Abstract
OBJECTIVES
Off-pump coronary artery bypass (OPCAB) surgery is a technically more demanding strategy of myocardial revascularization compared with the standard on-pump technique. Thoracic epidural anaesthesia, by reducing sympathetic stress, may ameliorate the haemodynamic changes occurring during OPCAB surgery. The aim of this randomized controlled trial was to evaluate the impact of thoracic epidural anaesthesia on intraoperative haemodynamics in patients undergoing OPCAB surgery.
METHODS
Two hundred and twenty-six patients were randomized to either general anaesthesia plus epidural (GAE) (n = 109) or general anaesthesia (GA) only (n = 117). Mean arterial blood pressure (MAP), heart rate (HR) and central venous pressure (CVP) were measured before sternotomy and subsequently after positioning the heart for each distal anastomosis.
RESULTS
Both groups were well balanced with respect to baseline characteristics and received a standardized anaesthesia. The MAP decreased in both groups with no significant difference (mean difference (GAE minus GA) −1.11, 95% CI −3.06 to 0.84, P = 0.26). The HR increased in both groups after sternotomy but was significantly less in the GAE group (mean difference (GAE minus GA) −4.29, 95% CI −7.10 to −1.48, P = 0.003). The CVP also increased in both groups after sternotomy, but the difference between the groups varied over time (P = 0.05). A difference was observed at the third anastomosis when the heart was in position for the revascularization of the circumflex artery (mean difference (GAE minus GA) +2.09, 95% CI 0.21–3.96, P = 0.03), but not at other time points. The incidence of new arrhythmias was also significantly lower in the GAE compared with the GA group (OR = 0.41, 95% CI 0.22–0.78, P = 0.01).
CONCLUSION
Thoracic epidural with general anaesthesia minimizes the intraoperative haemodynamic changes that occur during heart positioning and stabilization for distal coronary anastomosis in OPCAB surgery.
Keywords: Haemodynamics, Epidural anaesthesia and off-pump coronary artery surgery
INTRODUCTION
Coronary artery bypass surgery (CABG) via median sternotomy using opioid anaesthesia leads to sympathetic activation by surgical stress causing tachycardia, hypertension and increased oxygen demand and extraction by the myocardium [1]. This response is stabilized by thoracic epidural anaesthesia [2]. The clinical advantages of thoracic epidural anaesthesia in CABG using cardiopulmonary bypass (CPB) are earlier extubation, reduced postoperative pain and confusion as demonstrated in prospective randomized trials [3, 4]. Off-pump coronary artery bypass grafting (OPCAB) has been shown to lead to a reduction of the stress response and myocardial injury [5, 6]. Combining epidural anaesthesia with OPCAB surgery is expected to have a synergistic effect on reducing sympathetic stress. The variations in haemodynamics during heart positioning for OPCAB anastomosis adds complexity to intraoperative management and could have detrimental clinical sequelae. We hypothesized that thoracic epidural anaesthesia, by reducing sympathetic stress, could help stabilize the heart and facilitate the construction of the distal anastomosis, particularly in the posterior and lateral walls.
The aim of this study was to investigate the effects of thoracic epidural anaesthesia on central intraoperative haemodynamics during OPCAB surgery. The primary analysis of this trial has been reported previously [7].
