Abstract
OBJECTIVES
Aortic replacement is based on the aortic diameter in the absence of dissection or connective tissue diseases. Frequently, a number of different aortic-to-prosthetic anastomotic positions are possible depending on patient factors and surgeon preferences. High stress on residual aortic tissue may result in aneurysm formation or aneurysmal dilatation. Utilizing a computational fluid dynamic evaluation, we aimed to define possible optimal operative interventions with regard to the extent of aortic replacement.
METHODS
For proof of principle, a computational fluid dynamic (CFD) analysis, using Fluent 6.2 (Ansys UK Ltd, Sheffield, UK), was performed on a simplified ascending arch and descending aortic geometry. Wall shear stress in three dimensions was assessed for the standard operations: ascending aortic replacement, arch replacement and proximal descending aortic replacement.
RESULTS
Hermiarch replacement is superior to isolated ascending aortic replacement with regard to residual stress analysis on tissues (up to a 10-fold reduction). Aortic arch replacement with island implantation of the supra-aortic vessels may potentially result in high stress on the residual aorta (10-fold increase). Aortic arch replacement with individual supra-aortic vessel implantation may result in areas of high stress (10-fold increase) on native vessels if an inadequate length of supra-aortic tissue is not resected, regardless of it being aneurysmal.
CONCLUSIONS
Computational fluid dynamic evaluation, which will have to be patient-specific, 3D anatomical and physiological, potentially has enormous implications for operative strategy in aortic replacement surgery. CFD analysis may direct the replacement of normal-diameter aortas in the future.
Keywords: Aortic, Computational fluid dynamics, Arch
INTRODUCTION
Replacing aortic tissue from a proximal normal area to a distal normal area for an aneurysmal or dissected portion of aorta remains the cornerstone of modern aortic surgery [1–3]. This concept is extended in Marfan’s syndrome when the technique of aortic exclusion is used to remove all remnants of aortic tissue, particularly in the region of the aortic sinuses [4].
Computational fluid dynamics (CFD) is a technique to simulate, without the need to perform laboratory testing, the fluid flow, stresses and strains in the wall of the containing vessel, and has been previously described in aortic surgery [5–7].
We utilized three-dimensional CFD analysis to estimate the wall stresses in the remnant of aortic tissue left behind at the site of aortic-graft anastomosis after aortic replacement. Leaving behind aortic tissue that is subjected to high wall stress may result in subsequent dissection and/or aneurysmal formation [8, 9].
METHODS
Computational fluid dynamic model
A computer tomography (CT) scan was used to obtain a 3D geometric anatomy of a typical aortic arch to create a mesh for the CFD analysis (Fig. 1). The following flows were utilized: ascending aorta, 5 l/min, arch, descending aorta, 3.1 l/min, innominate artery, 1 l/min, left carotid, 0.75 l/min and left subclavian, 0.15 l/min, as determined via the use of MRI angiography [10].
Figure 1:
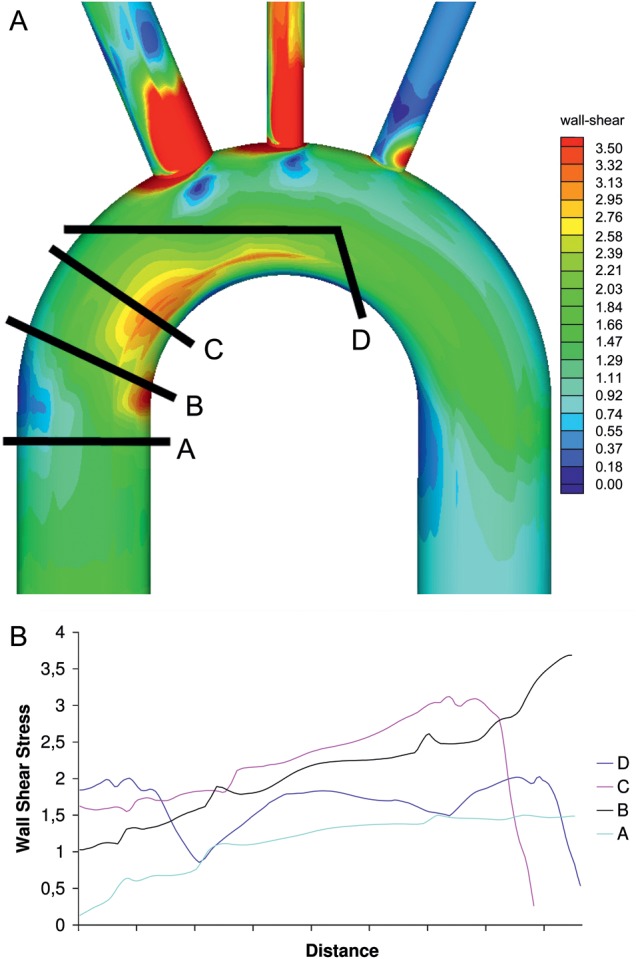
(A) Four possible prosthetic aorta anastomotic sites for replacement of the ascending aorta, (B) wall shear stress at the above anastomotic sites.
