Abstract
Although behavioral weight loss interventions generally have been shown to improve depressive symptoms, little is known as to whether some people with major depressive disorder experience worsening of depression during a weight loss intervention. We examined rates and predictors of change in depression symptoms among 148 obese women with major depressive disorder who participated in a trial comparing depression treatment plus behavioral weight loss treatment (Behavioral Activation; BA) to behavioral weight loss treatment alone (Lifestyle Intervention; LI). A statistically reliable change in depression was calculated as ≥ 9 points on the Beck Depression Inventory in this sample. At 6 months, 73% of participants in BA and 54% of participants in LI showed reliable improvement in depression symptoms and 1.5% of participants in BA and 1.3% of participants in LI showed reliable worsening in depression symptoms. Rates of reliable change were similar at 12 months. Participants who experienced reliable improvement in depression lost significantly more weight than those who did not in both conditions. In the LI condition, baseline psychiatric variables and change in physical activity during treatment were also related to reliable improvement in depression. We found no evidence for an iatrogenic effect of behavioral weight loss treatment on depressive symptoms among obese women with major depressive disorder; rather, behavioral weight loss treatment appears to be associated with significant concurrent improvement in depression. Even greater rates of reliable improvement were observed when depression treatment was added to weight loss treatment.
Introduction
Depression is commonly encountered in clinically obese patients (1, 2). Behavioral weight loss interventions result in improved depressive symptoms in samples not selected for depression (3). However, examining average pre to post-treatment changes in depression during treatment may mask patients experiencing an iatrogenic (i.e., worsening depression) response to weight loss treatment. Three recent studies have examined individual trajectories of depression change during behavioral weight loss treatment.
Faulconbridge and colleagues (2009)(4) examined depression symptom change in non-depressed participants who received one of four weight loss treatments (sibutramine alone, a behavioral weight loss treatment alone, sibutramine + a behavioral weight loss treatment, and sibutramine + brief lifestyle visits with a primary care provider). They found that 8.5% of patients in the behavioral weight loss treatment alone condition experienced a 5-point or greater increase on the Beck Depression Inventory-II (BDI(5)) over 12 months of treatment. During the same time frame, 34.0% of the patients in the behavioral weight loss treatment alone condition experienced a 5 BDI point or greater decrease in depression symptoms. Across all four conditions, they found that a psychiatric history predicted 5-point or greater increases in BDI and that those with increased depression symptoms during treatment also lost less weight. However, it is possible that these relationships were primarily driven by the three of four treatment conditions that included sibutramine (i.e., it is unclear if these relationships were similar specifically in the behavioral weight loss treatment alone condition).
Faulconbridge and colleagues (2012)(6) continued this line of work with a secondary analysis of the Look AHEAD study, which tested the efficacy of a behavioral weight loss treatment in a primarily non-depressed sample (82.8% had BDI < 10 at baseline) of overweight and obese participants with type 2 diabetes. The Look AHEAD randomized trial compared behavioral weight loss treatment to a minimal education and support control group. Among those that received the behavioral weight loss intervention, they found that 1) 6.3% patients who were non-depressed at baseline (i.e., BDI <10) had clinically significant depression symptoms (i.e., BDI ≥ 10) at 1 year follow-up and 2) 60.8% of those with clinically significant depression symptoms at baseline were non-depressed at 1 year follow-up. Change in depression status between baseline and follow-up was significantly associated with concurrent change in weight, such that development of clinically significant depression symptoms was associated with less weight loss and resolution of clinically significant depression symptoms was associated with greater weight loss.
In a sample of obese women with moderate or greater depression symptoms, Simon and colleagues (7) examined changes in depression in participants randomized to either a behavioral weight loss intervention or a behavioral weight loss intervention integrated with CBT for depression. They found that 10% of participants experienced increases in depression symptoms and 33% experienced decreases in depression symptoms during treatment, but they did not examine differences between treatment conditions. Similar to the two studies reviewed above, they found that change in depression symptoms during treatment (i.e. increasing vs. stable vs. decreasing) was positively associated with concurrent changes in weight such that those with decreases in depression lost the most weight, while those with increases in depression lost the least weight. In addition, they found that those with decreases in depression had the largest increases in physical activity, while those with increases in depression had the smallest increases in physical activity. Change in depression symptoms was not associated with change in energy intake
None of the above studies used an established method for setting a cut-off for meaningful change in depression symptoms. Although Faulconbridge and colleagues (2012)(6) used a well established cutoff for clinically significant symptoms on the BDI (i.e., BDI ≥ 10), a patient with very little change in BDI score over 1 year could be classified as developing clinically significant depression symptoms (e.g., a patient with a BDI of 8 at baseline and 10 at 1 year follow-up was classified as developing clinically significant depression symptoms when their BDI score only changed by two points).
Thus it is unclear whether the degree of change reported in these studies is within the range that would be expected simply due to the less than perfect reliability of instrument used to measure depression. Extensive previous work has developed equations for calculating statistically reliable change that take into account the reliability of the instrument used to measure depression (the “Reliable Change Index” (8, 9).