MATERIALS AND METHODS
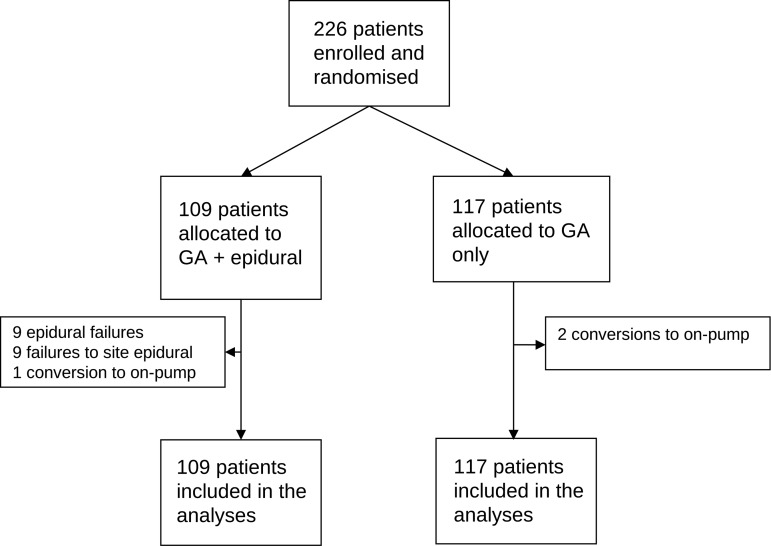
Eligible patients were adults (≥16 years) undergoing primary non-emergency OPCAB surgery without the use of CPB and cardioplegic arrest. Four fulltime OPCAB surgeons participated in the trial. Patients on intravenous heparin, warfarin or clopidogrel at the time of surgery or who suffered from bleeding diathesis were excluded. The study was approved by the Central and South Bristol Research Ethics Committee (registration number E5471), and written informed consent was obtained from all patients. Patients were randomized to receive either general anaesthesia plus epidural (GAE) or general anaesthesia (GA) only. Computer generated randomization was performed with allocations stratified by a consultant team in a 1:1 manner, using blocks of varying size. A flow chart of the allocations and numbers subject to analyses is shown in Fig. 1.
Figure 1:
Flow chart for all patients randomized to study.
Anaesthetic technique
Full details of the anaesthetic technique have been described previously [7]. Briefly, premedication with benzodiazepines, and induction with propofol at 0.5–1 mg/kg combined with fentanyl (10–20 μg/kg) was used in all patients. Anaesthesia was maintained with either isoflurane at 0.8–1.0 minimal anaesthetic concentration or IV propofol 3–4 mg/kg/h, at the discretion of the consultant anaesthetist.
In addition, patients in the GAE group had a thoracic epidural catheter sited in the operating theatre immediately before surgery at the T2–3 or T3–4 inter vertebral space. Bilateral neuraxial block was established from T1 to T10 with an initial bolus of 5 ml bupivacaine 0.5% followed by another 5 ml bolus after 10 min. After induction of GA and when central haemodynamic status was stable, a continuous infusion of 0.125% bupivacaine and 0.0003% clonidine (150 µg in 500 ml) was commenced at an initial rate of 10 ml/h in accordance with the protocol described by Scott et al. [3]. Vasoplegia related to epidural infusion was treated with norepinephrine infusion. These infusions were commenced prior to heart positioning and haemodynamic measurements where needed. Hypotension related to heart positioning was dealt with by repositioning and limited retraction rather than vasopressor or fluid administration.
Surgical technique
The surgical technique and the method of exposure and stabilization for performing anastomoses in patients undergoing OPCAB surgery have been described previously [8, 9]. Briefly, following median sternotomy, the pericardium is not hitched up to allow free movement of the heart. A half-folded swab (12 cm wide and 70 cm long) is snared to the posterior pericardium (using a single 0-silk suture) placed halfway between the inferior vena cava and the left pulmonary vein. Traction is then applied to the end of the snared suture caudally, which lifts the pericardium and the apex of the heart upwards. The left anterior descending (LAD) coronary artery is grafted first to provide protection against ischaemia and myocardial dysfunction that can result from lifting the heart to revascularize the posterior (second anastomosis onto the posterior descending coronary artery) and lateral walls (third anastomosis onto the circumflex coronary artery). All surgeons used 20° Trendelenburg with addition of right lateral tilt for the circumflex grafting. The two limbs of the swab are used to hold the heart in position by securing it to the drapes. We used the Estech Hercules Universal stabilizer (Estech USA, Canal Winchester, OH, USA) to construct our distal anastomoses, aided by intracoronary shunts and a CO2 mister blower.
Haemodynamic measurements
Continuous haemodynamic measurements were available from a peripheral pulse wave saturation monitor, central venous monitoring line and radial arterial monitoring line (Hospira, Lake Forest, IL, USA). The baseline measurements of heart rate (HR), mean arterial pressure (MAP) and central venous pressure (CVP) were recorded prior to sternotomy. Subsequent measurements were taken after the heart was positioned for OPCAB anastomosis prior to performing the arteriotomy. This process was repeated for each subsequent anastomosis. In the event of a second graft to the same territory, we recorded the measurements for both positions. Postoperative patient management was according to unit protocol as previously reported [9].
