Abstract
Reluctance to recommend lung cancer surgery for octogenarians is partly based on the expectation that the rate of complications and mortality is higher in this group of patients, and on the impression that the life expectancy of an octogenarian with lung cancer is limited by death from natural causes. Moreover, the belief that radiation therapy and observation yield similar results to surgery in early-stage disease have influenced low resection rates in this population. Nevertheless, advances in surgical techniques, anaesthesia and postoperative care have made surgical lung resection a safer procedure than it was in the past. Judging from the more recent findings, surgery should not be withheld because of postoperative mortality, but suboptimal or palliative treatment may be necessary in patients with poor physical or mental function. To enable informed decision-making, both patients and clinicians need information on the risks of surgical treatment. In this review, available information from the literature was collected in an effort to understand the real benefit of surgical treatment in octogenarians with non-small-cell lung cancer, and to determine what should be done or avoided during the selection course.
Keywords: Lung cancer, Octogenarian, Surgery, Mortality, Survival, Complications
INTRODUCTION
The incidence of non-small-cell lung cancer (NSCLC) in octogenarians is increasing in Western countries. This disease represents the second leading cause of cancer death in this age group, and it is also responsible for a substantial increment in morbidity and health-care costs [1]. Several studies [2, 3] have suggested that age per se should not be considered a risk factor for surgical mortality and morbidity in lung cancer patients and access to surgical treatment should not be denied only on the basis of age. Indeed, advanced age may represent an indicator of several factors such as comorbidity or poor physical performance, which in turn can increase surgical risk and dramatically reduce life expectancy [4]. The management of these patients requires an understanding of the predictable changes in pulmonary physiology occurring with surgery and anaesthesia as well as a knowledge of the factors associated with the development of postsurgical complications. The surgical procedure causes reduction of lung capacity and diaphragm dysfunction, and impairs gas exchange and cough and mucociliary clearance, leading to the development of micro-atelectasis and postoperative hypoxaemia [5]. These modifications are exacerbated in chronic obstructive pulmonary disease [6], as well as in older patients [7], survivors of recent myocardial infarction [8], in cases of starvation [9] and smoking patients [10]. Moreover, the octogenarian patients submitted to lung resection procedures developed severe and frequent postoperative complications, so these patients are frequently submitted to preoperative risk evaluation [11]. Advances in surgical and anaesthetic techniques, combined with a detailed preoperative and sophisticated perioperative assessment, have contributed to an increasing number of octogenarians undergoing a successful surgical treatment of NSCLC.
RATIONALE
Life expectancy represents an important component in surgical decision-making, alongside disease parameters and patient choice. Indeed, the issue of life expectancy can make the difference between patients receiving treatment and being denied it. The justification for major cancer operations in octogenarian patients depends on several factors [12, 13]. First, the life expectancy of the subjects must exceed their projected survival if the neoplasm is not treated or is addressed by non-operative modalities. Second, the operative mortality rate for the group should be sufficiently low that it does not negate or substantially blunt the long-term benefit. The third issue, quality of life, is of paramount concern to elderly people and must not be sacrificed for limited added longevity. A fourth concern is sensible resource utilization, an inescapable issue in the current era of cost containment. Finally, and especially relevant in lung cancer, is the need to define the boundaries of operative treatment in the older age group with respect to tumour stage and extent of resection.
RISK FACTORS
Octogenarians present frequently with several coexistent comorbid conditions [14]. In fact, previous studies have shown that >80% of octogenarians have at least one associated disease and >50% of octogenarians have two or more adverse prognostic comorbidities at the time of lung cancer diagnosis. [15]. Furthermore, comorbidity has been correlated with survival [16] and with postoperative complications [17]. In consideration of the strong relationship between number of comorbid conditions and advanced age, comorbidity may explain the increased mortality risk in octogenarians documented by several studies and confound the association between age and surgical complications. In fact, some authors consider advanced age as a risk factor for mortality and perioperative complications [18, 19], while others disagree with it [20, 21]. Despite early suggestions of an increased risk of pulmonary complications with advanced age, this is not an independent risk factor for pulmonary complications. The healthy status of a patient, mainly cardiorespiratory, seems to be much more important than age [6, 20].
Preoperative risk assessment and evaluation of lung function facilitate the selection of elderly patients who are suitable candidates for pulmonary resection. A detailed assessment based on history, symptoms and signs of chronic lung or heart disease, as well as chest X-ray, electrocardiogram, arterial blood gas analysis, pulmonary function test and biochemical and haematological panel can help in screening potential surgical patients. Preoperative appraisal of pulmonary function factors has been comprehensively studied to forecast morbidity and mortality after pulmonary resection. Many different values have been found to be predictive of pulmonary complications and mortality in patients undergoing lung resection. For example, forced expiratory volume in 1 s (FEV1), forced vital capacity, diffusion capacity of lung for carbon monoxide and postoperative predicted data have all been studied [22]. In the multivariate analysis, patients with percentage-predicted FEV1 <60 were found to experience significantly higher rates of postoperative complications when compared with patients whose percentage-predicted FEV1 was 80 or greater. Moreover, patients with severe chronic obstructive pulmonary disease (COPD) are six times more likely to have a major postoperative complication [23]. Elective surgery should be deferred in patients who are symptomatic, have poor exercise capacity or have acute exacerbation. A partial pressure of carbon dioxide in the arterial blood (PaCO2) of >45 mmHg often occurs in persons with severe COPD and indicates a high risk, although it is not necessarily prohibitive for surgery [24]. However, hypoxaemia was associated with cardiac complications, principally the occurrence of arrhythmias. Anaemic patients had more respiratory and infectious complications, probably due to nutritional deficiencies not identified during the preoperative evaluation. In fact, malnutrition is a risk of morbidity and mortality in critical-care and surgical patients [25], and perioperative blood transfusion was associated with increased postoperative complications [26].