CFD is a means of simulating fluid flow by solving the governing equations of fluid motion: the Navier–Stokes equations. These equations are essentially Newton's second law (i.e. force = mass × acceleration) but applied to a fluid element. To define the geometry, a volume mesh was constructed of ∼600 000 cells using the software Gambit (Ansys UK Ltd, Sheffield, UK). The CFD programme used was Fluent 6.2 (Ansys UK Ltd, Sheffield, UK). The fluid had a density of 1050 kg/m3 and viscosity of 0.0035 Pa's to represent blood. The walls of the models were assumed to be rigid as has been previously described [11, 12]. Each model was solved using steady flow into the ascending aorta to produce an initial solution. The results of CFD calculations are presented using Tecplot 9.0 (Tecplot, Inc. Bellevue, WA, USA).
Aortic scenarios
Three typical aortic scenarios were analysed, ascending aortic replacement, arch replacement and proximal descending aortic replacement. The effect of the aortic resection site on the stresses in the residual aorta and at the site of graft aortic anastomosis was studied. The aortic root was excluded from analysis.
Physiological variation
Typical flows in the ascending aorta, arch, descending aorta, innominate artery, left carotid and left subclavian arteries were determined via the use of MRI angiography in 9 patients undergoing scans for non-aneurysmal disease (spinal surgery).
RESULTS
Ascending aortic replacement
Four possible prosthetic aorta anastomotic sites were analysed (Fig. 1a). The wall stresses at the four sites are diagrammatically shown in Fig. 1b. It can be seen that a high wall stress point exists in the distal ascending aorta on the inner curvature. The most proximal resection relative to the aortic valve causes the least graft prosthesis-aortic shear stress, however an area of high stress remains within the remnant aorta. The root was not analysed due to the added complexity of valve and coronary, flow and motion.
Arch replacement
Three different prosthetic aorta anastomotic sites were analysed (Fig. 2a). The wall stresses at the three sites are diagrammatically shown in Fig. 2b. It can be seen that separate head and neck vessel reimplantation with a suitable length of vessel resection results in the lowest residual wall stress in the remnant aorta.
Figure 2:
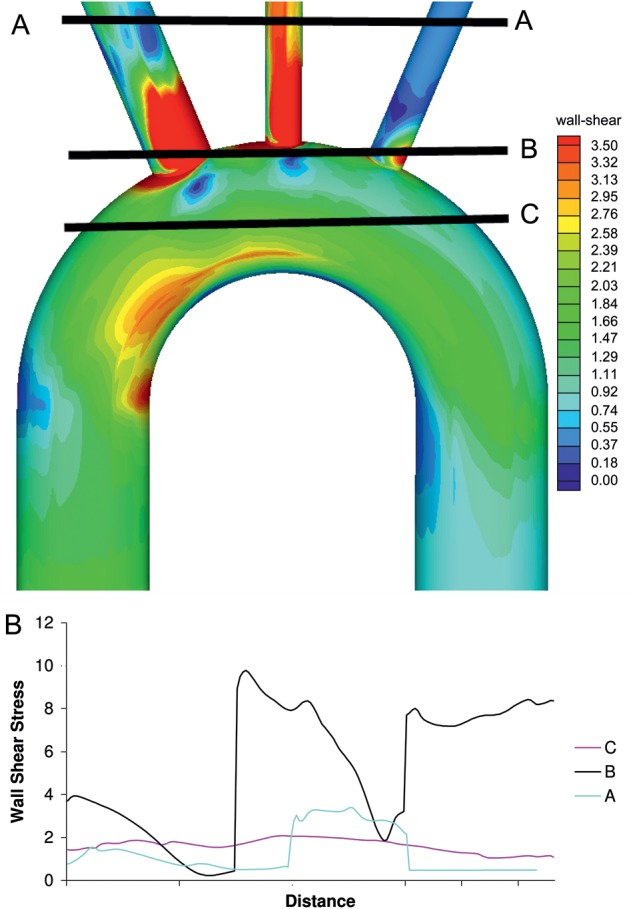
(A) Three possible prosthetic aorta anastomotic sites for replacement of the aortic arch, (B) wall shear stress at the above anastomotic sites.