Data from the Be Active trial (10, 11) provides an opportunity to examine the rates of statistically reliable improvement and worsening of depression symptoms among obese women with major depressive disorder (MDD) during either a behavioral weight loss intervention alone or one integrated with depression treatment. Secondarily, we explored baseline predictors of reliable changes in depression, with a particular focus on psychiatric factors (e.g., anti-depressant status, co-morbid psychiatric diagnoses) in order to follow-up on previous findings (4). Finally, we explored the association between reliable depression change and concurrent changes in energy intake, physical activity, and weight. We conducted analyses within each condition in order to present the first data on predictors of individual changes in depression during a behavioral weight loss treatment alone (i.e., not combined with other treatment). We also extend upon previous work by controlling for baseline depression in regression analyses.
Method and Procedures
Participants were enrolled in a randomized clinical trial testing whether adding behavioral treatment for depression to a behavioral weight loss intervention improves weight loss relative to a behavioral weight loss intervention alone in women with co-morbid obesity and depression (10). The design and methods of this trial have been described in detail elsewhere (11). All procedures were approved by the Internal Review Board of the University of Massachusetts Medical School.
Participants were women between the ages of 21 and 65 years who met criteria for MDD via a structured clinical interview (12), but were not actively suicidal, had not been hospitalized for psychiatric issues in the previous 12 months, and were not currently in psychotherapy. Other exclusion criteria included: smoking; bipolar disorder, psychotic disorder, bulimia, or post-traumatic stress disorder; diabetes; bariatric surgery; inability to walk unaided for 0.25 miles or lack of clearance from a primary care provider for physical activity; conditions prohibiting dietary changes; medical conditions likely to limit lifespan; current weight loss treatment; taking medications known to affect appetite and/or weight; pregnant or trying to become pregnant; and non-English speaking. Participants on antidepressant medication needed to have a period of no dose change of 3 months before enrolling in the intervention.
Treatment Conditions
Participants were randomized to one of two conditions; Behavioral Activation (BA; depression treatment followed by a lifestyle intervention) or a Lifestyle Intervention with attention control visits (LI). BA and LI conditions included a 6-month (weeks 1–24) Intensive Treatment phase (ten-60 minute individual sessions and sixteen 90-minute group sessions) and a 6 month Maintenance Phase (6 monthly 90-minute group sessions and 6 monthly 20-minute counseling phone calls). In both conditions, group sessions during the Intensive Treatment and Maintenance Phases followed the Diabetes Prevention Program (DPP(13)) Lifestyle Intervention protocol. All groups were led by either a dietitian or an exercise physiologist. The content of these groups was identical across conditions. The timing of group visits and the content of the individual intensive phase sessions and maintenance phase phone calls distinguished conditions.
BA Condition
In the BA condition, the 10 individual sessions during the intensive treatment phase were focused on behavioral depression treatment (see below) and were conducted by master’s- and doctoral-level counselors under the supervision of a licensed clinical psychologist. These individual sessions occurred weekly during the first 10 weeks of treatment (weeks 1–10). The group behavioral weight loss sessions during the intensive treatment phase started at week 9 and continued to the end of the intensive phase (weeks 9–24). During the maintenance phase for BA participants, the monthly 20-minute counseling phone calls also focused on depression treatment.
The depression treatment was based on the brief behavioral activation treatment developed by Lejuez and colleagues (14). Meta-analytic findings support the efficacy of this approach (15). Behavioral activation is posited to improve depression by increasing the patient’s contact with positive reinforcement through collaborative, structured activity scheduling. These out-of-session activity assignments are explicitly focused on the patient’s life values, and much of counseling sessions focus on problem-solving assignment completion. BA sessions were exclusively focused on depression treatment. Thus, BA therapists did not monitor or provide recommendations for diet or physical activity changes.
LI Condition
In the LI condition, both the 16 group weight loss sessions and the 10 individual sessions started at week 1 and were spread throughout the intensive phase (weeks 1–24). Both the intensive phase individual counseling sessions and the monthly maintenance phase phone calls focused on general health education (i.e., attention control). The content of health education sessions and calls were determined by the participant who could select from 23 health topics relevant to women’s health (e.g., skin health, proper footwear). These contacts were conducted by health educators with no training in psychological or lifestyle counseling.
Main Trial Results
In the main analyses of this trial, there were no differences between the BA and LI conditions in weight loss (10), however participants in the BA condition experienced better depression outcomes (16), including higher rates of response and remission on the BDI. Pagoto and colleagues (10) also reported that participants who experienced depression response or remission also experienced greater concurrent weight loss.
Measures
Depression, weight, energy intake, and physical activity were assessed at baseline, 6 months, and 12 months. We report on changes in these variables between baseline and the follow-up time points. Throughout the results, negative change scores indicate decreasing values and positive change scores indicate increasing values.