Several scoring systems include associated comorbidities for quantifying surgical risk in lung cancer patients. Harpole et al. [27] included American Society of Anesthesiologists (ASA) physical status in their multi-institutional outcome study of major pulmonary resections. Patients with a preoperative ASA status of 4 had a risk of death more than six times than that of those with an ASA status of 2 [28]. Boffa et al. [29] analysed the risk factors for patients with a lobectomy for lung cancer using the Society of Thoracic Surgeons General Thoracic Surgery Database. They indicated age, ASA score, male sex, Zubrod score, insulin-dependent diabetes, renal dysfunction, induction therapy, percentage-predicted FEV1 and smoking as risk factors. In France, a thoracic surgery scoring system for in-hospital mortality (Thoracoscore) was developed using data obtained from >15 000 patients who were enrolled in a nationally representative thoracic surgery database. Mortality risk factors included in the model were patient age, sex, dyspnoea score, ASA score, performance status, priority of surgery, diagnosis, procedure class and comorbid disease [30]. The model was subsequently validated on 1675 patients from the USA, where a similar accuracy was noted [31]. Pneumonectomy, Zubrod score and ASA class had the highest impact on short-term outcome.
In conclusion, the decisions on surgical indications, the method and the risk quantification for the selected surgical method should be evaluated separately. Functional data may therefore be relatively less important for determining the surgical indications and the optimal treatment method in comparison with comorbidity risk assessment.
SURGERY AND PATIENT SELECTION
In the past, age >80 years was considered a relative contraindication to pulmonary resection, prompting some to advocate non-operative management or a sublobar resection in this age group [32]. However, advances in preoperative and postoperative care and in surgical technique have encouraged many to offer surgical resection to the elderly population. In fact, during the last two decades, numerous studies (Table 1) involving lung resections in the octogenarian have dismissed older accounts of prohibitively high mortality rates, and have suggested that lobectomies, in particular, are safe and effective.
Table 1:
Surgical series in patients older than 80 years with non-small-cell lung cancer
| Reported | Author | No. of years (dates) | No. of patients | Male (%) | Mean age (years) | Preoperative comorbidity (%) | Stage I (%) | MLN evaluation (%) | Lobectomies (%) | Sublobar resections (%) | Pneumonectomy (%) | Complications (%) | Operative mortality (%) | 5-year survival (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | Personal data | 5 (2007–11) | 57 | 70.2 | 82.2 | 38.6 | 51.1 | 57.8 | 61.4 | 15.8 | 8.8 | 33.3 | 1.8 | – |
| 2011 | Fanucchi et al. [38] | 9 (2001–09) | 82 | 76.8 | 81.1 | 23.0 | 52.5 | 20.7 | 76.8 | 22.0 | 0 | 30.4 | 2.4 | 36.0 |
| 2009 | Okami et al. [39] | 1 (1999) | 367 | 63.2 | 82.0 | 27.8 | 60.9 | 34.6 | 66.8 | 33.2 | 0 | 8.4 | 1.4 | 56.1 |
| 2009 | Chida et al. [66] | 26 (1981–2006) | 48 | – | 81.7 | – | 62.5 | 100 | 89.5 | 6.3 | 4.2 | 68.8 | – | 35.0 |
| 2008 | Suemitsu et al. [67] | 26 (1981–2006) | 146 | 58.2 | 82.6 | – | 74.7 | 100 | 54.1 | 37.6 | 0.7 | – | – | 46.8 |
| 2008 | Mun and Kohno [50] | 8 (1999–2006) | 55 | 63.4 | 82.7 | 91.0 | 80.0 | – | 67.3 | 30.9 | 0 | 25.6 | 3.6 | 65.9 |
| 2008 | Bolukbas et al. [68] | 6 (1999–2004) | 47 | – | – | – | 38.3 | 100 | 66.0 | – | 4.3 | 38.2 | 4.3 | 41.0 |
| 2007 | Brokx et al. [69] | 16 (1989–2004) | 124 | 86.3 | 82.0 | – | 64.0 | – | 70.2 | 4.8 | 12.1 | – | 4.0 | 24.0 |
| 2007 | Koizumi et al. [70] | 20 (1982–2001) | 32 | 75.0 | 82.0 | 90.6 | 68.8 | 59.4 | 100 | 0 | 0 | 56.2 | 12.5 | 46.6 |
| 2007 | Hope et al. [71] | 6 (1999–2004) | 20 | 50.0 | 82.1 | – | 50.0 | – | 60.0 | – | 15.0 | 45.0 | 10 | 39.0 |
| 2006 | Dominguez et al. [65] | 20 (1985–2004) | 379 | 65.4 | 82.0 | 33.5 | 67.1 | 93.4 | 63.3 | 28.3 | 6.6 | 48.0 | 6.3 | – |
| 2005 | Matsuoka et al. [72] | 8 (1997–2004) | 40 | 75.0 | 82.0 | 17.5 | 87.5 | 40.0 | 60.0 | 30.0 | 0 | 20.0 | 0 | 56.9 |
| 2005 | McVay et al. [73] | 13 (1992–2004) | 159 | 38.0 | 83.0 | – | 100 | – | 96.0 | 2.0 | 2.0 | 18.0 | 1.8 | – |