STATISTICAL METHODS
Continuous variables are summarized using the mean and standard deviation (SD) (or median and interquartile range if the distribution was skewed), and categorical data are summarized as a number and percentage. Serial haemodynamic measurements were modelled using a mixed regression model, adjusted for baseline value, consultant team and number of grafts. Treatment by time interactions were examined, and if statistically significant at the 5% level, the differences in mean response between the two groups were reported separately for each time point, otherwise an overall treatment difference was given. All effect estimates were reported with 95% confidence intervals (CI).
Analyses were carried out on the basis of intention to treat. All analyses were adjusted for consultant team as the randomization was stratified by teams. Centre by treatment and consultant team by treatment interactions were examined and no evidence of differing treatment effects between centres and teams was found. The validity of the assumptions underpinning the models fitted was checked.
RESULTS
Between August 2003 and November 2007, 226 patients were enrolled in the study, 109 were randomly assigned to GAE and 117 to GA. Relevant summary of baseline characteristics are presented in Table 1. The mean ages were 65.9 and 65.5 years in the GAE and GA groups, respectively. Patient characteristics with respect to cardiovascular symptoms, cardiovascular risk factors, left ventricular function and EuroSCORE were similar between the two groups. The mean number of distal anastomoses performed was 2.7 (SD 0.7).
Table 1:
Baseline characteristics (reproduced from reference [7])
| Variable |
Randomized to GAE (n = 109) | Randomized to GA (n = 117) | |
|---|---|---|---|
| Age (years) | Mean (SD) | 65.9 (8.8) | 65.5 (8.6) |
| Males | n (%) | 102 (94) | 102 (87) |
| Body mass index | Mean (SD) | 27.4 (3.5) | 28.2 (4.1) |
| Q-wave myocardial infarction | n (%) | 48 (44) | 57 (49) |
| Diabetes mellitus | n (%) | 22 (20) | 28 (24) |
| Creatinine value (μmol/l) | Mean (SD) | 102 (21.3) | 108 (35.5) |
| Previous CVA/TIA | n (%) | 14 (13) | 12 (10) |
| Peripheral vascular disease | n (%) | 13 (12) | 15 (13) |
| Left ventricle function | Good (>50%) n (%) | 84 (77) | 82 (70) |
| Moderate (30–50%) n (%) | 23 (21) | 29 (25) | |
| Poor (<30%) n (%) | 0 (0) | 3 (3) | |
| Triple vessel coronary disease | n (%) | 78 (72) | 80 (68) |
| Left main stem disease | n (%) | 28 (26) | 36 (31) |
| EuroSCORE | Median (interquartile range) | 3 (2, 4) | 3 (1, 4) |
CVA: cerebral vascular accident; GA: general anaesthesia only; GAE: general anaesthesia plus epidural; TIA: transient ischaemic attack.
HAEMODYNAMIC DATA
The central haemodynamic changes from baseline through various heart positions for OPCAB anastomosis is shown in Table 2. MAP decreased from baseline when the heart was positioned to perform the first anastomosis onto the LAD. The reduction in MAP was higher in the GA group compared with the GAE group, which was consistent throughout all the anastomoses (Fig. 2). However, this difference was not statistically significant (P = 0.26). The resting HR increased between baseline and first anastomosis in response to sternotomy in both groups. In the GAE group, the rise was significantly less and throughout the procedure the increase in HR was consistently and significantly higher in the GA group (P = 0.003, Fig. 3). The right heart-filling pressures as estimated by CVP showed a general increase from baseline with heart positioning in both groups, but the difference in mean CVP between the two groups varied with time (P = 0.05, Fig. 4). The mean CVP was similar in the two groups, except at the third anastomosis of the circumflex coronary artery, when the mean CVP was significantly higher in the GAE group.