Proximal descending aortic replacement
Three different prosthetic aorta anastomotic sites were analysed (Fig. 3a). The wall stresses at the three sites are diagrammatically shown in Fig. 3b. It can be seen that resection of aortic tissue at the origin of the left subclavian artery is necessary to reduce residual areas of high stress on the native aorta.
Figure 3:
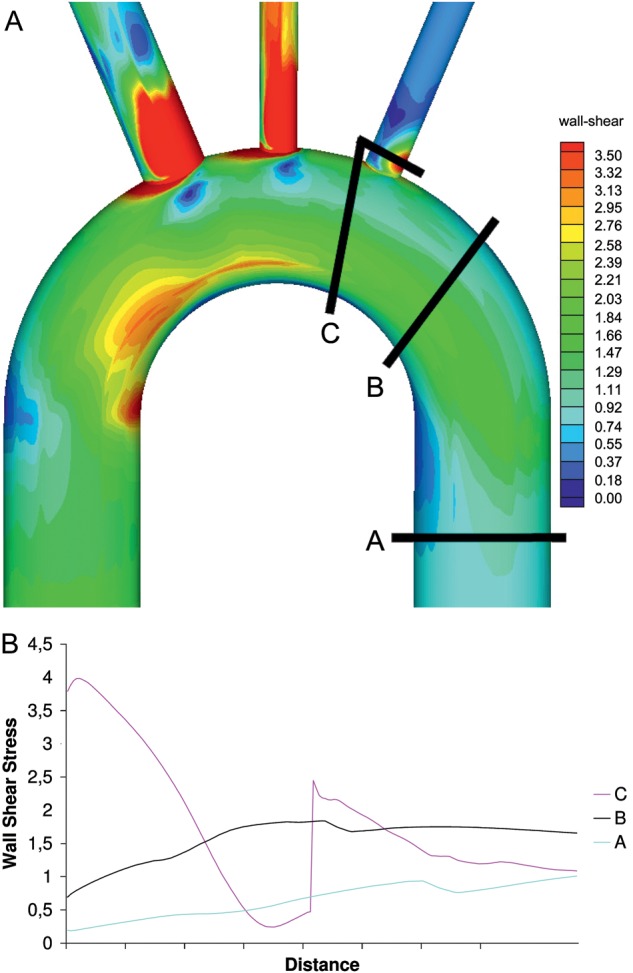
(A) Three possible prosthetic aorta anastomotic sites for replacement of the proximal descending aorta, (B) wall shear stress at the above anastomotic sites.
Physiological variation
Figure 4 demonstrates the large variation in flow rates obtained from MRI scanning. Wall stress is proportional to the dynamic viscosity, the flow velocity parallel to the vessel wall and inversely to the distance to the wall [13]. This means the analysis above only holds for the flow rates stipulated in the methods section (velocity = flow in artery/cross sectional area of artery). These results demonstrate why patient physiology and 3D anatomy need to be analysed together, as models based on anatomy are only fundamentally flawed from an engineering point of view. The difference in flows between MRI patients was statistically significant, ascending aorta, P < 0.0001, innominate artery, P = 0.01, left subclavian artery, P = 0.0002 and proximal descending aorta, P < 0.0001 (one sample t-test).