| 2004 | Brock et al. [28] | 23 (1980–2002) | 68 | 65.0 | 82.0 | 39.7 | 60.3 | 80.9 | 69.0 | 16.0 | 1.5 | 44.0 | 8.8 | 34.0 |
| 2004 | Port et al. [53] | 14 (1990–2003) | 61 | 41.0 | 82.0 | 36.1 | 44.0 | 100 | 75.4 | 8.2 | 6.5 | 38.0 | 1.6 | 38.0 |
| 2003 | Aoki et al. [74] | 17 (1985–2001) | 49 | 53.1 | 81.2 | 69.4 | 100 | 44.9 | 100 | 0 | 0 | 41.0 | 2.0 | 44.8 |
| 2000 | Aoki et al. [75] | 18 (1981–98) | 35 | 62.9 | 81.4 | 28.6 | 68.6 | 54.3 | 71.4 | 28.6 | 0 | 21.0 | 0 | 39.8 |
| 1999 | Hanagiri et al. [76] | 4 (1992–95) | 18 | – | – | – | 50.0 | – | 66.7 | 27.8 | 0 | 50.0 | 0 | 42.6 |
| 1997 | Pagni et al. [13] | 16 (1980–95) | 54 | 48.2 | 82.0 | 24.0 | 76.0 | – | 79.6 | 5.6 | 1.8 | 42.0 | 3.7 | 43.0 |
| 1994 | Naunheim et al. [77] | 11 (1981–91) | 37 | 55.0 | 82.7 | – | 95.0 | – | 70.2 | 15.0 | 13.5 | 45.0 | 16.0 | 30.0 |
| 1994 | Osaki et al. [62] | 18 (1974–91) | 33 | 75.8 | 82.4 | 67.0 | 48.0 | – | 66.7 | 15.2 | 9.1 | 67.0 | 21.2 | 32.3 |
| 1989 | Shirakusa et al. [78] | 10 (1978–87) | 33 | 78.8 | 82.0 | 45.5 | 54.5 | 100 | 63.6 | 6.1 | 9.1 | 51.0 | 13.0 | 55.0 |
In fact, evidence-based guidelines recommend that patients with lung cancer should not be denied resection on the grounds of age alone [33]. Treatment decisions for a patient with cancer are based on the performance status and stage of cancer. To predict surgical risk, cardiopulmonary function is assessed preoperatively [34, 35]; however, the surgical options are still restricted for elderly patients, who frequently present with multiple diseases.
The extent of pulmonary resection may influence outcomes in octogenarians [36]. The role of sublobar pulmonary resections remains a controversial area for both elderly and nonelderly patients with early-stage lung cancer, though some evidence is emerging, particularly for octogenarians [37, 38]. Okami et al. [39] found no difference in 5-year survival in octogenarians who underwent lobectomy compared with limited resection. However, in a study from the University of Pittsburgh [40], lobectomy was compared with segmentectomy for patients with Stage I NSCLC and segmentectomy was associated with reduced mortality (7.8 vs 2.8%) and improved 3-year survival in a subgroup of 99 octogenarians. So, modified procedures such as wedge resection or segmentectomy are usually recommended in high-risk patients. However, extended segmentectomy for low-risk patients might strike a balance between curability and a low rate of complications. Although specific evidence is lacking, limited surgeries may have a role in octogenarians with limited survival, or possibly for patients with small, peripherally located tumours, who are at significant risk of postoperative complications.
More-extensive surgeries require careful evaluation of the risks and benefits. In octogenarians, pneumonectomy, particularly right sided, is strongly associated with an increased risk of complications as compared with standard lobectomy or limited resection [41, 42]. Mizushima et al. [43] reported that operative mortality after pneumonectomy was 22.2% in patients ≥70 years and significantly differs from that in patients <70 years (3.2%). In fact, bronchoplasty should be performed whenever possible, even in elderly patients, to avoid pneumonectomy [44].
Video-assisted thoracoscopic surgery (VATS) has become popular in the treatment of lung cancer [45, 46]. This minimally invasive procedure is believed to substantially reduce morbidity and mortality in elderly patients. Nagahiro et al. [47] and Yim et al. [48] reported that VATS generates less pain and cytokine production, and offers better preservation of pulmonary function in the early postoperative phase. Recently, Cattaneo et al. [46] have reported that postoperative complications after pulmonary lobectomy in an elderly patient population occurs with a lower frequency with a minimally invasive VATS approach compared with a traditional, rib-spreading thoracotomy (28 vs 45%). This is an important finding because it is well established that operative morbidity and mortality rates for pulmonary resections rise with advancing patient age [49]. Several other authors [45, 46, 50] have reported the efficacy of VATS, which was demonstrated by the lower incidence of morbidity and mortality, and acceptable 5-year survival rate, in octogenarians.