Table 2:
Haemodynamic data
| Baseline (prior to sternotomy) | Randomized to GAE (n = 109) |
Randomized to GA (n = 117) |
Treatment difference | P-value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Mean arterial pressure (mmHg) | 92.2 | 16.9 | 93.0 | 16.7 | ||
| Heart rate (beats/min) | 59.8 | 11.6 | 63.2 | 11.3 | ||
| Central venous pressure (mmHg) | 9.20 | 2.96 | 8.57 | 3.31 | ||
| Intraoperative (after application of stabilizer) | Mean | SE | Mean | SE | ||
| Mean arterial pressure (mmHg) | ||||||
| First anastomosis (LAD) | 72.9 | 1.05 | 74.6 | 1.03 | –1.68 | |
| Second anastomosis (PDA) | 75.0 | 0.99 | 75.8 | 1.10 | –0.77 | |
| Third anastomosis (Cx) | 72.8 | 0.98 | 74.2 | 1.50 | –1.44 | |
| Postoperative | 75.4 | 0.90 | 75.9 | 1.01 | –0.54 | |
| Test for treatment × time interaction | 0.91 | |||||
| Overall estimate of treatment effect | –1.11 (–3.06, 0.84) | 0.26 | ||||
| Heart rate (beats/min) | ||||||
| First anastomosis (LAD) | 62.5 | 1.05 | 67.7 | 1.30 | –5.15 | |
| Second anastomosis (PDA) | 63.7 | 1.19 | 68.2 | 1.42 | –4.48 | |
| Third anastomosis (Cx) | 63.9 | 1.12 | 69.1 | 1.63 | –5.21 | |
| Postoperative | 66.5 | 1.13 | 68.8 | 1.05 | –2.32 | |
| Test for treatment × time interaction | 0.29 | |||||
| Overall estimate of treatment effect | –4.29 (–7.10, –1.48) | 0.003 | ||||
| Central venous pressure (mmHg) | ||||||
| First anastomosis (LAD) | 11.8 | 0.49 | 11.0 | 0.48 | 0.77 (–0.56, 2.10) | 0.25 |
| Second anastomosis (PDA) | 14.0 | 0.64 | 13.4 | 0.62 | 0.57 (–1.18, 2.32) | 0.52 |
| Third anastomosis (Cx) | 13.5 | 0.68 | 11.5 | 0.68 | 2.09 (0.21, 3.96) | 0.03 |
| Postoperative | 9.1 | 0.30 | 9.0 | 0.30 | 0.12 (–0.69, 0.94) | 0.76 |
| Test for treatment × time interaction | 0.05 | |||||
LAD: left anterior descending artery; PDA: posterior descending artery; Cx: circumflex coronary artery.
Figure 2:
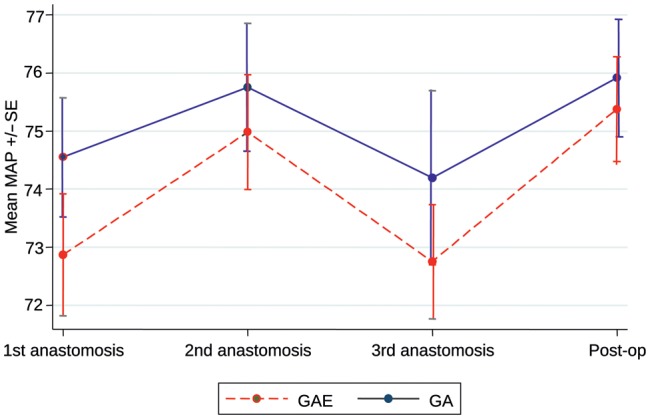
Mean arterial pressure.
Figure 3:
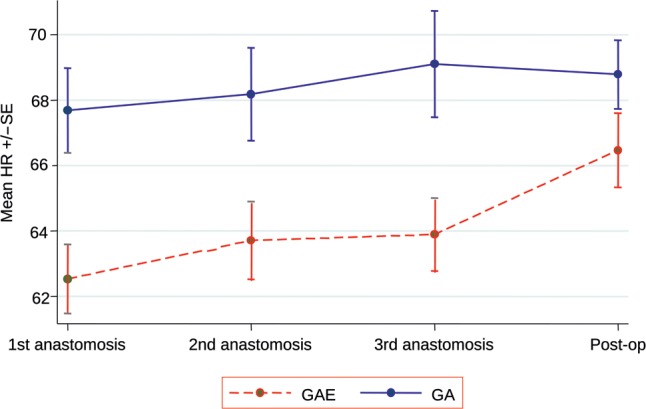
Heart rate.