Figure 4:
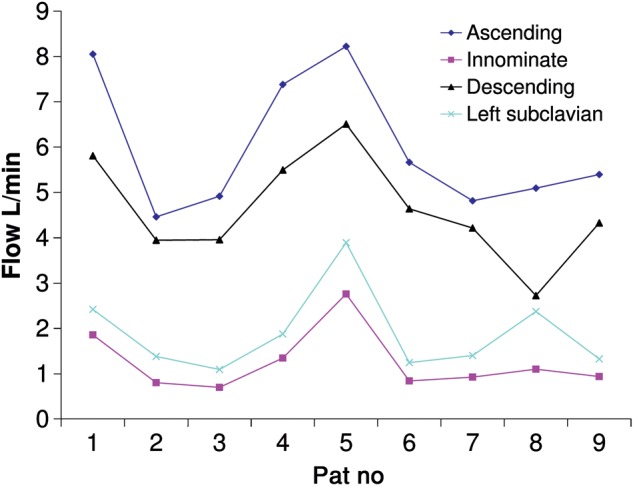
MRI derived flow in ascending aorta, innominate, left carotid and left subclavian artery in nine non-aortic individuals.
DISCUSSION
CFD analysis predicts that the stress at the prosthetic graft aortic anastomosis and in the residual aortic tissue depends on the extent of the surgical technique utilized. Physiological variation in flow rates in the absence of aneurysmal disease means that predictive modelling should be based on an individual’s 3D anatomy and physiology. Currently, aortic replacement is based on size criteria. However, as is currently practised in connective tissue disorders such as Marfan syndrome, replacement of normal-diameter sections of aorta in the aortic root may be necessary, the extent of which can only be predicted by CFD analysis.
Aortic exclusion is routinely utilized in patients with connective tissue disorders such as Marfan’s syndrome and Ehlers Danlos syndrome [4]. Removal of all remnant aortic tissue is not always possible or feasible in procedures that involve the aortic arch and its branches, however CFD analysis will potentially allow patient-directed aortic replacement.
We have made no attempt to accurately model the 3D anatomy of a human aorta as we present this paper as a concept. Even a simplistic model demonstrates that the surgical approach utilized has major implications from a biomechanical point of view. An engineering analysis should never override clinical/surgical acumen for a given patient, however, in patients who are fit enough to undergo potentially more-extensive surgery, resection of just the aneurysmal or dissected segment of aorta CFD may aid treatment and potentially long-term survival.
With regard to ascending aortic replacement, CFD analysis predicts that the proximal inner curve of the aortic arch is a wall-stress hot spot. This confirms that hemiarch replacement in Marfan’s syndrome is the optimal procedure to avoid high-stress areas on remnant aortic tissue. To date, this point is contentious in the surgical literature [14, 15].
Head and neck vessel management in arch replacement can be performed via island reimplantation or individual vessel anastomosis [16, 17]. Island reimplantation is associated with aneurysm formation in some patients with connective tissue disorders [18]. With regard to individual vessel reimplantation, the length of vessel to resect is unknown. CFD analysis predicts that island reimplantation and individual vessel reimplantation flush with the outer arch of the aorta will result in areas of high stress remaining on remnant aortic tissue.
Proximal descending aortic replacement should involve the origin of the left subclavian artery to avoid the risk of a dissection just distal to its origin. The length of subclavian artery to resect will depend on the native left subclavian flow, which is highly variable. Unfortunately, surgery on the left subclavian artery is complicated by its involvement in the spinal cord collateral network [19].
Our analysis assumes a rigid aortic wall. In the case of Dacron replacement, this is a reasonable assumption [20], and implies that the results for prosthetic-aorta anastosmosis are accurate. The results for wall stress in the remnant aortic tissue will be less accurate due to aortic compliance, however, assuming a rigid wall is common during CFD analysis of the aorta in the literature [5, 7]. Analysis accounting for aortic compliance is possible, but the errors due to the variation in aortic compliance between individuals (which are usually unknown) are probably higher than the increased accuracy that would result.