Depression
MDD diagnosis was determined before enrollment though the Structured Clinical Interview for DSM-IV (12) with a trained assessor. Severity of depression symptoms was measured using the Beck Depression Inventory-II (BDI; (5)), a 21-item self-report scale that assesses mood over the previous 2 weeks. Possible BDI scores range from 0–63 with higher scores indicating greater depressed mood. The reliability and validity of the BDI is well established (17) and the reliable change index has been calculated on the BDI in several past depression treatment studies (18–22).
Reliable change is calculated using a scale’s internal consistency and standard deviation within the sample for which it is to be applied (8). The cut-off for reliable change is calculated as 1.96 times the standard error of the difference between scores of a given measure administered on two occasions (SEDiff;). RCI cut off was calculated using the following formula:
SDpre represents total score standard deviation at baseline and α represents scale reliability. This sample was large enough that standard deviation and reliability at baseline provide reasonable estimates of these statistics among women with co-morbid obesity and depression. Thus, baseline BDI data, rather than general population psychometric data, were used to calculate a reliable change cut-off. Only participants with a baseline BDI and either a 6-month or 12-month BDI (n = 148) were included in this calculation (as only these participants were included in later depression change analyses). The standard deviation of the BDI at baseline (SDPre) was 5.74 and Cronbach’s alpha (α) was .73. A SEDiff of 4.22 and a reliable change cut-off of 8.27 were computed for the current sample. Thus, any change of ≥ 9 BDI points was considered a reliable change in the forthcoming analyses. Of note, our sample calculated reliable change cut-off ≥ 9 BDI points is the same as what has been suggested as by experts as a cut-off for statistically reliable change on the BDI in non-obese samples (23). We categorized participants as experiencing a reliable worsening of depression (≥ 9 BDI point increase), no reliable change (less than 9 point change in either direction), and reliable improvement in depression (≥ 9 BDI point decrease).
Weight
Weight was measured using a digital Tronix scale in light clothing and without shoes. Weight is reported in pounds (lbs).
Energy Intake
Average daily energy intake (kcal) was calculated at each time point using 24-hour diet recalls on 3 randomly-selected days (2 weekdays, 1 weekend day) within a 3-week window. Interviews were computer-guided and utilized a multiple-pass diet recall technique. Interviews were conducted by registered dietitians who were blind to treatment condition. The data produced by these interviews was analyzed using Nutrition Data System for Research (NDSR; version 2010, Nutrition Coordinating Center, University of Minnesota, MN;(24, 25)) to produce an average calories per day value (kcal).
Physical Activity
Average daily energy expended in sport and leisure physical activity (METs) was also assessed during the 24-hour recall phone calls described above. Daily METs were calculated based on participant report of time engaged in mild, moderate, hard, and very hard physical activity across multiple sport and leisure activities.
Other Psychiatric Variables
Five psychiatric variables were assessed at baseline. Participants self-reported current anti-depressant medication status (yes/no). Past MDD diagnosis (yes/no) and other axis-I psychiatric diagnoses (yes/no) were assessed using the Structured Clinical Interview for DSM disorders (12). Possible co-morbid diagnoses were: substance (including alcohol) abuse or dependence, panic disorder, social phobia, specific phobia, obsessive compulsive disorder, and generalized anxiety disorder. Note that some co-morbid psychiatric diagnoses (e.g., bipolar disorder) were exclusion criteria.
Attention deficit/hyperactivity disorder (ADHD) symptomatology was measured using the Adult ADHD Self-Report Scale (ASRS;(26)). While the total ASRS has 18 items, 6 items (ASRS part A) have been found to be most predictive of ADHD and are used as a dichotomous screener. Specifically, those likely to have ADHD (≥4 of 6 symptoms) are distinguished from those unlikely to have significant ADHD symptoms (<4 of 6 symptoms). An ADHD screener was included in the original trial and the current analysis due to recent research suggesting that ADHD is highly co-morbid with obesity and predicts poor outcomes in behavioral weight loss treatment (27–29).Hedonic capacity (i.e., potential to experience positive affect) was measured using the 36-item self-reported Fawcett-Clark Pleasure Capacity Scale (FCPS;(30)). Items ask about the degree of pleasure that would be experienced in several hypothetical pleasurable situations across several domains. Reliability and validity have been established in clinical and non-clinical samples (31). Hedonic capacity was measured in this trial because, its inverse anhedonia, is a hallmark symptom of depression and is important to BA’s purported mechanism of change. High scores on the FCPS indicate higher levels of hedonic capacity.
Analytic Plan
Of the 161 women enrolled in the Be Active trial, we excluded from our analyses women missing BDI scores at both 6 and 12 months (because no reliable change could be calculated). Rates of reliable depression change are presented for baseline to 6-month follow-up and baseline to 12-month follow-up. Because we were specifically interested in predictors of depression change category within each treatment group, we followed the recommendation of Rosenthal and colleagues (32) for “focused” analyses. Accordingly, we tested our hypotheses regarding prediction of depression change category through orthogonal a priori contrasts within both treatment groups.