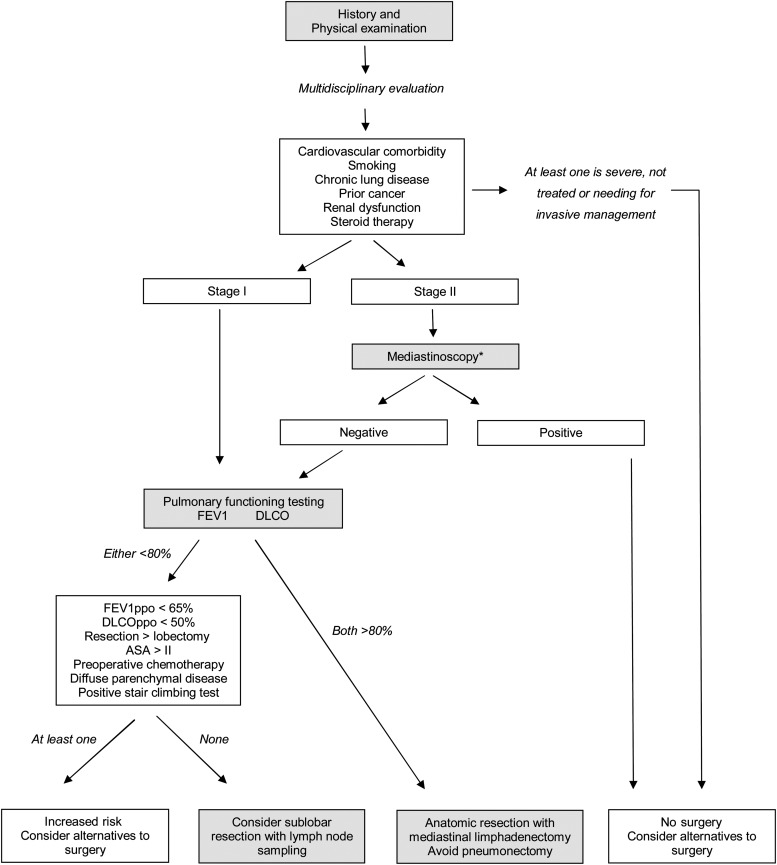
In conclusion, preoperative risk assessment helps in screening potential surgical patients and facilitates the selection of the right surgery for the right patient. Recently, we retrospectively analysed pulmonary resection in the octogenarian population at our institution (personal data not published), focusing on whether age, comorbidity and surgical strategy which influenced the prognosis of elderly patients with lung cancer, to establish the factors that should be considered in the decision to operate on elderly patients. We also formulated a functional decision-algorithm for the surgical management of octogenarian lung cancer patients (Fig. 1).
Figure 1:
Algorithm for functional evaluation of octogenarian patients being considered for surgical resection of lung cancer—based on recommendations relating to assessment of resectability from evidence-based guidelines for lung cancer proposed by the American College of Chest Physicians. Recommendations were adapted to a specific population and mainly based on physiological and comorbidity conditions. Authors describe how to manage patient selection and how to decide what extension of resection should be performed. The asterix indicates that mediastinoscopy is performed only if lung resection is considered; FEV1: forced expiratory volume in 1 second; DLCO: diffusing capacity of the lung for carbon monoxide; ppo: predicted postoperative; ASA: American Society of Anaesthesiologists physical status.
OPERATIVE MORBIDITY AND MORTALITY
Postoperative mortality after lung resection in octogenarians ranges from 0 to 21% depending on the type of surgery and selection of patients, with a long-term (5-year) survival ranging from 24 to 66% (Table 1). The major causes of death within 30 days have been pneumonia and cardiac complications [51, 52]. Possible reasons for the low perioperative mortality in some series are strict patient selection, improvements in surgical and anaesthetic technique and attempts to avoid pneumonectomy whenever possible [53]. Pneumonectomy is associated with a high incidence of complications, such as supraventricular tachyarrhythmias and respiratory complications (pneumonia, respiratory failure, empyema), and the rate of occurrence of these adverse events is higher for older than younger patients. In addition, in consideration of the lower cardiac and respiratory reserve consistent with physiological aging (not to mention the higher number of comorbidities), in older adults these complications turn out to be fatal in a higher proportion of cases [54]. Pulmonary complications caused by increased bronchial secretion and difficulty in expectoration directly increase mortality, making the prevention of such complications essential. Wound pain and drainage tubes cause elderly patients to restrict their movement and suppress coughing, increasing the risk of coexisting illness after operation. Pain control is mandatory after thoracotomy, particularly in elderly patients [55]. Postoperative pulmonary care including epidural analgesia, bronchoscopy, early ambulation and physical therapy should be extremely aggressive and early. In addition, patients should be instructed not to smoke and to perform deep respirations before operation. The impact of smoking cessation on perioperative outcome has been a matter of considerable debate [56, 57]. The beneficial effects of smoking cessation, including improvement in ciliary and small airway function and a decrease in sputum production, occur gradually over several weeks. The risk is highest in patients who were smoking within the last 2 months, and patients who had quit smoking for >6 months have a risk similar to those who do not smoke [21]. The ACCP recommends that all patients with lung cancer be counselled regarding smoking cessation [33]. ERS/ESTS guidelines recommend smoking cessation for at least 2–4 weeks before surgery, because this may change perioperative smoking behaviour and decrease the risk of postoperative complications [58]. Pulmonary rehabilitation in the perioperative period has been shown to improve measures of activity tolerance, allowing resection of marginal candidates, and improving functional outcomes after resection [59]. The ERS/ESTS guidelines state that early pre- and postoperative rehabilitation may produce functional benefits in resectable lung cancer patients [58].
Therefore, it should not be a surprise that guidelines recommend that patients with Stage I disease should be considered for surgical treatment regardless of age and underline the need for a careful assessment of comorbid conditions preoperatively [34]. Anyway, not all comorbidities have the same impact on survival and a careful selection of patients, based on the kind of comorbidities (in particular cardiovascular and respiratory diseases), more than their number, can be recommended as part of routine preoperative evaluation [60]. It is unclear whether operative morbidity increases with very advanced age, and there is no convincing evidence that patients of this age with Stage I disease have worse prognosis than younger patients. Unfortunately, there are no reports relating specifically to Stage II and III disease, and several studies have shown that in this age group, pneumonectomy is associated with a higher mortality risk. Therefore, in octogenarians, surgery for Stage II and III disease, pneumonectomy and extended resections should be considered only in exceptional cases.