Figure 4:
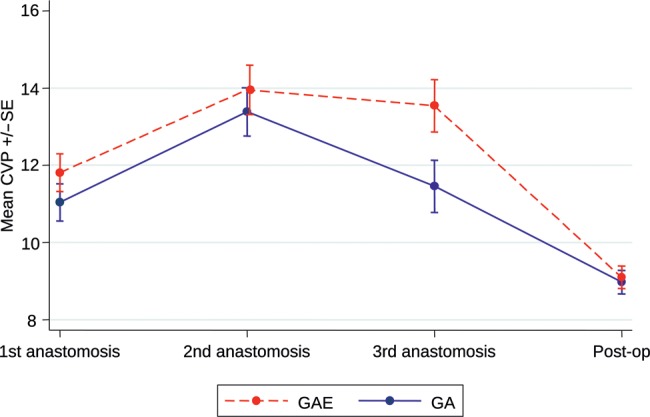
Central venous pressure.
There was 1 death in the GAE group and no mortality in the GA group. The intubation times were significantly shorter in the GAE group (HR = 1.73, 95% CI 1.31–2.27, P < 0.001), as was the in-hospital stay (HR = 1.39, 95% CI 1.06–1.82, P = 0.017). There was no difference in blood loss or infective and neurological complications between groups (P > 0.05 for all). The incidence of new arrhythmias were significantly lower in the GAE, compared with the GA, group (odds ratio (OR) = 0.41, 95% CI 0.22–0.78, P = 0.01), while patients in the GAE group were more likely to require vasoconstrictors intraoperatively than in the GA group (OR = 2.50, 95% CI 1.22–5.12, P = 0.012). The incidence of intraoperative myocardial infarction was higher in the GA group compared with the GAE group, but the difference was not statistically significant (OR = 0.49, 95% CI 0.14–1.73, P = 0.27).
DISCUSSION
The main finding reported here is that thoracic epidural anaesthesia seems to better stabilize the intraoperative haemodynamic during OPCAB surgery performed via a median sternotomy. OPCAB surgery demands more collaboration between the surgical and anaesthetic teams to maintain consistent haemodynamics by a combination of alterations in right heart-filling pressures (Trendelenberg/intravenous filling) and vasoconstrictor use to facilitate the construction of the distal anastomosis. Haemodynamic variability during heart positioning and stabilization may result in inaccuracy of the construction of the coronary anastomosis.
It has been suggested that the technical difficulties in performing the distal anastomosis may be associated with the less optimal mid-term outcomes after OPCAB surgery [10]. Data from our institution and others have shown an increase in HR, decrease in stroke volume and cardiac output and decrease in MAP with the biggest difference occurring during lateral wall revascularization [11, 12]. Improvements in techniques and stabilization devices along with experience has no doubt had significant impact on reducing technical error during construction of anastomosis with excellent long-term results [13]. However, better intraoperative haemodynamic stability with thoracic epidural should offer a further technical advantage, particularly in the early stage of a surgeon's learning curve or when teaching trainees.
Thoracic epidural anaesthesia reduces the sympathetic drive associated with intense surgical stimulation of CABG surgery via median sternotomy [14]. The result is stabilization of the HR throughout the intraoperative course, thus prolonging diastolic filling time and consequently improving coronary perfusion. Large epicardial arteries and coronary arterioles are densely innervated by adrenergic sympathetic nerve fibres, which cause vasoconstriction [15, 16]. Accordingly, improvements in coronary blood flow and increases in myocardial oxygen supply/demand ratio have been demonstrated in clinical studies with thoracic epidural [17, 18].
Another finding of this study previously reported [7] is the reduced incidence of postoperative new arrhythmias with the use of thoracic epidural, which has also been reported by other investigators [19, 20]. Atrial fibrillation (AF) after CABG has been observed in 30–40% of patients and has a significant clinical impact [21, 22]. Atrial ectopics seem to precede postoperative AF in the majority of cases [22]. One of the mechanistic predispositions is a heightened sympathetic tone and vagal rebound [23]. High regional anaesthesia of the first five thoracic segments resulting in blocks in cardiac afferent and efferent fibres and lower catecholamine release has been demonstrated in CABG patients [19, 20, 24, 25]. A consistent effect of thoracic epidural anaesthesia on the cardiovascular system is vasodilatation [14]. This resulted in larger vasoconstrictor requirement in the GAE group. Theoretically, this may jeopardize organ perfusion, but no such deleterious end-organ effects were observed between our study groups.
The main limitation of our study is the use of standard haemodynamic monitoring rather than more invasive cardiac output monitoring, which is not used routinely in our service.
CONCLUSION
The addition of a thoracic epidural to GA helps to stabilize the intraoperative haemodynamic changes that occur during heart positioning for distal coronary anastomosis in OPCAB surgery.