We present bivariate (i.e., t-tests, Mann-Whitney U Tests, and χ2 tests) and multivariate relationships between depression change category at 6 months and 1) baseline characteristics, and 2) change in weight, energy intake, and physical activity between baseline and 6 months. We chose 6 months because it marked the end of intensive treatment. For multivariate analyses, three separate, three-step hierarchical logistic regressions were run for each of the two treatment conditions (i.e., BA and LI) to test if baseline to 6-month changes in weight, energy intake, or physical activity were related to 6-month reliable change in depression after controlling for baseline variables. Baseline depression was entered in Step 1 for all models to control for any variance caused by floor (i.e., participants with lower baseline BDI scores may be less likely to fall by 9 points) or regression to the mean effects. Other baseline predictors included in Step 2 of all models were to control for potential confounders and were informed by bivariate relationships between baseline variables and depression change category. Specifically, baseline variables with a p < .10 bivariate relationship with reliable change in either condition were entered into Step 2. Step 3 added change in weight, energy intake, or physical activity during treatment as predictors, entered in separate models. Finally, in order to facilitate direct comparison with previous work with non-depressed samples using the BDI(4), we calculated the proportion of participants with a change of five or more BDI points in either direction between baseline and 12 month follow up.
Results
All participants in the Be Active trial (10, 11) with BDI scores at baseline and either 6-months or 12 months were included in the current study (n = 148, 91.9% of randomized sample). Participants were primarily married or living with a partner (67.6%), Caucasian (85.8%), employed full time (65.5%) and had some post-high school education (some college or trade school; 91.9%). Mean age was 46.0 years (SD = 10.95) and median household income was $50,000–60,000 per year. Baseline BDI in this sample ranged from 12 to 38 with a mean of 21.0. There were no significant differences between treatment conditions on any of these variables.
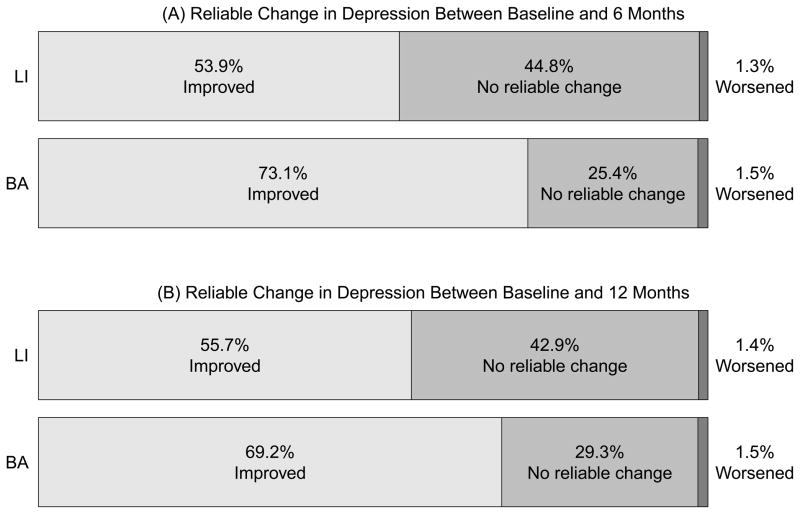
Figure 1a presents the rates of participants with reliable worsening in depression (1.5% in BA, 1.3% in LI), reliable improvement in depression (73.1% in BA, 53.9% in LI), and no reliable change in depression (25.4% in BA, and 44.8% in LI) between baseline and 6 month assessment. Figure 1b presents these categories between baseline and the 12 month assessment (reliable worsening: 1.5% in BA, 1.4% in LI; reliable improvement: 69.2% in BA, 55.7% in LI; no reliable change: 29.3% in BA, 42.9% in LI). The rate of reliable improvement was significantly higher in BA relative to LI between baseline and 6 months (χ2 = 5.62, p =.02), but no significant difference was observed between conditions on reliable improvement from baseline and 12 months (χ2 = 2.62; p = .11). Of those who had experienced a reliable improvement by 6 months, 86.4% in the BA condition and 76.3% in the LI condition maintained that improvement at 12 months.
Figure 1.
Rates of reliable changes in depression between baseline and 6 months (A) and between baseline and 12 months (B).
Because of the low rate of participants experiencing reliable worsening in depression (<1.5% of the total sample) both those with no reliable change in depression and those who experienced reliable worsening were collapsed into one category. For clarity, this combined group will be referred to as non-responders. Further analyses compared those experiencing non-response to those experiencing reliable improvement.
Table 1 presents the bivariate relationships between baseline and treatment variables and depression change category at 6 months. Among women in the BA condition greater weight loss during treatment was significantly associated with reliable improvement (t(65) = 2.47, p = .02) and, greater baseline physical activity was marginally associated with reliable improvement (METs; Z =1.93, p = .053)1. Among women in the LI condition, higher baseline depressive symptoms (BDI; t(72) = 3.33, p = .001), absence of baseline ADHD symptoms (χ2 = 4.82, p =.03), lower hedonic capacity (t(72) = 2.24, p = .03), and greater weight loss during treatment (t(73) = 3.66, p < .001) were significantly associated with reliable improvement in depression. Absence of co-morbid psychiatric diagnoses at baseline (χ2 = 3.12, p =.08) and greater reduction in energy intake (t(72) = 1.78, p = .08) were also marginally related to reliable improvement in the LI condition.
