1. INTRODUCTION
Photoreceptors and retinal pigment epithelial (RPE) cells, two adjacent cells types of the outer retina, interact with each other functionally in numerous ways. Maintenance of permanent retinal adhesion and cyclic phagocytosis of shed photoreceptor outer segment fragments (POS) by RPE cells are two forms of these interactions that are crucial for vision. RPE cells form a polarized monolayer and extend apical microvilli that ensheath photoreceptor outer segments. Outer segments consist of stacked membranous disks containing the phototransduction machinery and are permanently renewed. To maintain constant outer segment length photoreceptors eliminate their most aged tips by daily shedding (Young, 1967), which precedes a burst of phagocytosis by the RPE that efficiently clears POS from the subretinal space and recycles many of their components (Young and Bok, 1969). POS shedding and subsequent phagocytosis by RPE cells are critical for photoreceptor cell function and long term survival. Indeed, complete failure to ingest POS by RPE cells from the Royal College of Surgeons (RCS) rat strain causes debris accumulation and rapid photoreceptor degeneration (Mullen and LaVail, 1976; Edwards and Szamier, 1977). Clearing their daily load of POS renders post-mitotic RPE cells the most active phagocytes in the body.
Synchronized POS clearance is tightly regulated and any delay in completing the shedding or digestion process can cause accumulation of autofluorescent lipofuscin inclusion bodies containing a complex mix of proteins and lipids that likely result from incomplete turnover of POS material (Feeney, 1978). In vitro studies have recently shown that lipofuscin components may directly impair RPE function and viability (Finnemann et al., 2002; Schutt et al., 2006). These data suggest that defective digestion of POS by RPE cells may contribute to development or progression of age-related retinal diseases such as age-related macular degeneration.
Outer segment renewal in higher vertebrates is synchronized by circadian rhythms influenced by the daily dark-light cycle (Goldman et al., 1980). Animal studies in rod- or cone-dominant species revealed that rods mainly shed their POS within 2 hours after onset of light and cones shed within 2 hours after dusk (LaVail, 1976; Young, 1977). The increase in the number of phagosomes present in RPE cells at these two time points suggests a peak in phagocytic activity every 12 or 24 hours for RPE cells depending on whether they serve rods, cones or both. No untimely phagocytosis has been observed so far, suggesting that RPE cells may downregulate their phagocytic activity if not “on duty”.
Retinal adhesion is equally essential for vision as daily POS phagocytosis but must be maintained permanently. Different factors such as intraocular pressure and a net fluid transport from retina to RPE contribute to retinal adhesion. Additionally, receptors expressed at the RPE apical surface are thought to adhere to ligands in the interphotoreceptor matrix (IPM), a complex mix of proteins and proteoglycans filling the subretinal space and ensheathing cone and rod POS (Hageman et al., 1995; Hollyfield et al., 1989; Hollyfield et al., 1999). IPM proteoglycan rearrangement and RPE microvilli collapse are early responses to retinal detachment that, if persistent, result in RPE dedifferentiation and proliferation, POS degeneration and photoreceptor cell death by apoptosis (Cook et al., 1995).
Despite their obvious importance for vision, we still know little about mechanisms that regulate the rhythm of RPE phagocytosis and RPE surface receptors or IPM ligands that mediate retinal adhesion.