Funding
This research was funded by the British Heart Foundation (2003-2006). £110,529 was awarded to principle investigators Caputo M, Wolf A, Monk C, Angelini GD. Study title: The effects of epidural anaesthesia on inflammatory and stress responses, and clinical outcomes in patients undergoing off pump coronary surgery: a prospective randomised trial.
Conflict of interest: none declared.
REFERENCES
- 1.Heikkila H, Jalonen J, Arola M, Laaksonen V. Haemodynamics and myocardial oxygenation during anaesthesia for coronary artery surgery: comparison between enflurane and high-dose fentanyl anaesthesia. Acta Anaesthesiol Scand. 1985;29:457–64. doi: 10.1111/j.1399-6576.1985.tb02234.x. doi:10.1111/j.1399-6576.1985.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirno K, Friberg P, Grzegorczyk A, Milocco I, Ricksten SE, Lundin S. Thoracic epidural anaesthesia during coronary artery bypass surgery: effects on cardiac sympathetic activity, myocardial blood flow and metabolism, and central hemodynamics. Anesth Analg. 1994;79:1075–81. [PubMed] [Google Scholar]
- 3.Scott NB, Turfrey DJ, Ray DA, Nzewi O, Sutcliffe NP, Lal AB, et al. A prospective randomised study of the potential benefits of thoracic epidural anaesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:528–35. doi: 10.1097/00000539-200109000-00003. doi:10.1097/00000539-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Priestley MC, Cope L, Halliwell R, Gibson P, Chard RB, Skinner M, et al. Thoracic epidural anaesthesia for cardiac surgery: the effects on tracheal intubation time and length of hospital stay. Anesth Analg. 2002;94:275–82. doi: 10.1097/00000539-200202000-00009. table of content. [DOI] [PubMed] [Google Scholar]
- 5.Ascione R, Lloyd CT, Gomes WJ, Caputo M, Bryan AJ, Angelini GD. Beating versus arrested heart revascularisation: evaluation of myocardial function in a prospective randomised study. Eur J Cardiothorac Surg. 1999;15:685–90. doi: 10.1016/s1010-7940(99)00072-x. doi:10.1016/S1010-7940(99)00072-X. [DOI] [PubMed] [Google Scholar]
- 6.Ascione R, Lloyd CT, Underwood MJ, Lotto AA, Pitsis AA, Angelini GD. Inflammatory response after coronary revascularisation with or without cardiopulmonary bypass. Ann Thorac Surg. 2000;69:1198–204. doi: 10.1016/s0003-4975(00)01152-8. doi:10.1016/S0003-4975(00)01152-8. [DOI] [PubMed] [Google Scholar]
- 7.Caputo M, Alwair H, Rogers CA, Pike K, Cohen A, Monk C, et al. Thoracic epidural anaesthesia improves early outcomes in patients undergoing off-pump coronary artery bypass surgery: a prospective, randomised, controlled trial. Anesthesiology. 2011;114:380–90. doi: 10.1097/ALN.0b013e318201f571. doi:10.1097/ALN.0b013e318201f571. [DOI] [PubMed] [Google Scholar]
- 8.Pitsis AA, Angelini GD. Off pump coronary bypass grafting of the circumflex artery. Eur J Cardiothorac Surg. 1999;16:478–9. doi: 10.1016/s1010-7940(99)00292-4. doi:10.1016/S1010-7940(99)00292-4. [DOI] [PubMed] [Google Scholar]
- 9.Angelini GD, Taylor FC, Reeves BC, Ascione R. Early and midterm outcome after off-pump and on-pump surgery in Beating Heart against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet. 2002;359:1194–9. doi: 10.1016/S0140-6736(02)08216-8. doi:10.1016/S0140-6736(02)08216-8. [DOI] [PubMed] [Google Scholar]
- 10.Takagi H, Tanabashi T, Kawai N, Kato T, Umemoto T. Off-pump coronary artery bypass sacrifices graft patency: meta-analysis of randomised trials. J Thorac Cardiovasc Surg. 2007;133:e2–3. doi: 10.1016/j.jtcvs.2006.08.062. doi:10.1016/j.jtcvs.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 11.Watters MP, Ascione R, Ryder IG, Ciulli F, Pitsis AA, Angelini GD. Haemodynamic changes during beating heart coronary surgery with the ‘Bristol Technique’. Eur J Cardiothorac Surg. 2001;19:34–40. doi: 10.1016/s1010-7940(00)00603-5. doi:10.1016/S1010-7940(00)00603-5. [DOI] [PubMed] [Google Scholar]