Table 1.
Baseline characteristics and treatment variables by reliable improvement in depression (=9 BDI point decrease) at 6 months and treatment group, (mean +/−SD, median +/− IQR, or percent).
| BA (n=67) | LI (n = 76) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Reliable improvement (n = 49) | Non-Response (n = 18) | p | Reliable Improvement (n = 41) | Non-Response (n = 35) | p | |
| Baseline Variables | ||||||
|
| ||||||
| Weight (lbs) | 213.2 ± 27.8 | 207.1 ± 26.9 | 200.4 ± 20.3 | 207.6 ± 24.4 | ||
| Energy intake (kcal/day) | 2300.2 ± 826.3 | 2065.7 ± 1208.3 | 2079.4 ± 559.4 | 2053.9 ± 572.5 | ||
| Physical Activity (METs/day, Median ± IQR) | .36 ±1.9 | 0 ± .38 | p=.05 | 0 ± 1.1 | .44 ± 1.3 | |
| Baseline Depression | 22.1 ± 5.5 | 19.6 ± 5.9 | 22.4 ± 6.2 | 18.4 ± 4.4 | p =.001 | |
| Taking Anti-depressants | 26.5% | 38.9% | 29.3% | 31.4% | ||
| ADHD Symptoms | 29.2% | 22.2% | 24.4% | 48.6% | p = .03 | |
| Past-MDD | 67.3% | 55.6% | 70.7% | 65.7% | ||
| Other Psychiatric Diagnosis a | 40.8% | 55.6% | 34.1% | 54.3% | p = .08 | |
| Hedonic Capacity | 130.3 ± 18.0 | 122.9 ± 18.7 | 121.2 ± 19.6 | 131.1 ± 18.2 | p = .03 | |
|
| ||||||
| Treatment Variables – change from baseline to 6 months | ||||||
|
| ||||||
| Change in weight (lbs) | −8.2 (10.0) | −1.07 (11.6) | p = .02 | −11.5 (11.9) | −2.56 (8.6) | p < .001 |
| Change in energy intake (kcals/day) | −548.56 (794.1) | −497.83 (1014.4) | −579.52 (472.6) | −343.33 (664.4) | p =.08 | |
| Change in physical activity (METs/day) | .51 (1.98) | .48 (.74) | .83 (2.3) | .46 (1.4) | ||
Possible co-morbid diagnoses were: Substance (including alcohol) abuse or Dependence, Panic Disorder, Social phobia, Specific Phobia, Obsessive Compulsive Disorder, and Generalized Anxiety Disorder
Logistic regression models can be seen in Table 2. Based on bi-variate relationships, baseline physical activity (METs), ADHD symptoms, co-morbid psychiatric diagnosis, and hedonic capacity were included as predictors in Step 2. The level of multicollinearity among these baseline predictor variables did not preclude them from being entered into the same model (all rs < .35). In the BA condition, no step 1 or step 2 predictors were significant, but greater baseline physical activity was marginally related to reliable improvement (p = .06). In step 3 models in the BA condition, only change in weight was a marginally significant predictor (p = .07). In the LI condition, higher baseline depression significantly predicted reliable response in Step 1 (p < .01). In Step 2, the absence of ADHD symptoms significantly (p = .04) and the absence of other psychiatric diagnoses marginally (p = .07) predicted reliable response. Greater reduction in weight (p = .001) and greater increase in physical activity (p = .01) were each significant predictors of reliable response in step 3 models in the LI condition.
Table 2.
Predictors of reliable improvement in depression (≥9 BDI point decrease) between baseline and 6-month follow-up
| BA (n = 65) | LI (n = 72) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Odds Ratio | 95% CI | R2 | R2 change | Odds Ratio | 95% CI | R2 | R2change | |
| Step 1: Baseline Depression | 1.08 | (.97–1.21) | .04 | 1.16** | (1.05–1.28) | .13 | ||
|
| ||||||||
| Step 2: Step 1 + Other Baseline variables | .18 | .14 | .27 | .14 | ||||
| METS/day | 2.24* | (.96–5.25) | 1.52 | (.90–2.57) | ||||
| ADHD Symptoms | .79 | (.18–3.38) | .29** | (.09–.92) | ||||
| Other Psychiatric Diagnosis | .47 | (.13–1.66) | .35* | (.11–1.09) | ||||
| Hedonic Capacity | 1.03 | (.99–1.07) | .98 | (.95–1.01) | ||||
|
| ||||||||
| Step 3 (entered separately): | ||||||||
| Step 2 + Change in weight | .93* | (.86–1.01) | .23 | .05 | .89** | (.83–.96) | .42 | .15 |
| Step 2 + Change in kcal/day | 1.00 | (1.00–1.00) | .18 | .00 | 1.00 | (1.00–1.00) | .29 | .02 |
| Step 2 + Change in METs/day | 1.34 | (.78–2.32) | .20 | .02 | 1.59** | (1.11–2.28) | .34 | .07 |
R2= Cox & Snell’s pseudo R-squared.
p < .1,
p < .05.