The demonstration of a large variation in aortic and supra-aortic vessel blood flow rates identifies that patient-specific physiology needs to be analysed in conjunction with the 3D anatomy. The failure to appreciate the importance of the combined physiological and anatomical combination is widespread in the literature [21, 22]. MRI scanning is the only clinically practical method to obtain flow rates in the supra-aortic vessels.
CFD analysis may help direct surgical strategy, as randomized trials are virtually impossible in aortic surgery due to the small numbers involved, and the potentially large number of confounding factors.
No cut-off exists for wall stress and aneurysms and dissection formation, however it is commonly accepted that the higher the value, the more likely it is that an aortic pathology, dissection or aneurysm may be initiated [23].
CT scanning and 3D reconstruction remain common preoperative investigations in elective aortic surgery patients [24]. MRI is frequently utilized in patients undergoing serial monitoring to reduce radiation exposure. CT scanning is unable to measure blood-vessel flow rates, however, with a vascular package, MRI is able to measure flow if the appropriate sequences are performed when the aorta is scanned [10]. Relative flows in the aorta and head and neck vessels will not alter after surgery as the grafts have negligible resistance compared with the distal capillary resistance of the organ bed that they feed (Poiseuille law).
The use of wall shear stress in isolation is too simplistic as a solution. Wall shear stress has less effect on wall rupture as the diameter decreases due to Laplace’s Law. This explains the hot spots in the head and neck vessels after CFD analysis, though clinically they are rarely the site of a primary dissection entry tear.
As blood flows from the aortic root, through the arch and into the descending aorta, the flow patterns in the preceding section influence the flow patterns in the following section. Thus an ascending aorta that is dilated or elongated will influence the distal flow patterns and sites of maximal wall stress distally. Analysis thus would have to include the hypothesized operative intervention or lack of it, as an intervention may alter the distribution and magnitude of distal wall stresses. We speculate this may help explain aortic rupture in patients after the first stage of the elephant trunk waiting for the second stage.
Computational fluid dynamic evaluation, which will have to be patient-specific, 3D anatomical and physiological, potentially has enormous implications for operative strategy in aortic replacement surgery. CFD analysis may direct replacement of normal-diameter aorta in the future.
Limitations
Patient blood pressure and rate of change of blood pressure (dP/dt) are important factors with regard to aortic dissection initiation and progression [25]. We have not taken these into account. Blood pressure and dP/dt control, however, are well-known medical management issues in aortic patients.
Turbulent non-Newtonian flow is complex to model. The assumption of non-turbulent flow and Newtonian behaviour of blood is frequently made. The location of high stress areas does not need to be accurate to the exact millimetre, as the surgeon just needs to know roughly the extent of the resection.
We have assumed in this concept paper that flow through the aortic valve is not jet like due to a stenotic valve or a bicuspid valve, as these are not present post procedure. In clinical practice, modelling would have to include the effect of a mechanical or tissue valve replacement, with the complex flow streams associated with them, and cardiac arrhythmias such as atrial fibrillation.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr M. Grabenwoger (Vienna, Austria): This would be very interesting for surgeons if they know that to replace or not to replace the ascending aorta is dependent on the type of fluid dynamics of the ascending aorta. For example, if you have a bicuspid aortic valve and we know that the fluid dynamics in the ascending aorta are dependent on the aortic valve, or is there an influence of the diameter of the ascending aorta on the shear stress, where, of course, there should be a strong correlation. In your study you investigated only on the model of a regular ascending aorta and you did not investigate the influence of the aortic valve. Is this true?
Dr Poullis: Yes. We deliberately avoided the aortic root for a number of reasons. Firstly, the 3D conformation of the valve opening and the torsional flow coming out of the aorta, a random angle, and the curvature on the ascending aorta are very complex to model. We presented this as a concept. That is why I only talked about the distal ascending aorta. We are just presenting this as a principle. The 3D anatomy and the physiology of flow are important. You need to put the two together. And instead of coming up with some quite complicated root scenario, particularly as the root is almost half sorted, if you have Marfan syndrome, you replace the whole root. There is a big debate. If you have Marfan syndrome, how much distal ascending aorta and how much proximal arch do you replace? Based on this, if you can find the hot spots, you maybe should be replacing them. There was a paper earlier on in this meeting on ‘do you do island reimplantation or do you do a branch graft for the head and neck vessels?’. Again, if you have a Marfan's and you have great big high stress areas, maybe you need to be removing those. Again, it is just a concept. You can see that it is a very, very simple model we have done. Even this, you cannot do this on a normal computer. It had to be done on the mainframe at the university because of the three-dimensional component. You can do 2D on a laptop; you cannot do 3D because of the power needed.
