Abstract
Aortic valve reconstruction with fixed pericardium may occasionally be very useful when treating children with aortic valve disease. This is because diseased aortic valves in children are sometimes too dysmorphic for simple repair without the addition of material, their annulus may be too small for a prosthesis, and the Ross operation may be precluded due to other congenital anomalies such as pulmonary valvar or coronary malformations. Such reconstruction is usually technically demanding and requires much precision. We describe a simple alternative method, which we have carried out in 3 patients, aged 1 week, 3 years and 12 years, respectively, with good early results.
Keywords: Aortic valve, Aortic valve reconstruction, Aortic valve repair, Aortic valvuloplasty
INTRODUCTION
Aortic valve reconstruction with fixed pericardium is a useful tool, especially in children, for whom the options may be limited [1, 2]. Such a reconstruction is usually technically demanding and requires much precision [3–6]. We describe a simple alternative method.
METHODS
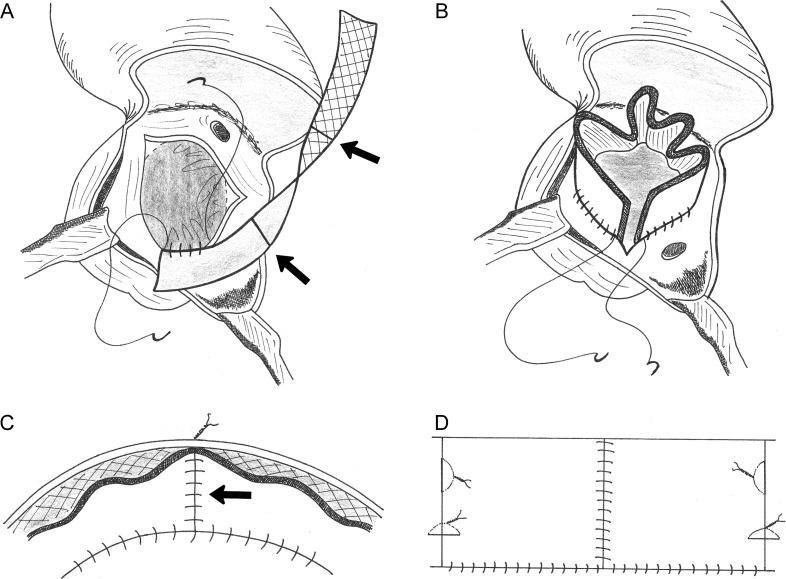
A rectangular patch of fixed autologous pericardium with the following dimensions is needed: width = the diameter of the aortic annulus and length = 4.5 times that diameter. To obtain such a long patch, the full length of the anterior pericardium must be harvested. This is fixed in 0.6% gluteraldehyde for 10 min before cutting it to the desired dimensions. Alternatively, commercially available bovine pericardium may be used. With a marker pen, two transverse lines are drawn across this long rectangular patch such that this is divided into three equal parts (arrows in Fig. 1A).
Figure 1:
Note that the patch is sutured in a horizontal plane: the suture line does not follow the hinge-point of the leaflets of the native aortic valve up to the tip of the commissures.
Through a transverse aortotomy, the native valve is excised. The surgeon must decide where to place the three commissures of the new valve. The exact sites are unimportant and independent of the number and sites of the commissures of the native valve. But the neocommissures should be evenly spaced, without obstructing the coronary arteries. These three sites are marked on the aortic root with a marker pen.
The pericardial patch is sutured to the lower margin of the aortic root, at the level of the nadir of the excised native cusps, with a continuous 6/0 polypropylene suture, with the shiny side of the patch facing upwards (Fig. 1A). The suture line should progress slightly more on the patch than on the aortic root in order to give some volume to the patch and allow for the growth of the annulus. Suturing should start at one end of the patch, connecting it to the site selected for one of the neocommissures. The progress of the suture line should be such that the two previously drawn transverse lines on the patch end on the other two neocommissural sites that were marked earlier on the aortic root.