2. STUDYING POS PHAGOCYTOSIS BY RPE CELLS IN VITRO AND IN VIVO
Studies seeking to identify the molecular machinery used by photoreceptors and RPE cells for photoreceptor outer segment renewal greatly benefit from the fact that RPE cells in tissue culture retain their phagocytic activity towards POS. Recording the binding and internalization kinetics of POS by RPE in culture ideally complements the classical microscopic characterization of OS uptake by RPE in vivo. First, OS recognition cannot be studied separately from OS internalization in vivo because shed OS in the subretinal space are juxtaposed to the RPE surface whether or not they are recognized or bound by RPE receptors. Second, far greater numbers of RPE cells can be evaluated in each sample in in vitro assays than in tissue sections. Therefore, small but significant alterations in RPE phagocytic activity may be detected in in vitro assays that may be missed in the light and electron microscopy studies of post-mortem tissues. Third, RPE cells in vitro can be studied following specific manipulation of their phagocytic mechanism by pharmacological compounds, recombinant proteins, protein overexpression or down-regulation, just to name a few. Gain- and loss-of-function approaches are well suited to unequivocally identify critical components of the RPE phagocytic machinery. Fourth, in vitro phagocytic challenge of RPE cells allows one to test directly the phagocytic activity of RPE cells towards POS, while altered photoreceptor shedding or IPM may indirectly alter the phagocytic activity by RPE cells in vivo. Importantly, however, RPE cells in vitro only provide a phagocytic assay system with relevance to RPE phagocytosis in vivo if cells are studied as differentiated, polarized epithelial monolayers that assemble their phagocytic machinery at their apical surface. Finally, while all evidence suggests that the phagocytic activity of polarized RPE cells in vitro retains the primary characteristics of the phagocytic activity of RPE cells in vivo, this is not the case for the nature of particle contact. In experimental phagocytosis assays, RPE cells must establish firm binding of isolated POS that is stable enough to withstand shear forces during sample processing, including vigorous washing steps. This is in sharp contrast to the contact of apical RPE receptors with shedding/shed POS in the subretinal space, where mechanical stress is absent and a stable binding event per se may not occur. Thus, comparison of in vivo and in vitro RPE phagocytosis counting fluorescence- or opsin antibody-labeled POS as illustrated in Figure 1 are both required to fully elucidate the phagocytic machinery of the RPE.
Figure 1.
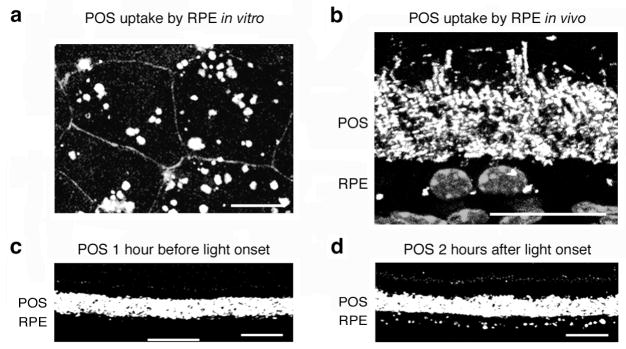
Fluorescence microscopy quantification of POS phagocytosis by RPE cells in vitro and in vivo. (a) Maximal projection of confocal microscopy sections of primary wild-type mouse RPE cells in culture. RPE cells show vigorous uptake of FITC-labeled POS (white) after 1 hour of phagocytic challenge. RPE cell junctions stained with ZO-1 (gray outlines). (b–d) Cryosections of 2-month-old wild-type mice eyecups labeled with rhodopsin antibody. (b) RPE-photoreceptor outer segment interface close-up showing intact rod outer segments and opsin-positive phagosomes (bright white dots) adjacent to RPE nuclei (gray). (c–d) Low magnification view of similar stainings without nuclei illustrates that these images can be used to count phagosome numbers in the RPE. (c) One hour before light onset, the RPE cell layer shows few opsin-labeled phagosomes. (d) Two hours after light onset, the RPE cell layer shows numerous opsin-labeled phagosomes confirming the daily burst of rod POS phagocytosis by the RPE. Scale bars: 20 μm. Modified from Finnemann and Chang with permission from Humana Press Inc.
3. RPE PHAGOCYTOSIS IN VITRO AND IN VIVO USES αvβ5 INTEGRIN
αvβ5 integrin expression at the apical surface of rodent RPE in vivo coincides with postnatal establishment of mature interactions between photoreceptors and RPE including the onset of POS renewal (Finnemann et al., 1997). Stable binding of POS to the cell surface of RPE cells in culture is largely dependent on αvβ5 integrin receptors (Finnemann et al., 1997). In addition to POS recognition or tethering RPE cells also use αvβ5 integrin receptors to activate signaling pathways through focal adhesion kinase that are necessary to activate MerTK (Finnemann, 2003; Nandrot et al., 2004). As β5 integrin only dimerizes with αv integrin subunits, β5 integrin knockout mice provide the opportunity to study RPE cells that permanently and exclusively lack αvβ5 receptors (Huang et al., 2000).