Dr Grabenwoger: As you said, in the beginning, you are a surgeon. Have these results of this investigational work already influenced your daily habit in the OR because you know now where the hot spots are?
Dr Poullis: Well, I have a slight conflict of interest here. I am not actually an aortic surgeon. I am a consultant cardiothoracic surgeon. I used to be an engineer. I have a research project with Rob Poole, who is an engineer. I am presenting this as a concept to people.
Dr Grabenwoger: The translation of this concept in the daily surgical routine would be of great interest if you can say to a surgeon in advance, ‘Please take care; he has a hot spot area here. You have to be very careful. You have to replace it or you can leave it.’
Dr Poullis: Yes, yes. You can only do this in an elective setting. These algorithms take a couple of hours to run, so you couldn't do it on a dissection or something silly that turns up in the middle of the night. It would have to be elective cases only. Also, you have to have MRI scanning. The CT will give you the 3D geometry just as an MRI will, but it won't give you flow data. There is also a ramification, and I'll expand slightly. When you do your MRI and get the flows in the head and neck vessels, they are highly variable, and actually this could be quite important for when you start doing selective cerebral perfusion on bypass. There is a publication that is coming out in JECT soon based on the same type of data we have. The flows are random, and at the moment, you just use a fixed flow rate.
Dr M. Zakkar (London, United Kingdom): When you say high is good and low is bad, in terms of shear stress, what do you mean by high is good and low is bad? We know that if you have a certain amount of high shear stress, it is actually good and it can cause anti-inflammation, while if you have low shear stress, it can contribute to inflammation and apoptosis.
Dr Poullis: Yes. That is slightly different. It is way more complicated than the wall shear stress. Again, I was educated by the engineer. He said there are about three different types of wall stress, and it is actually way more complicated than this. Again, that is where the literature gets confused. You are right, in apoptosis, atherosclerosis wall shear stress is the other way around, but in terms of tubes rupturing, high wall stress is bad, but wall stress is only one component of it and it is a three-dimensional concept, which is not what we have shown here because of the complexity of it. Certainly when engineers get involved, people like me don't fully understand this. But red is bad for surgeries. This is a surgical message.
Dr G. Soppa (London, United Kingdom): For me this is a fantastic idea and concept. I can see where you are going and where you want to take this. Now, just going back to the review on the flow in the aorta published by Professor Yacoub and Dr Cohn from the Brigham in Circulation in the early part of this decade, in that they showed that the actual vortex created by the torsion of the ventricle actually creates a torsional flow in the ascending aorta and the arch, and that is one of the main reasons why the stress is reduced. So if you do not have the root and that vortex kind of motion, I think all these could be flawed, because you are looking at cylindrical flow.
Dr Poullis: You are completely right. That's why I said this is a concept. You can make this as complicated as you want to, to get it as close to the real thing. This is just a concept. At the moment, how much aorta do you replace? Surgeon A always does an open distal anastomosis and Surgeon B doesn't when you have normal diameter, because the whole of aortic surgery, in the absence of dissection, is focused on diameter, and as a non-aortic surgeon, as a previous engineer, I think that is potentially wrong.
Dr Soppa: I can see that this is a very good concept. It is similar to using PET scan for looking at a heat map in the ascending aorta. Sure, things like this would have a great impact in the future, but I think right now it is probably limited by not using the flow from the ascending aorta because that actually is protected.
Dr Poullis: Yes, yes. You can actually get the 3D flows off an MRI scan. So, again, all of the information is there and it's all measured on all of these patients. The way I got this data here was actually from people. They collect the data but they don't put it on the PACS system. You just ask them for it. All the cardiac outputs and the flows are all there in the background. It's just not written down on the report.