QUALITY OF LIFE
Patient-centred outcomes are gaining importance in orienting health-care management. The focus of health-care providers and the public is gradually shifting from early postoperative end-points (such as morbidity and mortality) to long-term outcomes, such as survival, residual function and quality of life (QoL) [61]. For decades, surgeons’ attitude in evaluating surgical success has focused mainly on minimizing the risk of postoperative complications and death. Fortunately, in the most recent years, this trend has changed, and there is now a greater attention both to what patients really fear about their surgical experience and to the price they are willing to pay for increasing their chance of cure. Many of them are willing to accept even postoperative cardiopulmonary complications, but less so long-term functional disability [62]. Pompili et al. [61] found that a considerable proportion of patients experience a large decline in physical and emotional components of their QoL compared with their preoperative status. Furthermore, compared with the general population, nearly half of the patients displayed a depressed physical and emotional status 3 months after surgery. Patients with better preoperative physical functioning and bodily pain perception (less symptomatic) and those with worse mental health are those at higher risk of experiencing a large physical decline. The risk of perceived emotional decline was found to be greater in patients with lower preoperative FEV1 and higher preoperative social functioning and mental health scores. In general, these findings confirm that patients reporting a better preoperative physical fitness, but with more compromised mental/emotional status, are those more prone to experience severe deterioration of their physical condition. Little is known about the impact of surgical treatment on the quality of life in this age group. When treating patients whose life expectancy is obviously reduced, such as octogenarians, assuring a better quality of life is sometimes more important than trying to ameliorate survival. This should be a subject of future research.
SURVIVAL
Previous series reported 5-year survival rates ranging from 24 to 66% (Table 1). This wide range is likely due to a varying degree of three well-known confounding factors: (i) selection bias; (ii) small sample sizes in each series (range 18–379) and (iii) incompleteness of follow-up.
Even with Stage I disease, however, octogenarians have a lower 5-year survival than published data for younger patients [63, 64]. A lower 5-year survival rate in octogenarians is expected because these patients have a lower life expectancy than younger patients. In the Brock et al. [28] cohort, the 5-year survival was also significantly different between Stages Ia and Ib (61 vs 10%). The cumulative evidence suggests that accurate preoperative clinical staging is imperative, and implies a need for more liberal use of sensitive imaging modalities, such as PET scans, to stage octogenarians [13]. When analysing outcomes by extent of resection, the best 5-year survival is achieved in patients undergoing lobectomy or bilobectomy. Sublobar resections are associated with a poor 5-year survival [65]. Here again, a selection bias may be an important confounding factor. In retrospective series, it is likely that limited resections were a surrogate for patients with more severe pre-existing respiratory compromise, leading to less-extensive pulmonary resections. Those undergoing pneumonectomy also have a poorer long-term survival. This likely demonstrates the effect due to both the extent of disease requiring a pneumonectomy for complete resection and the limited ability of these elderly patients to withstand, over the long term, the loss of pulmonary function involved in a pneumonectomy.
CONCLUSION
In conclusion, octogenarians should not be denied surgery solely due to age because properly selected ≥80-year olds with NSCLC can be resected safely with acceptable long-term survival. When surgeons have to take the controversial decision of whether to offer resection to octogenarians, they should base their choice first on the stage of the disease, and then on an accurate assessment of the general clinical conditions, rather than on pulmonary function alone. Surgery offers the best chance of cure for older patients with early stages lung cancer, and mortality rates have reached acceptable levels. Based on the need of a multidisciplinary assessment to identify comorbidities and operative risk, a close collaboration among pneumologists, radiologists, oncologists, thoracic surgeons, anaesthesiologists, cardiologists, geriatric specialists and physical therapists is highly recommended. A concise and standardized preoperative evaluation can provide a suitable and safe postoperative prediction of complications in patients submitted to lung resection, and patients with COPD, hypoxaemic, older, anaemic patients, and in need of pneumonectomy must be classified as high-risk for developing postoperative complications.
Conflict of interest: none declared.