- 12.Mathison M, Edgerton JR, Horswell JL, Akin JJ, Mack MJ. Analysis of hemodynamic changes during beating heart surgical procedures. Ann Thorac Surg. 2000;70:1355–60. doi: 10.1016/s0003-4975(00)01590-3. doi:10.1016/S0003-4975(00)01590-3. [DOI] [PubMed] [Google Scholar]
- 13.Angelini GD, Culliford L, Smith DK, Hamilton MC, Murphy GJ, Ascione R, et al. Effects of on- and off-pump coronary artery surgery on graft patency, survival, and health-related quality of life: long-term follow-up of 2 randomised controlled trials. J Thorac Cardiovasc Surg. 2009;137:295–303. doi: 10.1016/j.jtcvs.2008.09.046. doi:10.1016/j.jtcvs.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemente A, Carli F. The physiological effects of thoracic epidural anaesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 2008;74:549–63. [PubMed] [Google Scholar]
- 15.Brown BG. Response of normal and diseased epicardial coronary arteries to vasoactive drugs: quantitative arteriographic studies. Am J Cardiol. 1985;56:23E–9E. doi: 10.1016/0002-9149(85)91172-5. doi:10.1016/0002-9149(85)91172-5. [DOI] [PubMed] [Google Scholar]
- 16.Buffington CW. Hemodynamic determinants of ischaemic myocardial dysfunction in the presence of coronary stenosis in dogs. Anesthesiology. 1985;63:651–62. doi: 10.1097/00000542-198512000-00016. doi:10.1097/00000542-198512000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Klassen GA, Bramwell RS, Bromage PR, Zborowska-Sluis DT. Effect of acute sympathectomy by epidural anaesthesia on the canine coronary circulation. Anesthesiology. 1980;52:8–15. doi: 10.1097/00000542-198001000-00003. doi:10.1097/00000542-198001000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Davis RF, DeBoer LW, Maroko PR. Thoracic epidural anaesthesia reduces myocardial infarct size after coronary artery occlusion in dogs. Anesth Analg. 1986;65:711–7. [PubMed] [Google Scholar]
- 19.Gonca S, Kilickan L, Dalcik C, Dalcik H, Bayindir O. The cardioprotective effects of thoracal epidural anesthesia are induced by the expression of vascular endothelial growth factor and inducible nitric oxide synthase in cardiopulmonary bypass surgery. J Cardiovasc Surg (Torino) 2007;48:93–102. [PubMed] [Google Scholar]
- 20.Royse CF. High thoracic epidural anaesthesia for cardiac surgery. Curr Opin Anaesthesiol. 2009;22:84–7. doi: 10.1097/ACO.0b013e32831a40b6. doi:10.1097/ACO.0b013e32831a40b6. [DOI] [PubMed] [Google Scholar]
- 21.Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612–8. doi: 10.1161/CIRCULATIONAHA.108.777789. doi:10.1161/CIRCULATIONAHA.108.777789. [DOI] [PubMed] [Google Scholar]
- 22.Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery. A meta-analysis of randomised control trials. Circulation. 1991;84(5 Suppl):III236–44. [PubMed] [Google Scholar]
- 23.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–8. doi: 10.1016/s0735-1097(03)00955-0. doi:10.1016/S0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 24.Moore CM, Cross MH, Desborough JP, Burrin JM, Macdonald IA, Hall GM. Hormonal effects of thoracic extradural analgesia for cardiac surgery. Br J Anaesth. 1995;75:387–93. doi: 10.1093/bja/75.4.387. doi:10.1093/bja/75.4.387. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarthy M, Nadiminti S, Krishnamurthy J, Thimmannagowda P, Jawali V, Royse CF, et al. Temporary neurologic deficits in patients undergoing cardiac surgery with thoracic epidural supplementation. J Cardiothorac Vasc Anesth. 2004;18:512–20. doi: 10.1053/j.jvca.2004.05.012. doi:10.1053/j.jvca.2004.05.012. [DOI] [PubMed] [Google Scholar]