No | 184 | 10 | 1.5 | 0.8 |
| Period | ||||
| 1980–89 | 97 | 8 | 1.2 | 0 |
| 1990–99 | 89 | 12 | 0 | 0 |
| 2000–09 | 37 | 12 | 5.1 | 0 |
T = T3 and T4 involvement; N = N2 involvement. Difference in survival according to the periods of time is significant (P = 0.013).
Table 5:
Survival of incomplete (R2) resections according to selected characteristics
| Number | Median survival (months) | 5-year survival (%) | 10-year survival (%) | |
|---|---|---|---|---|
| Cause of R2 resection | ||||
| T | 76 | 12 | 10.6 | 8.9 |
| N | 55 | 14 | 11.1 | 2.2 |
| T + N | 10 | 14 | 0 | 0 |
| Pleura | 37 | 13 | 11.2 | 2.8 |
| Miscellaneous | 19 | 23 | 19.7 | 0 |
| Induction therapy | ||||
| Yes | 43 | 15 | 8.5 | 4.2 |
| No | 154 | 13 | 11.5 | 4.6 |
| Period | ||||
| 1980–89 | 35 | 10 | 11.9 | 5.9 |
| 1990–99 | 97 | 13 | 11.1 | 4.5 |
| 2000–09 | 65 | 15 | 10.9 | 3.6 |
T = T3 and T4 involvement; N = N2 involvement. Differences in survival according to the periods of time (P = 0.70) and induction therapy (P = 0. 75) were not significant.
Patients with first-line ET underwent adjuvant treatment including chemotherapy in 46 (21%) cases, radiation therapy in 55 (25%) or a combination of both in 61 (27%). Despite these medical treatments, none of them were long-term survivors. Patients with first-line R2 resection underwent adjuvant therapy including chemotherapy in 29 cases (15%), radiation therapy in 58 (29%) or a combination of both in 29 (15%). Combining R2 resection and adjuvant treatment led to a median survival of 14 months, and a 5-year survival rate of 12.9%.
DISCUSSION
On one hand, this study demonstrates that the rate of ET is decreasing over time, down to 2% of thoracotomies and that this indication was more frequent in squamous cell carcinoma and concomitant large T + N involvements. It also demonstrates that ET resulted in postoperative complications and mortality in 10 and 5% of the cases, respectively, with no long-term survival. On the other hand, R2 resection was observed in 3% of thoracotomies. R2 resection was more frequent in adenocarcinoma and T1–T2 tumours and resulted in postoperative complications and mortality in 20 and 7% of the cases, respectively, with a 5-year survival rate of 11%, up to 15% if adjuvant treatments were performed, and 21% in the last decade.
R2 resections and ET proceed from the same failure to assess the complete resectability of the tumour preoperatively. The recent improvements in medical imaging and preoperative staging explain why the rate of ET has dropped over the last decades [6]. The influence of induction therapy in clinically advanced cases remains questionable, as it alters the preoperative staging [7] and perioperative assessment of surgical margins [8], and may explain the stable rate of R2 resections over time. As multidisciplinary tumour boards have been widely spread over the last decade and are now mandatory before scheduling the surgical resection of an NSCLC, the increased use of induction therapy counterbalances the improvement of medical imaging, resulting in a stable rate of R2 resections, thus arguing for a better assessment of first-line resectability during these meetings.
ET remains a major pitfall of the surgical management of NSCLC. In 1990, the French Society of Thoracic and Cardiovascular Surgery found 2962 ET for lung cancer, representing 11.7% of 25 591 operations performed over 10 years, with a significant decrease in the rate of ET over time [9]. Wada et al. [10] reported the same decrease in a period running from 1976 to 1990. More recently, Steinbaum et al. [11] noticed that more than one half of their ET could have been avoided by the refinement of imaging criteria and a judicious surgical approach to N2 disease. It is commonly accepted that the frequency of ET should be 1% or less [12].