For comparison to Faulconbridge and colleagues’ (2009) (4) non-depressed sample, we performed similar analyses using the cut-off they used. Recall that Faulconbridge and colleagues found that among their patients in the behavioral weight loss treatment alone condition, 34% experienced ≥5 BDI point reduction at 1 year, while 8.5% experienced a ≥5 BDI point increase at 1 year. In the LI condition of the current study 77.1% of participants experienced ≥5 BDI point reduction in depression and 4.29% experienced a ≥5 BDI point increase in depression between baseline and 1 year. In the BA condition, 81.5% of participants experienced ≥5 BDI point reduction in depression and 6.15% experienced a ≥5 BDI point increase in depression between baseline and 1 year.
Discussion
Results indicate that very few participants (<2%) experienced a reliable increase in depression symptoms during treatment. In fact, the majority of participants (63% of the total sample) experienced reliable improvement in depression (≥9 BDI point decrease) during treatment, even in the LI condition (54%) where depression was not specifically treated. These findings raise the possibility that behavioral weight loss treatment alone has significant antidepressant effects.
We also used a 5 BDI point or greater cut-off to allow for a direct comparison with Faulconbridge and colleagues (2009) (4). We found that participants in the LI condition were more likely to experience 5 BDI point improvements in depression at 1 year follow up (77.1%) than the non-depressed participants in Faulconbridge and colleagues in a similar behavioral weight loss treatment condition (34.0%). Further, worsening of depression by five or more BDI points was half as likely in our LI condition (4.29%) as in the Faulconbridge and colleagues behavioral weight loss treatment condition (8.5%). It is possible that these differences between Faulconbridge and colleagues’ non-depressed sample and the current study’s depressed sample were caused by regression to the mean or spontaneous recovery due to the natural episodic course of depression in the current study. Nonetheless, it appears that those with MDD are more likely to experience improvement and less likely to experience worsening of depression during a behavioral weight loss treatment than non-depressed patients.
Across both cut-off thresholds, results suggest that behavioral weight loss treatment does not exacerbate depression symptoms and is therefore not contraindicated in depressed patients. However, several studies have shown that those with depression lose less weight in behavioral weight loss treatments (e.g., (1, 33, 34)), and that improvement in depressive symptoms during behavioral weight loss treatment is associated with concurrent weight loss (4, 6, 7, 35). Therefore, depression still appears to pose a barrier to weight loss even though symptoms tend to improve during behavioral weight loss treatment. The parent study also revealed that depression response and remission were associated with greater weight losses (10).
In the LI condition, higher depression symptoms, lower hedonic capacity, and the absence of ADHD symptoms were significantly associated with greater likelihood of experiencing a reliable improvement in depression. The absence of a co-morbid psychiatric condition was also marginally (p<.10) predictive of experiencing reliable improvement in depression. The strong relationship between baseline depression and depression change category in the LI condition likely represents a combination of regression to the mean and floor effects (i.e., one is more likely to improve by ≥ 9 BDI points if starting from a BDI of 30 than from a BDI of 13). Further, the counterintuitive finding regarding hedonic capacity is likely an artifact of these effects, as anhedonia is a symptom of depression. This interpretation is supported by the finding that hedonic capacity was not predictive of reliable improvement after controlling for baseline depression in multivariate analyses (Step 2, Table 2).
Findings in the LI condition showing that co-morbid psychiatric diagnosis and ADHD symptoms were associated with non-response are consistent with the Faulconbridge and colleagues (2009) (4) finding that general psychiatric history at baseline predicted depression worsening during treatment. It is notable that a positive screen for ADHD symptoms at baseline was significantly associated with a lower likelihood of experiencing reliable improvement in depression in both bi-variate and multivariate analyses (Step 2, Table 2) because of the recently established link between obesity and ADHD (27–29).
In the BA condition, greater baseline leisure time physical activity was marginally related to reliable improvement in both bivariate and multivariate comparisons. It is possible that the focus of BA on activation was particularly helpful among those who were more physically active. The absence of psychiatric variables predicting depression change category in the BA condition in either bi-variate or multivariate analyses suggests that Behavioral Activation may buffer some of the negative effects emanating from pre-existing psychiatric diagnoses, although the processes by which this might happen are unclear.
Our observation that reliable improvement in depression in both conditions is related to greater concurrent weight loss replicates the findings of previous studies (4, 6, 7), but in a sample that was selected for MDD per a diagnostic interview. Unlike previous studies, we demonstrated this relationship in the LI condition after controlling for multiple baseline variables associated with depression change. Most importantly, the current study controlled for baseline depression (thus controlling for floor and regression to the mean effects) and psychiatric factors that predict depression change. However, because depression change and weight change were measured concurrently, no direct causal conclusions can be made from this data.
Consistent with Simon and colleagues (7), we found no significant relationships between depression change status and change in energy intake. In bivariate analyses we were unable to replicate Simon and colleagues finding that changes in physical activity are related to changes in depression symptoms. However, in the LI condition after controlling for baseline variables, greater increases in physical activity were significantly associated with higher rates of reliable improvement in depression. This finding is consistent with data supporting exercise as a promising treatment for depression (e.g., (36)). However, no causal conclusions can be drawn regarding physical activity change and depression change status due to concurrent measurement.