Once the whole length of the patch is sutured to the entire circumference of the aortic root, the two ends of the patch are finally adjacent to each other at the site of the first neocommissure (Fig. 1B). These two ends are sewn to each other and to the aortic wall, thus forming a pericardial sleeve within the aorta, tethered to the aortic wall. That tethering forms one neocommissure (arrow in Fig. 1C). The other two neocommissures are constructed by attaching the marked sites of the patch to their adjacent aortic wall, each with one high vertical, and another low horizontal, mattress suture with the knot tied outside the aorta (Fig. 1D). The objective of such an orientation of these two tethering sutures is to simulate the triangular shape of the natural commissures.
Lastly, a simple stitch is put at the top of each neocommissure to make sure that the valve closes well at those points (where the pericardium folds back on itself and may not shut completely during diastole). This stitch is passed through the aortic wall and tied outside the aorta (Fig. 2).
Figure 2:
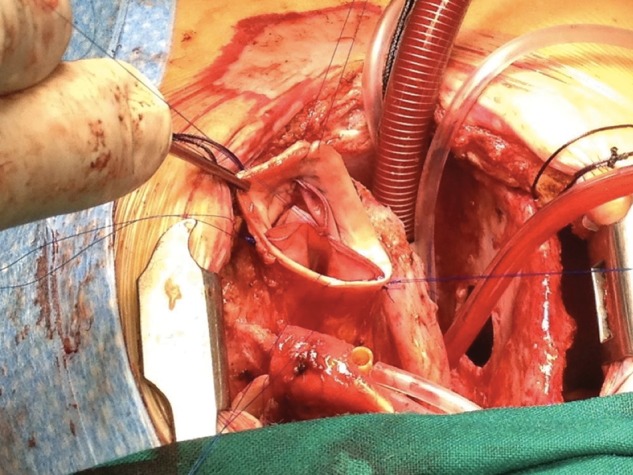
Intraoperative picture showing the final result, with the commissural stitches tied outside the aorta.
Three such reconstructions have been done in our centre, by one surgeon (A.R.H.): 1—a neonate with common arterial trunk (truncus arteriosus) with severe truncal valvar regurgitation. 2—a 12-year old boy with severe mixed aortic valve disease and a significant coronary artery crossing the infundibulum. 3—a 3-year old girl previously operated on for the correction of an aorto-ventricular tunnel, with severe mixed aortic valve disease, intramural right coronary artery, and gross dilatation of the aortic root and ascending aorta that we repaired (plicated) in the same operation. The immediate results were excellent in the first two, and there was mild (Grade I/IV) residual regurgitation in the third case. These remain echocardiographically unchanged at 5-, 3- and 2-month follow-up, respectively, with no stenosis (Supplementary Video 1).
Supplementary Video 1:
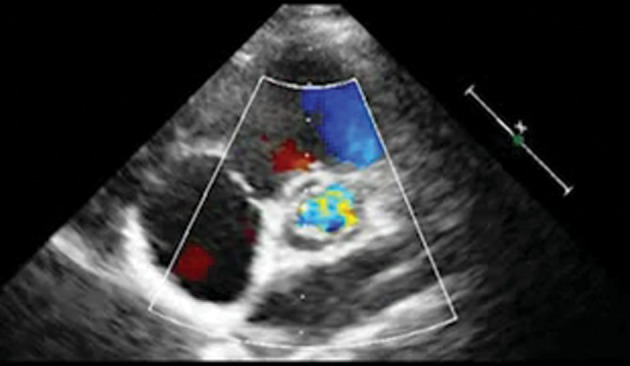
Transthoracic echocardiogram showing a well-functioning neo-aortic valve.
DISCUSSION
Aortic valve surgery in children is problematic. Simple repair without the addition of material would be ideal, but is often inadequate when the valve is severely dysmorphic. Prosthetic valve replacement is also often impossible due to a small annulus. The Ross procedure may also be precluded due to other additional malformations (pulmonary valvar or coronary anomalies). Another option is valvar reconstruction with fixed pericardium [1–6], fascia lata [7] and dura mater [8]. These have produced good early and mid-term, but poor long-term results. Recently, reconstruction with 0.1-mm thick polytetrafluoroethylene (PTFE) membrane has been described with good early results [9]. We were reluctant to use PTFE in view of its friability in response to suturing. However, the authors of the published report did not encounter this problem. We chose fixed pericardium as this is readily available. The methods used for such reconstruction have generally been technically demanding, requiring much precision. In contrast, our technique is simple and independent of the morphology of the native valve and previous repairs. In essence, it consists of first creating a pericardial sleeve within the aortic root, and then fashioning the commissures by tethering that sleeve to the aortic wall along three vertical lines.