Despite improvements in medical imaging and appropriate clinical staging, one must admit that some patients still undergo ET or R2 resection currently. Beyond surgical series, the survival of NSCLC patients should be considered as a whole from the time of diagnosis. Otherwise, strong selection bias could lead to a dramatic but futile improvement in surgical results, associated with stagnation in the survival of the overall population, as recently suggested by an exhaustive population-based analysis [2]. Therefore, despite the increasing frequency of R0 resection due to better operability assessment, ET and R2 resections still exist in unexpected cases, as witness to an aggressive surgical management.
ET is associated with a high rate of postoperative complication without any long-term survival. This poor prognosis is even worse than that of patients exclusively treated with chemoradiation who benefit from a 5-year survival rate of 20% in Stage IIIA-N2 disease [13]. Aside from medical complications, the psychological role of the absence of resection may be underestimated during the postoperative course. The diagnosis of NSCLC has been associated with a high rate of depression, leading in some cases to patients committing suicide within the first month [14]. This depression has been shown to exist preoperatively and to continue postoperatively [15]. In fact, the postoperative course of ET is often characterized by patients' resignation, tending to helplessness and hopelessness, and suggesting that the absence of resection after an initial hope may contribute to depress the patient. As the links between psychological behaviour, psychiatric disorders and outcome of NSCLC have already been outlined [16], this postoperative resignation may darken the prognosis, and patients undergoing ET should benefit from early palliative care.
It is commonly considered that R2 resection causes unnecessary pain, impairs the quality of life, and postpones subsequent radiation and/or chemotherapy, with no therapeutic advantage when compared with ET, and no 5-year survival [12, 17]. This study may contribute to soften these considerations. Regarding short-term outcome, the postoperative mortality reported here is similar between ET and R2, similar to the postoperative mortality reported by Debevec et al. [18], and slightly higher than those recommended by Ginsberg et al. [19] for R0 resection. Regarding long-term outcome, this study suggests that R2 resection leads to better 5-year survival than ET in every case of T or N uncompleted resection, contradicting previous reports [12, 17]. The only exception is when the uncompleted resection affects both T and N2, resulting in the absence of long-term survival.
The boundaries between R0, R1 and R2 resections can be subtle and depend on small details in the anatomical extension of the tumour and lymph nodes [20–22]. Therefore, when the surgical exploration reveals an unexpected extension of NSCLC, the thoracic surgeon faces a dilemma, between performing the resection and risking R2 resection, and closing the chest and facing an adverse outcome. Our findings suggest that doubtful resection could be advocated when the unexpected extension involves either the tumour or the nodes, whereas ET should be preferred when the unexpected extension affects both the tumour and the nodes.
However, this study has several limitations. The first is its retrospective and non-randomized design. The inclusion of patients with unexpected tumour extension in a prospective trial and their subsequent randomization between doubtful resection vs ET, are hard to conceive from both ethical and practical points of view. In this setting, a large retrospective study may still be informative, as the one-eyed man being king in the kingdom of the blind. The second limitation is the lack of precision in the elements arguing for a doubtful resection or an ET. This ambiguity reflects the reality of surgical management, with some surgeons being reluctant to risk R2 resection, and their counterparts refusing the shadow of an ET. We hope this study will help thoracic surgeons when facing this Hobson's choice.
Conflict of interest: none declared.