This study is the first to examine individual participant changes in depression during behavioral weight loss treatment among those with diagnosed MDD by structured interview. Strengths include the use of an established method of determining a cut-off for significant change in depression (“Reliable Change Index”; (8)) and control for baseline confounders. However, some study limitations are notable. First, this sample was restricted to women aged 21–65 years, so it is unknown if these findings generalize to men or women of other age groups. It is notable that the Simon and colleagues study (7) also only included women and the Faulconbridge and colleagues (2009) study (4) sample was about 80% women, while studies suggesting that weight loss can trigger significant increases in depression (37) included only men. Second, this study excluded the most severely depressed patients, thus this study does not speak to possible iatrogenic effects in patients with the most severe depression. Finally, the results described here should not be interpreted regarding the comparative efficacy of BA vs. LI on depression symptoms. The current analyses were specifically designed to explore the rate and predictors of significant changes in depression within each treatment. The main outcome paper for the Be-Active trial (10) should be the primary resource for questions regarding treatment efficacy.
Although this study and others have shed some light on the rates of depression change during behavioral weight loss treatment, the exact processes through which depression change occurs (improvement or worsening) are not well understood. For example the temporal relationship between changes in depression and changes in weight during treatment is unknown. Further, plausible physiological and psychological mediators of the relationship between weight and depression during weight loss treatment remain unexplored. For example, it is possible that the social reinforcement one receives for losing weight or body image changes following weight loss drive depression changes. It is also possible that behavioral weight loss treatment itself (e.g., social support, working toward weight loss goals, etc) is activating enough to have a significant effect on depression among those with MDD. These possibilities should be explored in future studies.
In summary, we found that the majority of women in our sample experienced reliable improvement in depression during the trial, regardless of whether they received depression treatment in addition to behavioral weight loss treatment. Less than 2% of all participants experienced a reliable worsening of depression. Our results suggest that behavioral weight loss treatments are unlikely to cause significant worsening of depression in obese women with MDD and may cause significant improvements. We also replicated previous findings indicating that improvement in depression during weight loss treatment is associated with better weight outcomes. The existence of a concurrent relationship between weight reduction and depression improvement during weight loss treatment is now well established and future research aimed at explaining this relationship is warranted.
Acknowledgments
The Be Active Trial was supported by R01 MH078012 to S. Pagoto. This secondary analysis was supported by K23HL107391 to A. Busch. Partial salary support for M. Waring is provided by 1U01HL105268.
Footnotes
Baseline physical activity data was significantly positively skewed. Thus, the non-parametric Mann-Whitney U Test was used, rather than a t-test. Median and IQR are reported in Table 1 rather than mean and SD.
Disclosure Statement
We have no conflicts to disclose.
References
- 1.Pagoto S, Bodenlos JS, Kantor L, Gitkind M, Curtin C, Ma Y. Association of major depression and binge eating disorder with weight loss in a clinical setting. Obesity (Silver Spring) 2007 Nov;15(11):2557–9. doi: 10.1038/oby.2007.307. [DOI] [PubMed] [Google Scholar]
- 2.Pagoto S, Schneider KL, Appelhans B, Curtin C, Hadjuk A. Psychological co-morbidities of obesity. In: Pagoto S, editor. Psychological Co-Morbidities of Physical Illness: A Behavioral Medicine Perspective. New York: Springer; in press. [Google Scholar]
- 3.Fabricatore AN, Wadden TA, Higginbotham AJ, Faulconbridge LF, Nguyen AM, Heymsfield SB, et al. Intentional weight loss and changes in symptoms of depression: a systematic review and meta-analysis. Int J Obes (Lond) 2011 Nov;35(11):1363–76. doi: 10.1038/ijo.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faulconbridge LF, Wadden TA, Berkowitz RI, Sarwer DB, Womble LG, Hesson LA, et al. Changes in symptoms of depression with weight loss: results of a randomized trial. Obesity (Silver Spring) 2009 May;17(5):1009–16. doi: 10.1038/oby.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 6.Faulconbridge LF, Wadden TA, Rubin RR, Wing RR, Walkup MP, Fabricatore AN, et al. One-Year Changes in Symptoms of Depression and Weight in Overweight/Obese Individuals With Type 2 Diabetes in the Look AHEAD Study. Obesity (Silver Spring) 2012 Apr;20(4):783–93. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon GE, Rohde P, Ludman EJ, Jeffery RW, Linde JA, Operskalski BH, et al. Association between change in depression and change in weight among women enrolled in weight loss treatment. Gen Hosp Psychiatry. 2010 Nov-Dec;32(6):583–9. doi: 10.1016/j.genhosppsych.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991 Feb;59(1):12–9. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 9.Wise EA. Methods for analyzing psychotherapy outcomes: a review of clinical significance, reliable change, and recommendations for future directions. J Pers Assess. 2004 Feb;82(1):50–9. doi: 10.1207/s15327752jpa8201_10. [DOI] [PubMed] [Google Scholar]