This technique, despite its ease and simplicity, has several weaknesses. First, fixed pericardium calcifies earlier than usual during growth such that other similar techniques have proven disappointing in the long term [2, 4, 10]. Secondly, the inflow suture is a continuous 6/0 polypropylene, which has to withstand the whole diastolic stress and may rupture due to fatigue over the years. Thirdly, the reconstruction is a cylindrical concept, which may not always fit the shape of the aortic root depending on the pathology, as seen in our third case. Fourthly, a lot of tissue is placed in an already small aortic root, which may result in a degree of stenosis in some patients, although this did not occur in any of our 3 patients. Overall, we consider this a last-resort technique when there is no other option. In such desperate situations, this technique may be a useful rescue procedure, and buy time for the patient to grow enough for a prosthesis.
Conflict of interest: none declared.
Supplementary Material
SUPPLEMENTARY MATERIAL
REFERENCES
- 1.Duran CM, Gometza B, Kumar N, Gallo R, Martin-duran R. Aortic valve replacement with freehand autologous pericardium. J Thorac Cardiovasc Surg. 1995;110:511–6. doi: 10.1016/S0022-5223(95)70248-2. doi:10.1016/S0022-5223(95)70248-2. [DOI] [PubMed] [Google Scholar]
- 2.Duran CM, Gometza B, Shahid M, Al-Halees Z. Treated bovine and autologous pericardium for aortic valve reconstruction. Ann Thorac Surg. 1998;66(6 Suppl):S166–9. doi: 10.1016/s0003-4975(98)01030-3. doi:10.1016/S0003-4975(98)01030-3. [DOI] [PubMed] [Google Scholar]
- 3.Odim J, Laks H, Allada Y, Child J, Wilson S, Gjertson D. Results of aortic valve sparing and restoration with autologous pericardial leaflet extension in congenital heart disease. Ann Thorac Surg. 2005;80:647–54. doi: 10.1016/j.athoracsur.2005.03.060. doi:10.1016/j.athoracsur.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 4.Alsoufi B, Karamlou T, Bradley T, Williams WG, Van Arsdell GS, Coles JG, et al. Short and midterm results of aortic valve cusp extension in the treatment of children with congenital aortic valve disease. Ann Thorac Surg. 2006;82:1292–9. doi: 10.1016/j.athoracsur.2006.04.039. doi:10.1016/j.athoracsur.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Baird CW, Myers PO, del Nido PJ. Aortic valve reconstruction in the young infants and children. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15:9–19. doi: 10.1053/j.pcsu.2012.01.004. doi:10.1053/j.pcsu.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki S, Kawase I, Yamashita H, Uchida S, Nozawa Y, Matsuyama T, et al. Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interact CardioVasc Thorac Surg. 2011;12:550–3. doi: 10.1510/icvts.2010.253682. doi:10.1510/icvts.2010.253682. [DOI] [PubMed] [Google Scholar]
- 7.Senning A. Fascia lata replacement of aortic valves. J Thorac Cardiovasc Surg. 1967;54:465–70. [PubMed] [Google Scholar]
- 8.Stolf NA, Puig LB, Zerbini EJ. Late results of replacemnt of cardiac valves by dura mater allograft. Int J Artif Organ. 1980;3:104–7. [PubMed] [Google Scholar]
- 9.Nosál M, Poruban R, Valentík P, Sagát M, Nagi AS, Kántorová A. Initial experience with polytetrafluoroethylene leafltet extensions for aortic valve repair. Eur J Cardiothorac Surg. 2012;41:1255–7. doi: 10.1093/ejcts/ezr214. doi:10.1093/ejcts/ezr214. [DOI] [PubMed] [Google Scholar]
- 10.Al-Halees Z, Al-Shahid M, Al-Sanei A, Sallehuddin A, Duran C. Up to 16 years follow-up of aortic reconstruction with pericardium: a stentless readily available cheap valve? Eur J Cardiothorac Surg. 2005;28:200–5. doi: 10.1016/j.ejcts.2005.04.041. doi:10.1016/j.ejcts.2005.04.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.