REFERENCES
- 1.American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Manual. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, editors. Cancer staging handbook. 7th edn. Chicago: Springer; 2010. pp. 299–323. [Google Scholar]
- 2.van der Drift MA, Karim-Kos HE, Siesling S, Groen HJ, Wouters MW, Coebergh JW, et al. Progress in standard of care therapy and modest survival benefits in the treatment of non-small-cell lung cancer patients in the Netherlands in the last 20 years. J Thorac Oncol. 2012;7:291–8. doi: 10.1097/JTO.0b013e31823a01fb. [DOI] [PubMed] [Google Scholar]
- 3.Oldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC cancer staging project: proposals for the revision of the TNM Stage Groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–23. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski F. SEM (Statistiques, Epidémiologie, Médecine): un outil de gestion informatique et statistique adapté à la recherche en cancérologie. Bull Cancer. 2000;87:715–21. [PubMed] [Google Scholar]
- 6.Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32–9. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg. 2006;131:1229–35. doi: 10.1016/j.jtcvs.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 8.Van Schil P, Hendriks J, De Maeseneer M, Vandenbroeck Ch, Lauwers P. Decision making about operability in non-small cell lung cancer. Acta Chir Belg. 2007;107:495–9. doi: 10.1080/00015458.2007.11680109. [DOI] [PubMed] [Google Scholar]
- 9.Morand G, Roeslin N. Exploratory thoracotomy in lung cancer surgery. Ann Chir: Chir Thorac Cardiovasc. 1990;44:133–7. [PubMed] [Google Scholar]
- 10.Wada H, Tanaka F, Yanagihara K, Ariyasu T, Fukuse T, Yokomise H, et al. Time trends and survival after operations for primary lung cancer from 1976 through 1990. J Thorac Cardiovasc Surg. 1996;112:349–55. doi: 10.1016/S0022-5223(96)70261-1. [DOI] [PubMed] [Google Scholar]
- 11.Steinbaum SS, Uretzky ID, McAdams HP, Torrington KG, Cohen AJ. Exploratory thoracotomy for nonresectable lung cancer. Chest. 1995;107:1058–61. doi: 10.1378/chest.107.4.1058. [DOI] [PubMed] [Google Scholar]
- 12.LoCicero J., III . Surgical treatment of non-small-cell lung cancer. In: Shields TW, LoCicero J III, Reed CE, Feins RH, editors. General Thoracic Surgery. 7th edn. Philadelphia: Lippincott Williams & Wilkins, Wolters Kluwer; 2009. pp. 1387–425. [Google Scholar]
- 13.Albain KS, Swann RS, Rusch VW, Turrisi AT, III, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang F, Fall K, Mittleman MA, Sparén P, Ye W, Adami HO, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366:1310–8. doi: 10.1056/NEJMoa1110307. [DOI] [PubMed] [Google Scholar]
- 15.Oh S, Miyamoto H, Yamazaki A, Fukai R, Shiomi K, Sonobe S, et al. Prospective analysis of depression and psychological distress before and after surgical resection of lung cancer. Gen Thorac Cardiovasc Surg. 2007;55:119–24. doi: 10.1007/s11748-006-0084-4. [DOI] [PubMed] [Google Scholar]
- 16.Pirl WF, Greer JA, Traeger L, Jackson V, Lennes IT, Gallagher ER, et al. Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care. J Clin Oncol. 2012;30:1310–5. doi: 10.1200/JCO.2011.38.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara N, Ohta M, Tanaka K, Ichinose Y, Noge S, Miyazaki K, et al. Assessment of the role of surgery for stage III bronchogenic carcinoma. J Surg Oncol. 1984;25:153–8. doi: 10.1002/jso.2930250304. [DOI] [PubMed] [Google Scholar]
- 18.Debevec L, Erzen J, Debeljak A, Crnjac A, Kovac V. Exploratory thoracotomy and its influence on the survival of patients with lung cancer. Wien Klin Wochenschr. 2006;118:479–84. doi: 10.1007/s00508-006-0638-6. [DOI] [PubMed] [Google Scholar]
- 19.Ginsberg RJ, Hill LD, Eagan RT, Thomas P, Mountain CF, Deslauriers J. Modern thirty-day operative mortality for surgical resection in lung. J Thorac Cardiovasc Surg. 1983;86:654–8. [PubMed] [Google Scholar]
- 20.Riquet M, Achour K, Foucault C, Le Pimpec Barthes F, Dujon A, Cazes A. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann Thorac Surg. 2010;89:870–6. doi: 10.1016/j.athoracsur.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Rami-Porta R, Wittekind C, Goldstraw P for the International Association for the Study of Lung Cancer Staging Committee. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49:25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Rami-Porta R, Mateu-Navarro M, Freixinet J, de la Torre M, Torres-García AJ, Pun YW, et al. Type of resection and prognosis in lung cancer. Experience of a multicentre study. Eur J Cardiothorac Surg. 2005;28:622–8. doi: 10.1016/j.ejcts.2005.06.026. [DOI] [PubMed] [Google Scholar]