Dr G. Laufer (Vienna, Austria): This is a very interesting study. I have a very simple question for you. You showed this one example being the left carotid in a complete red colour, so that would mean we have to replace the whole left carotid because the shear stress is so high. How do you explain that?
Dr Poullis: I asked the engineer that as well, because my understanding was, do you have to replace it up to the brain or something, and he said no, no, no. The wall shear stress is different depending on the diameter of the tube, apparently, and the bigger diameter tubes are more affected by the same wall stress. When you have a smaller diameter tube, you can have a higher wall stress and it won't rupture because of the rule of Laplace. I'm virtually repeating that answer of the engineer word for word, because it was beyond my brain. But what it does mean is that you can't leave the little red bit on the arch of the aorta. You have to go up the vessel. So you can't do an island implantation on it if you believe this is the case.
Dr S. Vachev (Penza, Russian Federation): You showed the measurement of wall shear stress. You showed dynamic viscosity.
Dr Poullis: Yes.
Dr Vachev: How can you measure this parameter?
Dr Poullis: There are a number of ways. The way Rob did it, he got out the literature on previous publications. But I'm presenting just the concept. The numbers on these graphs are just a concept here. The bottom line is the graph there - flow and diameter, where the flow is the physiology and the diameter is the 3D anatomy. It is the two together that you need. This is just a concept. Don't get hung up. The dynamic viscosity actually varies. I didn't tell you about the other bit. This is modelled as a Newtonian fluid. Blood is not Newtonian. When I asked the engineer, who does actually do non-Newtonian analysis, he said ‘yes’, but that will take the mainframe a week to do. It can do it and they do do this modelling, but he said this is so close and it is just a concept anyway, so just do it as Newtonian. It gets more and more and more complicated as you go along. It is just a concept.
REFERENCES
- 1.de la Cruz KI, Coselli JS, LeMaire SA. Open aortic arch replacement: a technical odyssey. J Extra Corpor Technol. 2012;44:42–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Spielvogel D, Strauch JT, Minanov OP, Lansman SL, Griepp RB. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg. 2002;74:S1810–4. doi: 10.1016/s0003-4975(02)04156-5. [DOI] [PubMed] [Google Scholar]
- 3.Sundt TM, III, Orszulak TA, Cook DJ, Schaff HV. Improving results of open arch replacement. Ann Thorac Surg. 2008;86:787–96. doi: 10.1016/j.athoracsur.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Shahcheraghi N, Dwyer HA, Cheer AY, Barakat AI, Rutaganira T. Unsteady and three-dimensional simulation of blood flow in the human aortic arch. J Biomech Eng. 2002;124:378–87. doi: 10.1115/1.1487357. [DOI] [PubMed] [Google Scholar]
- 6.Tse KM, Chiu P, Lee HP, Ho P. Investigation of hemodynamics in the development of dissecting aneurysm within patient-specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J Biomech. 2011;44:827–36. doi: 10.1016/j.jbiomech.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Tse KM, Chang R, Lee HP, Lim SP, Venkatesh SK, Ho P. A computational fluid dynamics study on geometrical influence of the aorta on haemodynamics. Eur J Cardiothorac Surg. 2013;43:829–38. doi: 10.1093/ejcts/ezs388. [DOI] [PubMed] [Google Scholar]
- 8.Beller CJ, Gebhard MM, Karck M, Labrosse MR. Usefulness and limitations of computational models in aortic disease risk stratification. J Vasc Surg. 2010;52:1572–9. doi: 10.1016/j.jvs.2010.05.117. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan FM, Luechinger R, Kurtcuoglu V, Sarikaya H, Poulikakos D, Baumgartner RW. Wall stress of the cervical carotid artery in patients with carotid dissection: a case-control study. Am J Physiol Heart Circ Physiol. 2011;300:H1451–8. doi: 10.1152/ajpheart.00871.2010. [DOI] [PubMed] [Google Scholar]