REFERENCES
- 1.Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–7. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, D'Arco C, Fusco D, Bernabei R. Preoperative assessment and risk factors in the surgical treatment of lung cancer: the role of age. Rays. 2004;29:407–11. [PubMed] [Google Scholar]
- 3.Nugent WC, Edney MT, Hammerness PG, Dain BJ, Maurer LH, Rigas JR. Non-small cell lung cancer at the extremes of age: impact on diagnosis and treatment. Ann Thorac Surg. 1997;63:193–7. doi: 10.1016/s0003-4975(96)00745-x. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard EM, Arnaoutakis K, Hesketh PJ. Lung cancer in octogenarians. J Thorac Oncol. 2010;5:909–16. doi: 10.1097/jto.0b013e3181d89b48. [DOI] [PubMed] [Google Scholar]
- 5.Wright CD, Gaissert HA, Grab JD, ÓBrien SM, Peterson ED, Allen MS. Predictors of prolongated length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg. 2008;85:1857–65. doi: 10.1016/j.athoracsur.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Brunelli A. Risk assessment for pulmonary resection. Semin Thorac Cardiovasc Surg. 2010;22:2–13. doi: 10.1053/j.semtcvs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Mazzone P. Preoperative evaluation of the lung resection candidate. Cleve Clin J Med. 2012;79:S17–22. doi: 10.3949/ccjm.79.s2.04. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson MK, Celauro AD, Vigneswaran WT. Validation of a modified scoring system for cardiovascular risk associated with major lung resection. Eur J Cardiothorac Surg. 2012;41:598–602. doi: 10.1093/ejcts/ezr081. [DOI] [PubMed] [Google Scholar]
- 9.Antoun S, Merad M, Raynard B, Ruffie P. Evaluating the nutritional status of a lung cancer patient is an important element in patient management. Rev Pneumol Clin. 2008;64:92–8. doi: 10.1016/j.pneumo.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Zaman M, Bilal H, Mahmood S, Tang A. Does getting smokers to stop smoking before lung resections reduce their risk? Interact CardioVasc Thorac Surg. 2012;14:320–3. doi: 10.1093/icvts/ivr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bapoje SR, Whitaker JF, Schulz T, Chu ES, Albert RK. Preoperative evaluation of the patient with pulmonary disease. Chest. 2007;132:1637–45. doi: 10.1378/chest.07-0347. [DOI] [PubMed] [Google Scholar]
- 12.Pagni S, McKelvey A, Riordan C, Federico JA, Ponn RB. Pulmonary resection for malignancy in the elderly: is age still a risk factor? Eur J Cardiothorac Surg. 1998;14:40–4. doi: 10.1016/s1010-7940(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 13.Pagni S, Federico JA, Ponn RB. Pulmonary resection for lung cancer in octogenarians. Ann Thorac Surg. 1997;63:785–9. doi: 10.1016/s0003-4975(96)01150-2. [DOI] [PubMed] [Google Scholar]
- 14.Satoh H, Kurishima K, Nakamura R, Ishikawa H, Kagohashi K, Ohara G, et al. Lung cancer in patients aged 80 years and over. Lung Cancer. 2009;65:112–8. doi: 10.1016/j.lungcan.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004;57:597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Battafarano RJ, Piccirillo JF, Meyers BF, Hsu HS, Guthrie TJ, Cooper JD, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123:280–7. doi: 10.1067/mtc.2002.119338. [DOI] [PubMed] [Google Scholar]
- 17.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–62. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Winter H, Meimarakis G, Pirker M, Spelsberg F, Kopp R, Rüttinger D, et al. Predictors of general complications after video-assisted thoracoscopic surgical procedures. Surg Endosc. 2008;22:640–5. doi: 10.1007/s00464-007-9428-0. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest. 1993;104:1445–51. doi: 10.1378/chest.104.5.1445. [DOI] [PubMed] [Google Scholar]
- 20.Vaporciyan AA, Merriman KW, Ece F, Roth JA, Smythe WR, Swisher SG, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg. 2002;73:420–5. doi: 10.1016/s0003-4975(01)03443-9. [DOI] [PubMed] [Google Scholar]
- 21.Wyser C, Stulz P, Solèr M, Tamm M, Müller-Brand J, Habicht J, et al. Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med. 1999;159:1450–6. doi: 10.1164/ajrccm.159.5.9809107. [DOI] [PubMed] [Google Scholar]
- 22.Kearney DJ, Lee TH, Reilly JJ, DeCamp MM, Sugarbaker DJ. Assessment of operative risk in patients undergoing lung resection: importance of predicted pulmonary function. Chest. 1994;105:753–9. doi: 10.1378/chest.105.3.753. [DOI] [PubMed] [Google Scholar]
- 23.Licker MJ, Widikker I, Robert J. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81:1830–7. doi: 10.1016/j.athoracsur.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 24.Busch E, Verazin G, Antkowiak JG, Driscoll D, Takita H. Pulmonary complications in patients undergoing thoracotomy for lung carcinoma. Chest. 1994;105:760–6. doi: 10.1378/chest.105.3.760. [DOI] [PubMed] [Google Scholar]
- 25.Morice RC, Peters EJ, Ryan MB, Putnam JB, Ali MK, Roth JA. Exercise testing in the evaluation of patients at high risk for complications from lung resection. Chest. 1992;101:356–61. doi: 10.1378/chest.101.2.356. [DOI] [PubMed] [Google Scholar]
- 26.Weber RS, Jabbour N, Martin RC., II Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol. 2008;15:34–45. doi: 10.1245/s10434-007-9502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harpole DH, Jr, DeCamp MM, Jr, Daley J, Hur K, Oprian CA, Henderson WG, et al. Prognostic models of thirty-day, mortality and morbidity after major pulmonary resection. J Thorac Cardiovasc Surg. 1999;117:969–79. doi: 10.1016/S0022-5223(99)70378-8. [DOI] [PubMed] [Google Scholar]
- 28.Brock MV, Kim MP, Hooker CM, Alberg AJ, Jordan MM, Roig CM, et al. Pulmonary resection in octogenarians with stage I nonsmall cell lung cancer: a 22-year experience. Ann Thorac Surg. 2004;77:271–7. doi: 10.1016/s0003-4975(03)01470-x. [DOI] [PubMed] [Google Scholar]