- 10.Pagoto S, Schneider KL, Whited MC, Oleski JL, Merriam P, Appelhans B, et al. Sequential depression and weight loss treatment for obese women with clinical depression: The Be Active Trial. under review. [Google Scholar]
- 11.Schneider KL, Bodenlos JS, Ma Y, Olendzki B, Oleski J, Merriam P, et al. Design and methods for a randomized clinical trial treating comorbid obesity and major depressive disorder. BMC Psychiatry. 2008;8:77. doi: 10.1186/1471-244X-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992 Aug;49(8):624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002 Dec;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lejuez CW, Hopko DR, Hopko SD. A brief behavioral activation treatment for depression. Treatment manual. Behav Modif. 2001 Apr;25(2):255–86. doi: 10.1177/0145445501252005. [DOI] [PubMed] [Google Scholar]
- 15.Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med. 2008 May;38(5):611–23. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001 Mar;20(2):112–9. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 18.de Graaf LE, Gerhards SA, Arntz A, Riper H, Metsemakers JF, Evers SM, et al. Clinical effectiveness of online computerised cognitive-behavioural therapy without support for depression in primary care: randomised trial. Br J Psychiatry. 2009 Jul;195(1):73–80. doi: 10.1192/bjp.bp.108.054429. [DOI] [PubMed] [Google Scholar]
- 19.Hopko DR, Armento ME, Robertson SM, Ryba MM, Carvalho JP, Colman LK, et al. Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: randomized trial. J Consult Clin Psychol. 2011 Dec;79(6):834–49. doi: 10.1037/a0025450. [DOI] [PubMed] [Google Scholar]
- 20.Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. Br J Psychiatry. 2011 Jan;198(1):66–72. doi: 10.1192/bjp.bp.110.079111. [DOI] [PubMed] [Google Scholar]
- 21.Ransom D, Heckman TG, Anderson T, Garske J, Holroyd K, Basta T. Telephone-delivered, interpersonal psychotherapy for HIV-infected rural persons with depression: a pilot trial. Psychiatr Serv. 2008 Aug;59(8):871–7. doi: 10.1176/ps.2008.59.8.871. [DOI] [PubMed] [Google Scholar]
- 22.Titov N, Andrews G, Davies M, McIntyre K, Robinson E, Solley K. Internet treatment for depression: a randomized controlled trial comparing clinician vs. technician assistance. PLoS One. 2010;5(6):e10939. doi: 10.1371/journal.pone.0010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogles BM, Lambert MJ, Fields SA. In: Essentials of Outcome Assessment. Kaufman AS, Kaufman NL, editors. New York: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 24.Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products - A research perspective. J Food Comp and Anal. 2001;14:315–22. [Google Scholar]
- 25.Schakel SF, Buzzard IM, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Comp and Anal. 1997;10:102–14. [Google Scholar]
- 26.Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005 Feb;35(2):245–56. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- 27.Cortese S, Angriman M, Maffeis C, Isnard P, Konofal E, Lecendreux M, et al. Attention-deficit/hyperactivity disorder (ADHD) and obesity: a systematic review of the literature. Crit Rev Food Sci Nutr. 2008 Jun;48(6):524–37. doi: 10.1080/10408390701540124. [DOI] [PubMed] [Google Scholar]
- 28.Pagoto SL, Curtin C, Bandini LG, Anderson SE, Schneider KL, Bodenlos JS, et al. Weight loss following a clinic-based weight loss program among adults with attention deficit/hyperactivity disorder symptoms. Eat Weight Disord. 2010 Sep;15(3):e166–72. doi: 10.1007/BF03325296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008 Jul;122(1):e1–6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 30.Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983 Jan;40(1):79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- 31.Clark DC, Fawcett J, Salazar-Grueso E, Fawcett E. Seven-month clinical outcome of anhedonic and normally hedonic depressed inpatients. Am J Psychiatry. 1984 Oct;141(10):1216–20. doi: 10.1176/ajp.141.10.1216. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research: A correlational approach. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 33.Clark MM, Niaura R, King TK, Pera V. Depression, smoking, activity level, and health status: pretreatment predictors of attrition in obesity treatment. Addict Behav. 1996 Jul-Aug;21(4):509–13. doi: 10.1016/0306-4603(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 34.Pekarik G, Blodgett C, Evans RG, Wierzbicki M. Variables related to continuance in a behavioral weight loss program. Addict Behav. 1984;9(4):413–6. doi: 10.1016/0306-4603(84)90044-3. [DOI] [PubMed] [Google Scholar]
- 35.Elder CR, Gullion CM, Funk KL, Debar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes (Lond) 2012 Jan;36(1):86–92. doi: 10.1038/ijo.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999 Oct 25;159(19):2349–56. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 37.Chaput JP, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiol Behav. 2005 Sep 15;86(1–2):224–32. doi: 10.1016/j.physbeh.2005.07.014. [DOI] [PubMed] [Google Scholar]