To determine whether αvβ5 integrin plays a role in RPE phagocytosis, we used the technology outlined above to quantify phagocytosis of photoreceptor outer segment fragments (POS) by β5-deficient RPE in vivo and in vitro (Nandrot et al., 2004). Indeed, RPE cells in β5 integrin knockout mice in vivo retain basal levels of phagocytic activity but lack the characteristic burst of phagocytosis upon early morning rod shedding. Moreover, αvβ5 integrin is essential for POS binding in vitro, as RPE cells derived from β5 integrin knockout mice in primary culture largely fail to bind isolated POS. These data demonstrate that αvβ5 integrin has a key function in RPE phagocytosis.
4. STUDYING THE STRENGTH OF RETINAL ADHESION
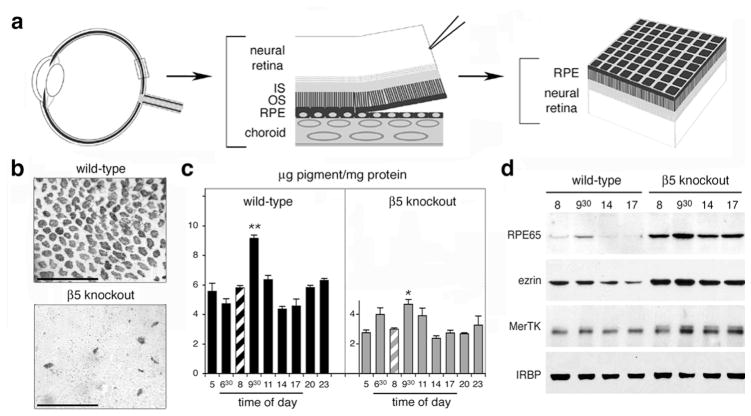
Integrins can directly mediate adhesive interactions of cells with their extracellular substrate or with neighboring cells. Because of the fragility of isolated neural retinas, in vitro assays that study let alone quantify adhesive interactions between photoreceptor outer segments and RPE cells of mammalian origin do not exist. Furthermore, retinal adhesion of intact tissue is known to rapidly decline postmortem in rodent retina. To measure strength of retinal adhesion in retinal tissue immediately post-mortem, we adapted a published protocol to directly compare strength of retinal adhesion from wild-type and knockout mice of different ages as depicted in figure 2a (Endo et al., 1988; Nandrot et al., 2006).
Figure 2.
Retinal adhesion is dramatically reduced in β5 knockout mice. (a) After enucleation and lens/cornea removal, retinas are swiftly peeled off opened eyecups creating shearing forces to assess retinal adhesion. In retinas with normal retinal adhesion, apical cellular domains of RPE largely remain attached to the outer surface of the neural retina. (b) Whole-mount of peeled retina, shown outer retina up, demonstrating that β5 knockout retina retains significantly less RPE pigment than wild-type retina. (c) Quantification of RPE pigment in retina peeled at different times of day. β5 knockout retina shows reduced pigment contents and attenuated adhesiveness peak compared to wild-type retina. (d) Representative immunoblots of individual peeled retina confirming the melanin pigment results. Modified from Finnemann and Chang and from Nandrot et al. (2006) with permission from Humana Press Inc. and the American Physiological Society.
During peeling, the apical domain of RPE cells separates from the basal domain and fractions with the isolated neural retina from wild-type mice if adhesion between the photoreceptors and RPE microvilli is sufficiently strong (Figure 2b). After peeling, both proteins and melanin pigment derived from RPE cells can be analyzed and quantified. Control experiments established that neural retina content of the RPE specific protein RPE65 and of melanin directly correlated with each other and very well represented the number of RPE apical patches attached to the neural retina. Interestingly, both melanin pigment quantification (Figure 2c) and analysis of proteins on western blots (Figure 2d) indicated that retinal adhesion, like retinal phagocytosis, followed a diurnal rhythm with maximum strength of adhesion at ~9.30 AM (3.5 hours after light onset).