- 29.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 30.Falcoz PE, Conti M, Brouchet L, Chocron S, Puyraveau M, Mercier M, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg. 2007;133:325–32. doi: 10.1016/j.jtcvs.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Chamogeorgakis TP, Connery CP, Bhora F, Nabong A, Toumpoulis IK. Thoracoscore predicts midterm mortality in patients undergoing thoracic surgery. J Thorac Cardiovasc Surg. 2007;134:883–7. doi: 10.1016/j.jtcvs.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Breyer RH, Zippe C, Pharr WF, Jensik RJ, Kittle CF, Faber LP. Thoracotomy in patients over age seventy years: ten-year experience. J Thorac Cardiovasc Surg. 1981;81:187–93. [PubMed] [Google Scholar]
- 33.Colice GL, Shafazand S, Griffin JP, Keenan R, Bollinger CT. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edn) Chest. 2007;132:161S–77S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 34.Nagamatsu Y, Iwasaki Y, Kashihara M, Nishi T, Yoshiyama K, Yamana H, et al. Selection of pulmonary resection procedures to reduce postoperative complications in 200 patients. Surg Today. 2011;41:780–6. doi: 10.1007/s00595-010-4350-9. [DOI] [PubMed] [Google Scholar]
- 35.Sekine Y, Suzuki H, Nakajima T, Yasufuku K, Yoshida S. Risk quantification for pulmonary complications after lung cancer surgery. Surg Today. 2010;40:1027–33. doi: 10.1007/s00595-009-4182-7. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez-Ventura A, Cassivi SD, Allen MS, Wigle DA, Nichols FC, Pairolero PC, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Eur J Cardiothorac Surg. 2007;32:370–4. doi: 10.1016/j.ejcts.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Audisio RA, Zbar AP, Jaklitsch MT. Surgical management of oncogeriatric patients. J Clin Oncol. 2007;25:1924–9. doi: 10.1200/JCO.2006.10.2533. [DOI] [PubMed] [Google Scholar]
- 38.Fanucchi O, Ambrogi MC, Dini P, Lucchi M, Melfi F, Davini F, et al. Surgical treatment of non-small cell lung cancer in octogenarians. Interact CardioVasc Thorac Surg. 2011;12:749–53. doi: 10.1510/icvts.2010.259002. [DOI] [PubMed] [Google Scholar]
- 39.Okami J, Higashiyama M, Asamura H, Goya T, Koshiishi Y, Sohara Y, et al. Japanese Joint Committee of Lung Cancer Registry. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer. J Thorac Oncol. 2009;4:1247–53. doi: 10.1097/JTO.0b013e3181ae285d. [DOI] [PubMed] [Google Scholar]
- 40.Tomaszek SC, Kim Y, Cassivi SD, Jensen MR, Shen KH, Nichols FC, et al. Bronchial resection margin length and clinical outcome in non-small cell lung cancer. Eur J Cardiothorac Surg. 2011;40:1151–6. doi: 10.1016/j.ejcts.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 41.Okada M, Yoshikawa K, Hatta T, Tsubota N. Is extended segmentectomy an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71:956–60. doi: 10.1016/s0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa K, Tsubota N, Kodama K, Ayabe H, Taki T, Mori T. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg. 2002;73:1055–8. doi: 10.1016/s0003-4975(01)03466-x. [DOI] [PubMed] [Google Scholar]
- 43.Mizushima Y, Noto H, Sugiyama S, Kusajima Y, Yamashita R, Kashii T, et al. Survival and prognosis after pneumonectomy for lung cancer in elderly. Ann Thorac Surg. 1997;64:193–8. doi: 10.1016/s0003-4975(97)82827-5. [DOI] [PubMed] [Google Scholar]
- 44.Okada M, Yamagishi H, Satake S, Matsuoka H, Miyamoto Y, Yoshimura M, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. Thorac Cardiovasc Surg. 2000;119:814–9. doi: 10.1016/S0022-5223(00)70018-3. [DOI] [PubMed] [Google Scholar]
- 45.Igai H, Takahashi M, Ohata K, Yamashina A, Matsuoka T, Kameyama K, et al. Surgical treatment for non-small cell lung cancer in octogenarians—the usefulness of video-assisted thoracic surgery. Interact CardioVasc Thorac Surg. 2009;9:274–7. doi: 10.1510/icvts.2008.199455. [DOI] [PubMed] [Google Scholar]
- 46.Cattaneo SM, Park BJ, Wilton AS, Seshan VE, Bains MS, Downey RJ, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–5. doi: 10.1016/j.athoracsur.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 47.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–5. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 48.Yim Ap, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg. 2000;70:243–7. doi: 10.1016/s0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- 49.Damhuis RA, Schutte PR. Resection rates and postoperative mortality in 7899 patients with lung cancer. Eur Resp J. 1996;9:7–10. doi: 10.1183/09031936.96.09010007. [DOI] [PubMed] [Google Scholar]
- 50.Mun M, Kohno T. Video-assisted thoracic surgery for clinical stage I lung cancer in octogenarians. Ann Thorac Surg. 2008;85:406–11. doi: 10.1016/j.athoracsur.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 51.British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osaki T, Shirakusa T, Kodate M, Nakanishi R, Mitsudomi T, Ueda H. Surgical treatment of lung cancer in the octogenarian. Ann Thorac Surg. 1994;57:188–92. doi: 10.1016/0003-4975(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 53.Port JL, Kent M, Korst RJ, Lee PC, Levin MA, Flieder D, et al. Surgical resection for lung cancer in the octogenarian. Chest. 2004;126:733–8. doi: 10.1378/chest.126.3.733. [DOI] [PubMed] [Google Scholar]
- 54.Ploeg AJ, Kappetein AP, van Tongeren RB, Pahlplatz PV, Kastelein GW, Breslau PJ. Factors associated with perioperative complications and long-term results after pulmonary resection for primary carcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:26–9. doi: 10.1016/s1010-7940(02)00655-3. [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka H, Tsubota N, Yoshimura M, Kubota M, Murotani A. Epidural fentanyl infusion for pain relief after thoracotomy. Jpn J Chest Surg. 1991;5:706–11. [Google Scholar]