- 10.Muzzarelli S, Meadows AK, Ordovas KG, Higgins CB, Meadows JJ. Usefulness of cardiovascular magnetic resonance imaging to predict the need for intervention in patients with coarctation of the aorta. Am J Cardiol. 2012;109:861–5. doi: 10.1016/j.amjcard.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Lam SK, Fung GS, Cheng SW, Chow KW. A computational study on the biomechanical factors related to stent-graft models in the thoracic aorta. Med Biol Eng Comput. 2008;46:1129–38. doi: 10.1007/s11517-008-0361-8. [DOI] [PubMed] [Google Scholar]
- 12.Morris L, Delassus P, Callanan A, Walsh M, Wallis F, Grace P, et al. 3-D numerical simulation of blood flow through models of the human aorta. J Biomech Eng. 2005;127:767–75. doi: 10.1115/1.1992521. [DOI] [PubMed] [Google Scholar]
- 13.Arheden H, Stahiberg F. Blood flow measurments. In: Higgins CB, De Roos A, editors. MRI and CT of the Cardiovascular System. Philadelphia, PA: Lippincott Williams and Wilkins; 2005, 78–78. [Google Scholar]
- 14.Pugliese P, Pessotto R, Santini F, Montalbano G, Luciani GB, Mazzucco A. Risk of late reoperations in patients with acute type A aortic dissection: impact of a more radical surgical approach. Eur J Cardiothorac Surg. 1998;13:576–80. doi: 10.1016/s1010-7940(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 15.Tagusari O, Ogino H, Kobayashi J, Bando K, Minatoya K, Sasaki H, et al. Should the transverse aortic arch be replaced simultaneously with aortic root replacement for annuloaortic ectasia in Marfan syndrome? J Thorac Cardiovasc Surg. 2004;127:1373–80. doi: 10.1016/j.jtcvs.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Minale C, Splittgerber FH, Reifschneider HJ. Replacement of the entire thoracic aorta in a single stage. Ann Thorac Surg. 1994;57:850–5. doi: 10.1016/0003-4975(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 17.Safi HJ, Miller CC, III, Estrera AL, Villa MA, Goodrick JS, Porat E, et al. Optimization of aortic arch replacement: two-stage approach. Ann Thorac Surg. 2007;83:S815–8. doi: 10.1016/j.athoracsur.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Svensson LG, Crawford ES. Cardiovascular and Vascular Disease of the Aorta. Philadelphia, PA: W.B. Saunders Co.; 1997. [Google Scholar]
- 19.Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg. 2007;83:S865–9. doi: 10.1016/j.athoracsur.2006.10.092. [DOI] [PubMed] [Google Scholar]
- 20.Hofer M, Rappitsch G, Perktold K, Trubel W, Schima H. Numerical study of wall mechanics and fluid dynamics in end-to-side anastomoses and correlation to intimal hyperplasia. J Biomech. 1996;29:1297–308. doi: 10.1016/0021-9290(96)00036-x. [DOI] [PubMed] [Google Scholar]
- 21.Nathan DP, Xu C, Gorman JH, III, Fairman RM, Bavaria JE, Gorman RC, et al. Pathogenesis of acute aortic dissection: a finite element stress analysis. Ann Thorac Surg. 2011;91:458–63. doi: 10.1016/j.athoracsur.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DP, Xu C, Plappert T, Desjardins B, Gorman JH, III, Bavaria JE, et al. Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg. 2011;92:1384–9. doi: 10.1016/j.athoracsur.2011.04.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karmonik C, Partovi S, Muller-Eschner M, Bismuth J, Davies MG, Shah DJ, et al. Longitudinal computational fluid dynamics study of aneurysmal dilatation in a chronic DeBakey type III aortic dissection. J Vasc Surg. 2012;56:260–3. doi: 10.1016/j.jvs.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PT, Horton KM, Fishman EK. Aortic valve and ascending thoracic aorta: Evaluation with isotropic MDCT. AJR Am J Roentgenol. 2010;195:1072–81. doi: 10.2214/AJR.09.2668. [DOI] [PubMed] [Google Scholar]
- 25.O'Gara PT. Acute aortic dissection. Curr Treat Options Cardiovasc Med. 1999;1:11–8. doi: 10.1007/s11936-999-0002-z. [DOI] [PubMed] [Google Scholar]