5. A ROLE FOR αvβ5 INTEGRIN IN RETINAL ADHESION
To determine whether αvβ5 integrin receptors at the surface of RPE cells participated in retinal adhesion, we used the neural retina separation technique to quantify strength of retinal adhesion in age- and background-matched β5 knockout and wild-type mice (Nandrot et al., 2006). RPE pigment was transferred onto peeled retina from β5 knockout mice to a much lower extent, showing a decreased adhesion due to the absence of αvβ5 integrin receptors (Figure 2b).
Furthermore, melanin and RPE protein quantification revealed that retinal adhesion was significantly reduced at all times in β5 knockout mice (Figure 2c, d). Interestingly the daily cyclic rhythm of retinal adhesion was attenuated, as the peak was less prominent compared to other time points in these mice. These data demonstrate that αvβ5 integrin receptors at the surface of RPE cells contribute significantly to retinal adhesion.
6. PERSPECTIVES
Our results show that lack of αvβ5 integrin receptors eliminates the rhythm of POS phagocytosis in the retina and weakens retinal adhesion. The two functions may be largely independent of each other since both are defective immediately following the establishment of mature RPE-photoreceptor interactions in β5 knockout mouse retina. We also found that retinal adhesion and POS phagocytosis, each a crucial function for vision, follow independent diurnal rhythms that peak at different times of day.
αvβ5 integrin can recognize a number of extracellular ligands such as thrombospondin, vitronectin and MFG-E8. It is therefore an intriguing possibility that apical αvβ5 receptors of the RPE may respond to two different ligands in the subretinal space to either promote POS phagocytic signaling or to mediate retinal adhesion. We are currently exploring candidates to identify the ligand for each of αvβ5 integrin’s function in the retina in vivo.
Acknowledgments
This work was supported by NIH grant EY13295 and the Dyson Foundation. S.C.F. is the recipient of a William and Mary Greeve Scholarship by Research To Prevent Blindness, Inc., and of an Irma T. Hirschl Career Scientist Award.
References
- Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–996. [PubMed] [Google Scholar]
- Edwards RB, Szamier RB. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197:1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- Endo EG, Yao XY, Marmor MF. Pigment adherence as a measure of retinal adhesion: dependence on temperature. Invest Ophthalmol Vis Sci. 1988;29:1390–1396. [PubMed] [Google Scholar]
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Chang Y. Photoreceptor-RPE interactions: Physiology and Molecular Mechanisms. In: Tombran-Tink J, Barnstable CJ, editors. Visual Transduction and Non Visual Light Perception. Humana Press; Totowa, Nerw Jersey: In press. [Google Scholar]
- Goldman AI, Teirstein PS, O’Brien PJ. The role of ambient lighting in circadian disc shedding in the rod outer segment of the rat retina. Invest Ophthalmol Vis Sci. 1980;19:1257–1267. [PubMed] [Google Scholar]
- Hageman GS, Marmor MF, Yao XY, Johnson LV. The interphotoreceptor matrix mediates primate retinal adhesion. Arch Ophthalmol. 1995;113:655–660. doi: 10.1001/archopht.1995.01100050123041. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Varner HH, Rayborn ME, Osterfeld AM. Retinal attachment to the pigment epithelium. Retina. 1989;9:59–68. [PubMed] [Google Scholar]
- Hollyfield JG. Hyaluronan and the functional organization of the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1999;40:2767–2769. [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Sircar M, Finnemann SC. Novel role for αvβ5-integrin in retinal adhesion and its diurnal peak. Am J Physiol Cell Physiol. 2006;290:C1256–C1262. doi: 10.1152/ajpcell.00480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz FG, Dithmar S, Volcker HE, Kopitz J. Accumulation of A2-E in mitochondrial membranes of cultured RPE cells. Graefes Arch Clin Exp Ophthalmol. 2006 doi: 10.1007/s00417-006-0376-5. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruct Res. 1977;61:172–185. doi: 10.1016/s0022-5320(77)80084-1. [DOI] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]