- 56.Barrera R, Shi W, Amar D, Thaler HT, Gabovich N, Bains MS, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest. 2005;127:1977–83. doi: 10.1378/chest.127.6.1977. [DOI] [PubMed] [Google Scholar]
- 57.Mason DP, Subramanian S, Nowicki ER, Grab JD, Murthy SC, Rice TW, et al. Impact of smoking cessation before resection of lung cancer. A Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg. 2009;88:362–71. doi: 10.1016/j.athoracsur.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 58.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. European Respiratory Society and European Society of Thoracic Surgeons joint task force on fitness for radical therapy. ERS/ESTS clinical guidelines on fi tness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 59.Cesario A, Ferri L, Galetta D, Cardaci V, Biscione G, Pasqua F, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57:118–9. doi: 10.1016/j.lungcan.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Ambrogi V, Pompeo E, Elia S, Pistolese GR, Mineo TC. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small-cell lung cancer. Eur J Cardiothorac Surg. 2003;23:811–7. doi: 10.1016/s1010-7940(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 61.Pompili C, Brunelli A, Xiumé F, Refai M, Salati M, Sabbatini A. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardiothorac Surg. 2011;39:732–7. doi: 10.1016/j.ejcts.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 62.Cykert S, Kissling G, Hansen CJ. Patient preferences regarding possible outcomes of lung resection. What outcomes should preoperative evaluations target? Chest. 2000;117:1551–9. doi: 10.1378/chest.117.6.1551. [DOI] [PubMed] [Google Scholar]
- 63.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 64.Naruke T, Goya T, Tsuchiya R, Suemasu K. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96:440–7. [PubMed] [Google Scholar]
- 65.Dominguez-Ventura A, Allen MS, Cassivi SD, Nichols FC, III, Deschamps C, Pairolero PC. Lung cancer in octogenarians: factor affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg. 2006;82:1175–9. doi: 10.1016/j.athoracsur.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 66.Chida M, Minowa M, Karube Y, Eba S, Okada Y, Miyoshi S, et al. Worsened long-term outcomes and postoperative complications in octogenarians with lung cancer following mediastinal lymph-node dissection. Interact CardioVasc Thorac Surg. 2009;8:89–92. doi: 10.1510/icvts.2008.193383. [DOI] [PubMed] [Google Scholar]
- 67.Suemitsu R, Yamaguchi M, Takeo S, Ondo K, Ueda H, Yoshino I, et al. Favorable surgical results for patients with nonsmall cell lung cancer over 80 years old: a multicenter survey. Ann Thorac Cardiovasc Surg. 2008;14:154–60. [PubMed] [Google Scholar]
- 68.Bölükbas S, Beqiri S, Bergmann T, Trainer S, Fisseler-Eckhoff A, Schirren J. Pulmonary resection of non small cell lung cancer: is survival in the elderly not affected by tumor stage after complete resection? Thorac Cardiovasc Surg. 2008;56:476–81. doi: 10.1055/s-2008-1038963. [DOI] [PubMed] [Google Scholar]
- 69.Brokx HA, Visser O, Postmus PE, Paul MA. Surgical treatment for octogenarians with lung cancer: results from a population-based series of 124 patients. J Thorac Oncol. 2007;2:1013–7. doi: 10.1097/JTO.0b013e3181559fdf. [DOI] [PubMed] [Google Scholar]
- 70.Koizumi K, Haraguchi S, Hirata T, Hirai K, Mikami I, Fukushima M, et al. Lobectomy by video-assisted thoracic surgery for lung cancer patients aged 80 years or more. Ann Thorac Cardiovasc Surg. 2003;9:14–21. [PubMed] [Google Scholar]
- 71.Hope WW, Bolton WD, Kalbaugh CA, Blackhurst DW, Stephenson JE, Taylor SM. Lung cancer resection in octogenarians: a reasonable approach for our aging population. Am Surg. 2007;73:22–4. [PubMed] [Google Scholar]
- 72.Matsuoka H, Okada M, Sakamoto T, Tsubota N. Complications and outcomes after pulmonary resection for cancer in patients 80 to 89 years of age. Eur J Cardiothorac Surg. 2005;28:380–3. doi: 10.1016/j.ejcts.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 73.McVay CL, Pickens A, Fuller C, Houck W, McKenna R., Jr VATS anatomic pulmonary resection in octogenarians. Am Surg. 2005;71:791–3. [PubMed] [Google Scholar]
- 74.Aoki T, Tsuchida M, Watanabe T, Hashimoto T, Koike T, Hirono T, et al. Surgical strategy for clinical stage I non-small cell lung cancer in octogenarians. Eur J Cardiothorac Surg. 2003;23:446–50. doi: 10.1016/s1010-7940(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 75.Aoki T, Yamato Y, Tsuchida M, Watanabe T, Hayashi J, Hirono T. Pulmonary complications after surgical treatment of lung cancer in octogenarians. Eur J Cardiothorac Surg. 2000;18:662–5. doi: 10.1016/s1010-7940(00)00573-x. [DOI] [PubMed] [Google Scholar]
- 76.Hanagiri T, Muranaka H, Hashimoto M, Nagashima A, Yasumoto K. Results of surgical treatment of lung cancer in octogenarians. Lung Cancer. 1999;23:129–33. doi: 10.1016/s0169-5002(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 77.Naunheim KS, Kesler KA, D'Orazio SA, Fiore AC, Judd DR. Lung cancer surgery in the octogenarian. Eur J Cardiothorac Surg. 1994;8:453–6. doi: 10.1016/1010-7940(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 78.Shirakusa T, Tsutsui M, Iriki N, Matsuba K, Saito T, Minoda S, et al. Results of resection for bronchogenic carcinoma in patients over the age of 80. Thorax. 1989;44:189–91. doi: 10.1136/thx.44.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]